
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jakub Smiechowicz | + 2126 word(s) | 2126 | 2022-02-09 04:15:06 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2126 | 2022-02-14 03:28:28 | | |
Video Upload Options
Lipopolysaccharide, the main component of the outer membrane of Gram-negative bacteria is a highly potent endotoxin responsible for organ dysfunction in sepsis. It is present in the blood stream not only in Gram-negative infections, but also in Gram-positive and fungal infections, presumably due to sepsis-related disruption of the intestinal barrier. Various pathways, both extra- and intracellular, are involved in sensing endotoxin and non-canonical activation of caspase-mediated pyroptosis is considered to have a major role in sepsis pathophysiology. Endotoxin induces specific pathological alterations in several organs, which contributes to poor outcomes. The adverse consequences of endotoxin in the circulation support the use of anti-endotoxin therapies, yet more than 30 years of experience with endotoxin adsorption therapies have not provided clear evidence in favor of this treatment modality. The results of small studies support timely endotoxin removal guided by measuring the levels of endotoxin; unfortunately, this has not been proven in large, randomized studies. The presence of endotoxemia can be demonstrated in the majority of patients with COVID-19, yet only case reports and case series describing the effects of endotoxin removal in these patients have been published to date. The place of blood purification therapies in the treatment of septic shock has not yet been determined.
1. Introduction
2. Lipopolysaccharide Sensing Pathways
2.1. Toll-like Receptor 4–Myeloid Differentiation Protein 2 (TLR4-MD-2) Pathway
2.2. Transient Receptor Potential (TRP) Ion Channels
2.3. Intracellular LPS Sensing
3. Organ Damage Caused by Sensing Endotoxin
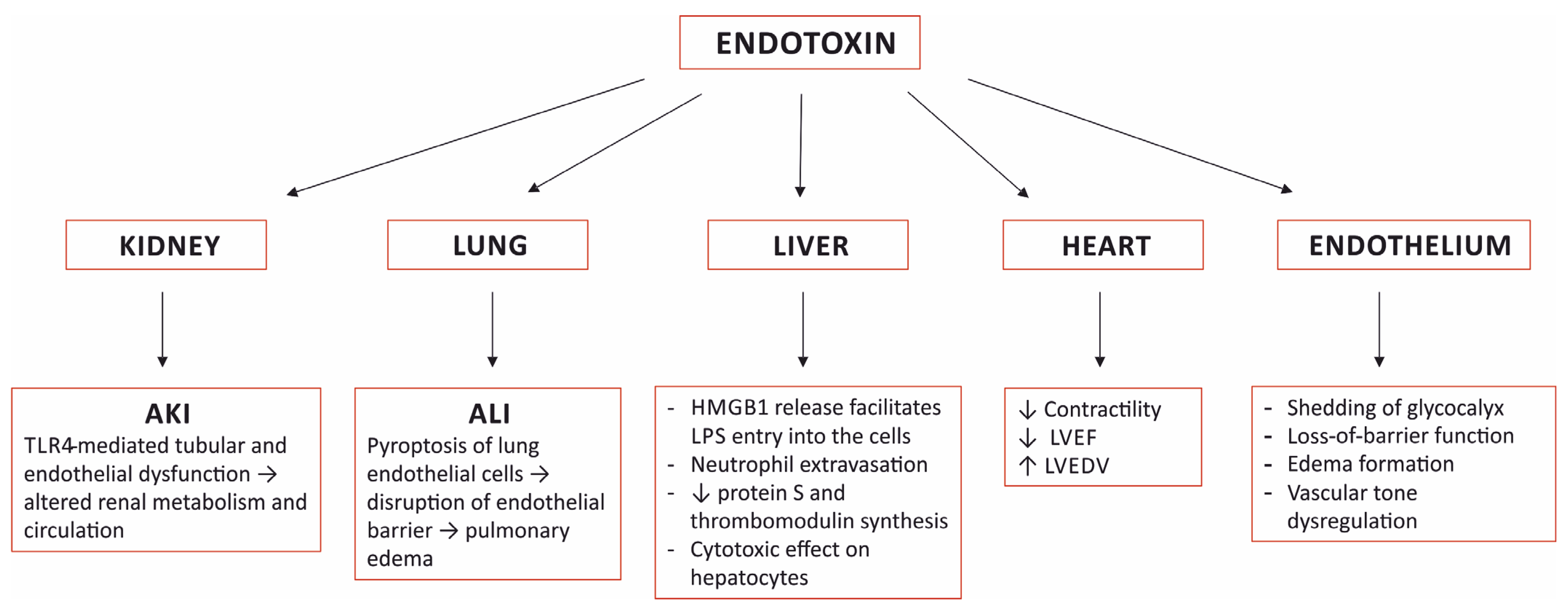 Figure 1. Selected organ damage induced by sensing endotoxin. AKI—acute kidney injury; ALI—acute lung injury; TLR4—toll-like receptor 4; HMGB1—high mobility group box-1; LPS—lipopolysaccharide; LVEF—left ventricular ejection fraction; LVEDV—left ventricular end diastolic volume.
Figure 1. Selected organ damage induced by sensing endotoxin. AKI—acute kidney injury; ALI—acute lung injury; TLR4—toll-like receptor 4; HMGB1—high mobility group box-1; LPS—lipopolysaccharide; LVEF—left ventricular ejection fraction; LVEDV—left ventricular end diastolic volume.3.1. The Kidney
3.2. The Lung
3.3. The Heart
3.4. The Liver
3.5. The Vascular Endothelium
4. Investigating Aspects of Endotoxin Removal
4.1. Timing of the Initiation of Endotoxin Adsorption
4.2. Extended Endotoxin Adsorption Treatment
4.3. Endotoxin Removal Treatment Guided by Measuring the Endotoxin Level
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211.
- Markwart, R.; Saito, H.; Harder, T.; Tomczyk, S.; Cassini, A.; Fleischmann-Struzek, C.; Reichert, F.; Eckmanns, T.; Allegranzi, B. Epidemiology and Burden of Sepsis Acquired in Hospitals and Intensive Care Units: A Systematic Review and Meta-Analysis. Intensive Care Med. 2020, 46, 1536–1551.
- Putzu, A.; Schorer, R.; Lopez-Delgado, J.C.; Cassina, T.; Landoni, G. Blood Purification and Mortality in Sepsis and Septic Shock. Anesthesiology 2019, 131, 580–593.
- Vincent, J.-L. Septic Shock. In Textbook of Critical Care; Vincent, J.-L., Abraham, E., Moore, F.A., Kochanek, P.M., Fink, M.P., Eds.; Elsevier: Philadelphia, PA, USA, 2017; pp. 843–848. ISBN 978-0-323-37638-9.
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247.
- Palsson-McDermott, E.M.; O’Neill, L.A.J. Signal Transduction by the Lipopolysaccharide Receptor, Toll-like Receptor-4. Immunology 2004, 113, 153–162.
- Rosadini, C.V.; Kagan, J.C. Early Innate Immune Responses to Bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19.
- Aluri, J.; Cooper, M.A.; Schuettpelz, L.G. Toll-Like Receptor Signaling in the Establishment and Function of the Immune System. Cells 2021, 10, 1374.
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273.
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.-A.; Fernández-Peña, C.; Talavera, A.; Kichko, T.; et al. TRPA1 Channels Mediate Acute Neurogenic Inflammation and Pain Produced by Bacterial Endotoxins. Nat. Commun. 2014, 5, 3125.
- Alpizar, Y.A.; Boonen, B.; Sanchez, A.; Jung, C.; López-Requena, A.; Naert, R.; Steelant, B.; Luyts, K.; Plata, C.; De Vooght, V.; et al. TRPV4 Activation Triggers Protective Responses to Bacterial Lipopolysaccharides in Airway Epithelial Cells. Nat. Commun. 2017, 8, 1059.
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. IJMS 2020, 21, 379.
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate Immunity to Intracellular LPS. Nat. Immunol. 2019, 20, 527–533.
- Zamyatina, A.; Heine, H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front. Immunol. 2020, 11, 585146.
- Cheng, K.T.; Xiong, S.; Ye, Z.; Hong, Z.; Di, A.; Tsang, K.M.; Gao, X.; An, S.; Mittal, M.; Vogel, S.M.; et al. Caspase-11–Mediated Endothelial Pyroptosis Underlies Endotoxemia-Induced Lung Injury. J. Clin. Investig. 2017, 127, 4124–4135.
- Zhang, W.; Coopersmith, C.M. Dying as a Pathway to Death in Sepsis. Anesthesiology 2018, 129, 238–240.
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of Acute Kidney Injury in Critically Ill Patients: The Multinational AKI-EPI Study. Intensive Care Med. 2015, 41, 1411–1423.
- Uchino, S. Acute Renal Failure in Critically Ill PatientsA Multinational, Multicenter Study. JAMA 2005, 294, 813.
- Vincent, J.-L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Le Gall, J.-R.; Payen, D. Sepsis in European Intensive Care Units: Results of the SOAP Study. Crit. Care Med. 2006, 34, 344–353.
- Kellum, J.A.; Chawla, L.S.; Keener, C.; Singbartl, K.; Palevsky, P.M.; Pike, F.L.; Yealy, D.M.; Huang, D.T.; Angus, D.C. The Effects of Alternative Resuscitation Strategies on Acute Kidney Injury in Patients with Septic Shock. Am. J. Respir. Crit. Care Med. 2016, 193, 281–287.
- Fenhammar, J.; Rundgren, M.; Forestier, J.; Kalman, S.; Eriksson, S.; Frithiof, R. Toll-Like Receptor 4 Inhibitor TAK-242 Attenuates Acute Kidney Injury in Endotoxemic Sheep. Anesthesiology 2011, 114, 1130–1137.
- Anderberg, S.B.; Luther, T.; Frithiof, R. Physiological Aspects of Toll-like Receptor 4 Activation in Sepsis-Induced Acute Kidney Injury. Acta Physiol. 2017, 219, 575–590.
- Kalakeche, R.; Hato, T.; Rhodes, G.; Dunn, K.W.; El-Achkar, T.M.; Plotkin, Z.; Sandoval, R.M.; Dagher, P.C. Endotoxin Uptake by S1 Proximal Tubular Segment Causes Oxidative Stress in the Downstream S2 Segment. J. Am. Soc. Nephrol. 2011, 22, 1505–1516.
- Ince, C.; Mayeux, P.R.; Nguyen, T.; Gomez, H.; Kellum, J.A.; Ospina-Tascón, G.A.; Hernandez, G.; Murray, P.; De Backer, D. The Endothelium in Sepsis. Shock 2016, 45, 259–270.
- Czaikoski, P.G.; Mota, J.M.S.C.; Nascimento, D.C.; Sônego, F.; Castanheira, F.V.e.S.; Melo, P.H.; Scortegagna, G.T.; Silva, R.L.; Barroso-Sousa, R.; Souto, F.O.; et al. Neutrophil Extracellular Traps Induce Organ Damage during Experimental and Clinical Sepsis. PLoS ONE 2016, 11, e0148142.
- Gomez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A Unified Theory of Sepsis-Induced Acute Kidney Injury: Inflammation, Microcirculatory Dysfunction, Bioenergetics, and the Tubular Cell Adaptation to Injury. Shock 2014, 41, 3–11.
- Boyd, J.; Mathur, S.; Wang, Y.; Bateman, R.; Walley, K. Toll-like Receptor Stimulation in Cardiomyoctes Decreases Contractility and Initiates an NF-ΚB Dependent Inflammatory Response. Cardiovasc. Res. 2006, 72, 384–393.
- Suffredini, A.F.; Fromm, R.E.; Parker, M.M.; Brenner, M.; Kovacs, J.A.; Wesley, R.A.; Parrillo, J.E. The Cardiovascular Response of Normal Humans to the Administration of Endotoxin. N. Engl. J. Med. 1989, 321, 280–287.
- Martin, L.; Derwall, M.; Al Zoubi, S.; Zechendorf, E.; Reuter, D.A.; Thiemermann, C.; Schuerholz, T. The Septic Heart. Chest 2019, 155, 427–437.
- Li, W.; Deng, M.; Loughran, P.A.; Yang, M.; Lin, M.; Yang, C.; Gao, W.; Jin, S.; Li, S.; Cai, J.; et al. LPS Induces Active HMGB1 Release From Hepatocytes Into Exosomes Through the Coordinated Activities of TLR4 and Caspase-11/GSDMD Signaling. Front. Immunol. 2020, 11, 229.
- Deng, M.; Tang, Y.; Li, W.; Wang, X.; Zhang, R.; Zhang, X.; Zhao, X.; Liu, J.; Tang, C.; Liu, Z.; et al. The Endotoxin Delivery Protein HMGB1 Mediates Caspase-11-Dependent Lethality in Sepsis. Immunity 2018, 49, 740–753.e7.
- Wang, Y.; Liu, Y. Neutrophil-Induced Liver Injury and Interactions Between Neutrophils and Liver Sinusoidal Endothelial Cells. Inflammation 2021, 44, 1246–1262.
- Takeyama, N.; Noguchi, H.; Hirakawa, A.; Kano, H.; Morino, K.; Obata, T.; Sakamoto, T.; Tamai, F.; Ishikura, H.; Kase, Y.; et al. Time to Initiation of Treatment with Polymyxin B Cartridge Hemoperfusion in Septic Shock Patients. Blood Purif. 2012, 33, 252–256.
- Chihara, S.; Masuda, Y.; Tatsumi, H.; Nakano, K.; Shimada, T.; Murohashi, T.; Yamakage, M. Early Induction of Direct Hemoperfusion with a Polymyxin-B Immobilized Column Is Associated with Amelioration of Hemodynamic Derangement and Mortality in Patients with Septic Shock. J. Artif. Organs 2017, 20, 71–75.
- Tanaka, T.; Tabata, T.; Fujino, K.; Tsujita, Y.; Eguchi, Y. Impact of Timing of Polymyxin B-immobilized Fiber Column Direct Hemoperfusion on Outcome in Patients with Septic Shock: A Single-center Observational Study. Acute Med. Surg. 2020, 7, e446.
- Yamashita, C.; Hara, Y.; Kuriyama, N.; Nakamura, T.; Nishida, O. Clinical Effects of a Longer Duration of Polymyxin B-Immobilized Fiber Column Direct Hemoperfusion Therapy for Severe Sepsis and Septic Shock: Longer Duration of PMX-DHP Therapy. Apher. Dial. 2015, 19, 316–323.
- Miyamoto, K.; Kawazoe, Y.; Kato, S. Prolonged Direct Hemoperfusion Using a Polymyxin B Immobilized Fiber Cartridge Provides Sustained Circulatory Stabilization in Patients with Septic Shock: A Retrospective Observational before-after Study. J. Intensive Care 2017, 5, 19.
- Mitaka, C.; Kusao, M.; Kawagoe, I.; Satoh, D.; Iba, T.; Ronco, C. Impact of Extended Duration of Polymyxin B-Immobilized Fiber Column Direct Hemoperfusion on Hemodynamics, Vasoactive Substance Requirement, and Pulmonary Oxygenation in Patients with Sepsis: An Observational Study. Blood Purif. 2021, 51, 62–69.
- Novelli, G.; Ferretti, G.; Ruberto, F.; Morabito, V.; Pugliese, F. Early Management of Endotoxemia Using the Endotoxin Activity Assay and Polymyxin B-Based Hemoperfusion. In Contributions to Nephrology; Ronco, C., Piccinni, P., Rosner, M.H., Eds.; KARGER: Basel, Switzerland, 2010; Volume 167, pp. 91–101. ISBN 978-3-8055-9484-4.
- Rachoin, J.-S.; Foster, D.; Giese, R.; Weisberg, L.S.; Klein, D.J. Importance of Endotoxin Clearance in Endotoxemic Septic Shock: An Analysis from the Evaluating Use of PolymyxinB Hemoperfusion in a Randomized Controlled Trial of Adults Treated for Endotoxemic Septic Shock (EUPHRATES) Trial. Crit. Care Explor. 2020, 2, e0083.




