Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Keke Zhi | + 2335 word(s) | 2335 | 2021-12-10 04:52:09 | | | |
| 2 | Catherine Yang | Meta information modification | 2335 | 2022-01-24 11:21:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhi, K. Activation Persulfate by Various Iron-Based Catalysts. Encyclopedia. Available online: https://encyclopedia.pub/entry/18701 (accessed on 07 February 2026).
Zhi K. Activation Persulfate by Various Iron-Based Catalysts. Encyclopedia. Available at: https://encyclopedia.pub/entry/18701. Accessed February 07, 2026.
Zhi, Keke. "Activation Persulfate by Various Iron-Based Catalysts" Encyclopedia, https://encyclopedia.pub/entry/18701 (accessed February 07, 2026).
Zhi, K. (2022, January 24). Activation Persulfate by Various Iron-Based Catalysts. In Encyclopedia. https://encyclopedia.pub/entry/18701
Zhi, Keke. "Activation Persulfate by Various Iron-Based Catalysts." Encyclopedia. Web. 24 January, 2022.
Copy Citation
Advanced oxidation technology of persulfate is a new method to degrade wastewater. As the economy progresses and technology develops, increasingly more pollutants produced by the paper industry, printing and dyeing, and the chemical industry are discharged into water, causing irreversible damage to water. Methods and research directions of activation persulfate for wastewater degradation by a variety of iron-based catalysts are reviewed. This entry describes the merits and demerits of advanced oxidation techniques for activated persulfate by iron-based catalysts. In order to promote the development of related research work, the problems existing in the current application are analyzed.
iron-based catalysts
activation persulfate
degrading wastewater
1. MeFe2O4 (Me = Cu, Co, Zn, etc.)
In terms of activation mechanism, transition metal compounds react with PS to produce a large amount of ·SO4−; the reaction equation follows:
Mn+ + S2O82− → M(n+1)+ +·SO4− + SO42−
As can be seen from the above reaction, metal ions are in a free state dispersed in the solution during the reaction process. Although the wastewater can be degraded by the activation persulfate mechanism, it belongs to homogeneous catalysis; metal ions will be dissolved in the aqueous solution, which causes difficult separation from solution. Therefore, the production cost is greatly increased due to its difficult recycling nature, and it is easy to cause secondary pollution to the environment. Therefore, MeFe2O4 with a low metal leaching rate has become a new research direction. Through PS/PMS [1] heterogeneous catalytic technology, these problems can be effectively solved [2][3][4].
At present, there are several common methods for preparing iron-based catalysts: hydrothermal, solvothermal, sol–gel preparation, and coprecipitation methods.
In the hydrothermal method, the solute is dispersed into the solution, stirred, and heated in the reactor, and finally washed and dried to obtain the required product [5].
Similar to the hydrothermal method, the solvothermal method changes water into an organic solvent. By dissolving one or more precursors in a nonaqueous solvent, the reaction occurs in liquid phase or supercritical conditions [6].
The sol–gel method is to dissolve the metal alkoxides in organic solvents, form homogeneous solutions, add other components, react at a certain temperature to form gels, and finally make products by drying [7].
Coprecipitation is an important method to prepare composite oxide ultrafine powder containing a large variety of metal elements [8].
The electron transfer between transition metal oxides is much higher [9] than that between single transition metal oxides. Generally, AB2O4 [10][11] structure is referred to as spinel structure. CuFe2O4 is a typical spinel ferrite with a magnetic structure, which has high chemical stability and low metal leaching rate. Taking CuFe2O4 as an example, compared with single transition metal oxides, Fe and Cu elements can play a role in the reaction; respectively, they can also activate PS to produce ·OH and ·SO4−.
G. Xian et al. [12] comprehensively compared the catalytic degradation effects of CoFe2O4, CuFe2O4, MnFe2O4, and ZnFe2O4. In detail, CuFe2O4 presented the best and fastest catalytic performance in organics removal. Almost 87.6% azo dye acid orange 7 (AO7) was removed in PS solution coupled with CuFe2O4 [12]. Additionally, it was known that CuFe2O4 had the best catalytic effect. Moreover, through the quenching experiment, it was not ·OH but ·SO4− that played a major role in the reaction.
Table 1 shows the degradation effects of some different MeFe2O4-activated PS/PMS on different kinds of wastewater. It can be seen from the table that the iron-based catalyst with spinel structure mainly acts on ·SO4− in the mechanism of activation persulfate; the effect of ·OH is slightly worse [13]. Of course, there are also some nonfree radical pathways, which degrade pollutants in water by generating singlet oxygen 1O2 [14][15][16].
Table 1. Effect of Different MeFe2O4-activated PMS on degradation of different wastewater [5][6][7][8][13][17][18][19].
| Catalyst | Pollution | Main Mechanism | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate/% | Number of Cycles | Synthesis Techniques |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PbFe2O4 | Thionine | 1O2 | 10 μM | 0.4 g/L | PMS | 400 μM | 20 | 100 | Not mentioned | Solution combustion | [17] |
| CoFe2O4–loaded quartz sand | Sulfachloropyridazine sodium |
·SO4− ·OH |
2 g/L | 10 g | PMS | 75 mg/L | 150 | 90 | Not mentioned | Citrate combustion | [18] |
| CoFe2O4-SAC | Norfloxacin (NOF) | ·SO4− ·OH |
10 mg/L | 0.1 g/L | PMS | 0.15 g/L | 120 | TOC reduction 81 |
5 (>80%) |
Hydrothermal | [13] |
| The biochar loaded with CoFe2O4 nanoparticles | Bisphenol A (BPA) |
·SO4− ·OH |
10 mg/L | 0.05 g/L | PMS | 0.5 g/L | 8 | 93 | Not mentioned | Hydrothermal | [5] |
| C3N4@MnFe2O4-graphene | Metronidazole | ·SO4− ·OH |
20 mg/L | 1.0 g/L | PS | 0.01 M | 90 | 94.5 | 5 (>80%) |
Solvothermal | [6] |
| Zn0.8Cu0.2Fe2O4 | Atrazine | ·SO4− | 4.4 μM | 200 mg/L | PS | 0.5 mM | 30 | 95 | Not mentioned | Sol–gel | [7] |
| CuFe2O4/O3 | 2,4-Dichlorophenoxyacetic acid (2,4-D) |
Not mentioned | 20 mg/L | 0.20 g/L | PMS O3 |
PMS 2.0 mM; O3 16.0 mg/L; |
40 | 88.9 | 5 (>80%) |
Coprecipitation | [8] |
| CoFe2O4 | Atrazine (ATZ) |
·SO4− | 10 mg/L | 0.4 g/L | PMS | 0.8 mM | 30 | >99 | 5 (>60%) |
Hydrothermal | [19] |
2. MeFe2O4 Combined with the Carrier
The carrier recombination method can increase the specific surface area and increase the contact of chemical sites [20], thus greatly improving the rate of chemical reaction. At present, SiO2 [20][21], black phosphorus [22][23], and rGO [24][25] (reduced graphene oxide) are commonly used as carriers. After compositing with the carrier, it is closely combined with the carrier by van der Waals force [24] or electrostatic interaction [26], making it difficult to fall off the surface of the carrier.
Pure graphene is a benzene-ring-like two-dimensional nanomaterial consisting of sp2 hybrid orbitals. However, its high production cost limits its large-scale application. Afterward, by improving Hummer’s method, a large number of oxygen-containing functional groups were linked at the edge of the plane by a strong oxidant, hence the name GO (graphene oxide) (Figure 1); rGO (Figure 2) was obtained by sodium borohydride and other means of reduction, which has low synthesis cost and is suitable for use as a good carrier of catalysis.
Figure 1. Plane structure (left) and solid structure (right) of GO (bond line type).

Figure 2. Plane structure (left) and solid structure (right) of rGO (bond line type).
Taking CuFe2O4, a representative of MeFe2O4, as an example, by comparing the effect of pure CuFe2O4 with that of CuFe2O4 combined with the carrier, it can be seen that the latter has a stronger catalytic effect under acidic and photoinduced conditions [27]. CuFe2O4 in CuFe2O4–rGO is closely combined with the oxygen-containing groups on rGO through electrostatic interaction, as shown in Figure 3. Images from a scanning electron microscope are shown in Figure 4.
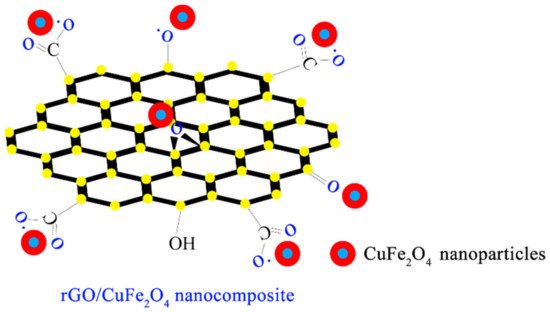
Figure 3. Chemical structural formula of CuFe2O4-rGO [26].
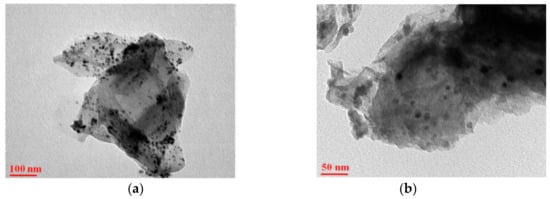
Figure 4. TEM images of (a,b) rGO/CuFe2O4 nanostructures under different magnifications [26].
Table 2 shows the degradation effects of some CuFe2O4 and rGO composite materials on different kinds of wastewater. It can be seen from the table that the composite catalyst can still produce good effects even without the presence of PS. Not only the Cu, Fe, and other elements in the catalyst can produce pure chemical catalytic effect, but the carrier rGO can produce electron transition under the light condition, promoting the transfer of electrons, and plays a part of the photocatalytic effect [28][29]. Table 2 contains some other carriers, which can also greatly influence degradation of different kinds of wastewater.
Table 2. Effects of partial MeFe2O4 and carrier composite materials on degradation of different kinds of wastewater [27][30][31][32][33][34][35][36].
| Catalyst | Pollution | Main Mechanism | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate /% | Number of Cycles | Synthesis Techniques |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CuFe2O4- 20%rGO |
Methylparaben | SO4−· ·OH |
10 mg/L | 0.2 mg/L | PS | 5 mM | 120 | 96 | Not mentioned | Sol-gel | [30] |
| CuFe2O4- 1% (w/w) rGO |
Phenol | ·OH | 20 ppm | 5 mL | 30% H2O2 |
6 mg/L | 240 | 100 | Not mentioned | Coprecipitation | [27] |
| CuFe2O4/g-C3N4 | Propranolol | SO4−· | 0.02 mM | 1 g/L | PS | 1 mM | 120 | 82.2 | Not mentioned | Sol-gel | [31] |
| CoFe2O4/CCNF | Dimethyl phthalate | SO4−· | 0.05 mM | 0.5 g/L | PMS | 1.5 mM | 60 | >90 | 5 (>90%) |
Sol-gel | [32] |
| TiO2@CuFe2O4/UV | 2,4-D | SO4−· | 20 mg/L | 0.1 g/L | PMS | 0.3 mM | 60 | 97.2 | 5 (>90%) |
Sol-gel | [33] |
| ZnS-ZnFe2O4 | Rhodamine B | SO4−· | 20 mg/L | 20 mg | PS | 5 mg | 90 | 97.67 | 3 (>95%) |
Hydrothermal | [34] |
| Fe2O3@CoFe2O4 | NOF | SO4−· ·OH |
15 μM | 0.3 g/L | PMS | 0.4 mM | 25 | 89.8 | 4 (90%) |
Hydrothermal | [35] |
| Nitrogen and sulfur codoped CNTs-COOH loaded CuFe2O4 | 2-Phenylbenzimidazole-5-sulfonic acid | SO4−· | 5 mg/L | 50 mg/L | PMS | 1:100 (molar ratio) | 40 | 98 | 5 (>95%) |
Coprecipitation | [36] |
@: the composite of two materials.
3. Activation Persulfate by Fe0
In recent years, activation persulfate based on Fe0 (zero-valent iron, ZVI) have been widely used in chemical production and environmental remediation [37][38]. As mentioned above, the activation persulfate/Fe (II) mechanism can cause secondary pollution to water, so ZVI/PS [39][40] is used instead to reduce a series of problems caused by the reduction of Fe2+ content due to the change of pH and other factors in water [37].
ZVI/PS system has strong reducibility (Fe0,E0 = −0.44 V) [41]. Compared with CuFe2O4, its reaction process is more complex, as shown in Figure 5. Fe0 is first converted to Fe2+ in the presence of acid and oxidant, then further oxidized to Fe3+ by Fe2+, and finally to Fe(IV) [42][43]. The reaction mechanism follows [44]: According to the reaction equation, the reaction is easily affected by pH, and the reaction will gradually slow with the increase of pH. Weng et al. [45] point out that the Fe0/PS system exhibits two-stage kinetics. The kinetic first stage is mostly attributed to a heterogeneous reaction occurring on the surface of the Fe0 aggregate. As the reaction proceeds, decolorization shifts from the slow kinetic first stage to the fast kinetic second stage when sufficient Fe2+ ions are maintained in the system [46].
Fe0 + 2H+ → Fe2+ + H2
2Fe0 + O2 + 2H2O → 2Fe2+ + 4OH−
Fe0 + S2O82− → Fe2+ + 2SO42−
Fe0 + HSO5− → Fe2+ + SO42− + OH−
Fe2+ + S2O82− → Fe3+ + SO42− + ·SO4−
Fe2+ + HSO5− → Fe3+ + SO42− + ·SO4−
Fe0 + S2O82− → Fe2+ + 2·SO4− + SO42−
Fe0+2 HSO5− → Fe2+ + 2OH− + 2·SO4−
Fe2+ + S2O82− + H2O → FeIVO2+ + 2SO42− + 2H+
Fe2+ + HSO5− → FeIVO2+ + SO42− + H+
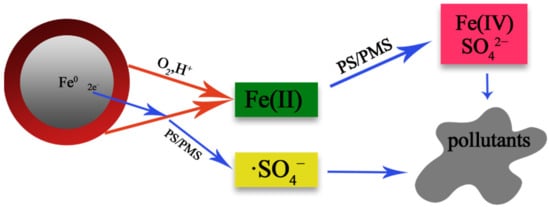
Figure 5. Schematic of the formation of ·SO4− and Fe(IV) in nZVI/persulfate systems containing methyl phenyl sulfoxide [47].
Figure 6 shows the proposed degradation pathway of 2,4-D [48]. By examining Figure 6, it can further confirm that macromolecular organic matter is decomposed into small molecular organic matter, which is gradually mineralized.
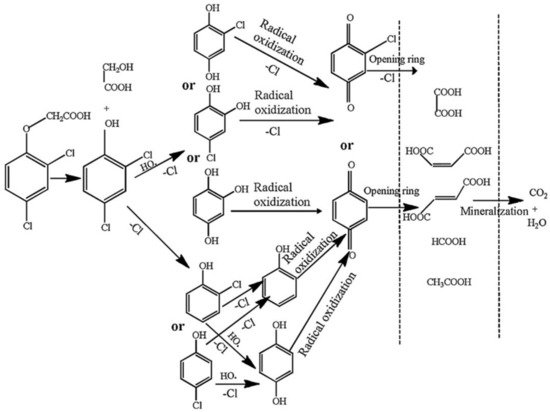
Figure 6. The proposed degradation pathway of 2,4-D [48].
Table 3 shows the degradation effects of various types of polluted water bodies activated by PS/PMS based on elemental iron. Usually, an appropriate amount of H2O2 [49] will be added to the water when PS is activated by Fe0, so as to reduce the cost of oxidant. Through the analysis of the table, it can be seen that the effect of ZVI when used alone [50] is worse than when it is combined with the carrier or when other conditions exist.
Table 3. Degradation effect of different kinds of wastewater based on PS/PMS activated by different kinds of iron [51][52][53][54][55][56][57][58].
| Catalyst | Pollution | Main Mechanism | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate /% | Number of Cycles | Synthesis Techniques |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| nZVI | Sulfamethazine | ·OH ·SO4− |
50 mg/L | 2 mM | PS H2O2 |
1 mM 0.5 mM |
30 | 96 | Not mentioned | Sol-gel | [54] |
| CN-Fe | Sulfamethazine | ·SO4− ·OH 1O2 |
50 μM | 0.5 g/L | PMS | 1 mM | 15 | 82 | Not mentioned | Carbothermal | [53] |
| Carbon-coated nZVI | 4-chlorophenol | ·SO4− ·OH |
150 μM | 0.25 g/L | PMS | 1 mM | 120 | 96 | Not mentioned | Commercially available | [52] |
| US-nZVI | Chloramphenicol | ·SO4− ·OH |
5 mg/L | 0.5 g/L | PMS | 1 mM | 90 | 98.1 | Not mentioned | Liquid phase reduction | [51] |
| Fe0@Fe3O4 | Dibutyl phthalate | ·OH ·SO4− |
18 μM | 0.5 g L−1 | PS | 1.8 mM | 180 | 94.7 | 6 (>68%) |
Calcination | [55] |
| Fe0@Fe3O4 | Atrazine | ·OH ·SO4− |
500 μg/L | 25 mg/L | PMS | 1 mM | 2 | 100 | Not mentioned | Reduction | [56] |
| Fe@C | Bisphenol S | ·OH ·SO4− |
5 mg/L | 0.5 g/L | PMS | 1.0 mM | 60 | 92.8 | Not mentioned | Resin carbonization | [57] |
| Fe@C/PB | 2,4-DichloroPhenol | ·OH ·SO4− |
20 mg/L | 0.6 g/L | PMS | 2.0 g/L | 50 | 99.4 | Not mentioned | Calcination | [58] |
@: the composite of two materials.
4. Fe3O4
Fe3O4 magnetite, also known as magnetic iron oxide, is a black crystal with a rotating spinel structure (Figure 7). In magnetite, Fe2+ and Fe3+ are disordered on the ferrite octahedron, so electrons can transfer rapidly between Fe2+ and Fe3+; thus, reversible redox reactions can occur at the same position on the octahedron.
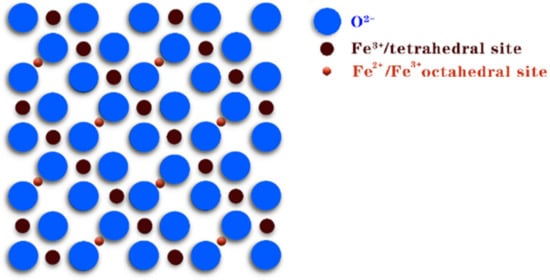
Figure 7. Crystal structure of Fe3O4.
However, since Fe3O4 is easy to accumulate in solution and contact sites are reduced after agglomeration, single Fe3O4 is rarely used. Using the composite carrier method [59] can not only solve these problems, but also speeds the reaction rate, making it more cost effective when applied in industrial production. He et al. [60] pointed out that the Fe3O4/GO/Ag composite microspheres are formed using magnetic Fe3O4 as cores, followed by coating an internal layer of GO and an outer layer of Ag nanoparticles, as Figure 8 shows. The synthesized Fe3O4/GO/Ag composite catalyst under the action of NaBH4, methylene blue, and ciprofloxacin can be completely degraded within 12 min. Figure 8 shows SEM images of Fe3O4/GO/Ag composite catalyst. In Figure 9, we can clearly observe that Ag has been completely attached to the Fe3O4/GO surface, which can increase the specific surface area and improve the chemical reaction rate.
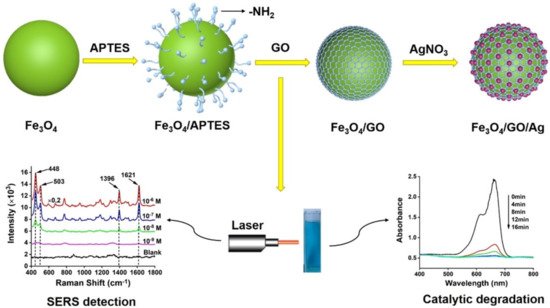
Figure 8. Illustration of the fabrication of Fe3O4/GO/Ag composite microspheres [60].
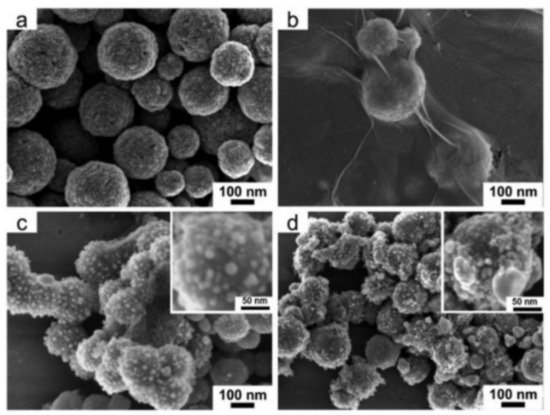
Figure 9. Typical FESEM images of (a) Fe3O4, (b) Fe3O4/GO, (c) Fe3O4/GO/Ag, and (d) Fe3O4/Ag microspheres. Inserts are magnified FESEM images of Fe3O4/GO/Ag and Fe3O4/Ag microspheres [60].
Table 4 shows the research progress of Fe3O4 and its composite materials on the degradation of different pollutants reported at present. According to the data in the table, when Fe3O4 is compounded with the carrier, the catalytic performance is greatly improved.
| Catalyst | Pollution | Main Mechanism | Pollutant Concentration | Catalyst Concentration | Oxidant | Oxidation Concentration | T/min | Degradation Rate /% | Number of Cycles | Synthesis Techniques |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4 | BPA | ·SO4− ·OH |
20 mg/L | 2.0 g/L | PMS | 5 mM | 30 | 27.53 | Not mentioned | Commercially available | [61] |
| CuO-Fe3O4-BC | BPA | ·SO4− ·OH |
20 mg/L | 2.0 g/L | PMS | 5 mM | 30 | 100 | 4 (>85%) |
Coprecipitation | [62] |
| rGO-Fe3O4 | NOF | 1O2 ·OH ·SO4− |
20 mg/L | 0.5 g/L | PS | 1 g/L | 30 | 89.69 | Not mentioned | Coprecipitation | [62] |
| Fe3O4 | Sulfamonomethoxine | ·SO4− | 0.06 mM | 2.4 mM | PS | 1.2 mM | 15 | 100 | Not mentioned | Coprecipitation | [63] |
| Fe3O4@Zn/Co-ZIFs | Carbamazepine | ·SO4− | 5 mg/L | 25 mg/L | PMS | 0.4 mM | 30 | 100 | Not mentioned | Solvothermal | [64] |
| Fe3O4/microwave irradiation (3 kW/L) | p-Nitrophenol | ·SO4− | 20 mg/L | 2.5 g/L | PS | 15:1 (molar ratio) | 28 | 94.2 | Not mentioned | Not mentioned | [65] |
| Fe3O4/MC | p-Hydroxybenzoic acid | ·SO4− | 1.0 g/L | 0.2 g/L | PS | 1.0 g/L | 30 | 100 | Not mentioned | Sol-gel | [66] |
| Fe3O4/graphene aerogels | Malachite green | Not mentioned | 20 mg/L | 0.2 g/L | PS | 1.0 mM | 12 | 91.7 | Not mentioned | Sol-gel | [67] |
@: the composite of two materials.
References
- Fan, X.; Wang, Y.; Zhang, D.; Guo, Y.; Gao, S.; Li, E.; Zheng, H. Effects of acid, acid-ZVI/PMS, Fe(II)/PMS and ZVI/PMS conditioning on the wastewater activated sludge (WAS) dewaterability and extracellular polymeric substances (EPS). J. Environ. Sci. 2020, 91, 73–84.
- Li, Y.; Zhu, W.; Guo, Q.; Wang, X.; Zhang, L.; Gao, X.; Luo, Y. Highly efficient degradation of sulfamethoxazole (SMX) by activating peroxymonosulfate (PMS) with CoFe2O4 in a wide pH range. Sep. Purif. Technol. 2021, 276, 119403.
- Wei, W.; Zhou, D.; Feng, L.; Li, X.; Hu, L.; Zheng, H.; Wang, Y. The graceful art, significant function and wide application behavior of ultrasound research and understanding in carbamazepine (CBZ) enhanced removal and degradation by Fe0/PDS/US. Chemosphere 2021, 278, 130368.
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517.
- Li, Y.; Ma, S.; Xu, S.; Fu, H.; Li, Z.; Li, K.; Sheng, K.; Du, J.; Lu, X.; Li, X.; et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4. Chem. Eng. J. 2020, 387, 124094.
- Wang, X.; Wang, A.; Ma, J. Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites. J. Hazard. Mater. 2017, 336, 81–92.
- Huang, Y.; Han, C.; Liu, Y.; Nadagouda, M.N.; Machala, L.; O’Shea, K.E.; Sharma, V.K.; Dionysiou, D.D. Degradation of atrazine by ZnxCu1−xFe2O4 nanomaterial-catalyzed sulfite under UV–vis light irradiation: Green strategy to generate SO4−. Appl. Catal. B Environ. 2018, 221, 380–392.
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Efficient degradation of 2,4-dichlorophenoxyacetic acid by peroxymonosulfate/magnetic copper ferrite nanoparticles/ozone: A novel combination of advanced oxidation processes. Chem. Eng. J. 2017, 320, 436–447.
- Song, Y.; Yang, Y.; Mo, S.; Guo, D.; Liu, L. Fast construction of (Fe2O3) heterostructure nanosheets as highly active catalyst for water oxidation. J. Alloys Compd. 2022, 892, 162149.
- Lyu, L.; Won Kim, C.; Seong, K.-D.; Kang, J.; Liu, S.; Yamauchi, Y.; Piao, Y. Defect engineering induced heterostructure of ZnMn2O4 nanocrystal for flexible asymmetric supercapacitor. Chem. Eng. J. 2021, 133115.
- Xie, X.; Wang, B.; Wang, Y.; Ni, C.; Sun, X.; Du, W. Spinel structured MFe2O4 (M = Fe, Co, Ni, Mn, Zn) and their composites for microwave absorption: A review. Chem. Eng. J. 2022, 428, 131160.
- Xian, G.; Kong, S.; Li, Q.; Zhang, G.; Zhou, N.; Du, H.; Niu, L. Synthesis of Spinel Ferrite MFe2O4 (M = Co, Cu, Mn, and Zn) for Persulfate Activation to Remove Aqueous Organics: Effects of M-Site Metal and Synthetic Method. Front. Chem. 2020, 8.
- Yang, Z.; Li, Y.; Zhang, X.; Cui, X.; He, S.; Liang, H.; Ding, A. Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate. Chem. Eng. J. 2020, 384, 123319.
- Mostafa, S.; Rosario-Ortiz, F.L. Singlet oxygen formation from wastewater organic matter. Environ. Sci. Technol. 2013, 47, 8179–8186.
- Yi, Q.; Ji, J.; Shen, B.; Dong, C.; Liu, J.; Zhang, J.; Xing, M. Singlet Oxygen Triggered by Superoxide Radicals in a Molybdenum Cocatalytic Fenton Reaction with Enhanced REDOX Activity in the Environment. Environ. Sci. Technol. 2019, 53, 9725–9733.
- Mikrut, M.; Mazuryk, O.; Macyk, W.; van Eldik, R.; Stochel, G. Generation and photogeneration of hydroxyl radicals and singlet oxygen by particulate matter and its inorganic components. J. Environ. Chem. Eng. 2021, 9, 106478.
- Liu, F.; Li, W.; Wu, D.; Tian, T.; Wu, J.-F.; Dong, Z.-M.; Zhao, G.-C. New insight into the mechanism of peroxymonosulfate activation by nanoscaled lead-based spinel for organic matters degradation: A singlet oxygen-dominated oxidation process. J. Colloid Interface Sci. 2020, 572, 318–327.
- Gao, D.; Junaid, M.; Lin, F.; Zhang, S.; Xu, N. Degradation of sulphachloropyridazine sodium in column reactor packed with CoFe2O4−loaded quartz sand via peroxymonosulfate activation: Insights into the amorphous phase, efficiency, and mechanism. Chem. Eng. J. 2020, 390, 124549.
- Li, J.; Xu, M.; Yao, G.; Lai, B. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation. Chem. Eng. J. 2018, 348, 1012–1024.
- Ratanaphain, C.; Viboonratanasri, D.; Prompinit, P.; Krajangpan, S.; Khan, E.; Punyapalakul, P. Reactivity characterization of SiO2-coated nano zero-valent iron for iodoacetamide degradation: The effects of SiO2 thickness, and the roles of dehalogenation, hydrolysis and adsorption. Chemosphere 2022, 286, 131816.
- Ding, S.; Wan, J.; Wang, Y.; Yan, Z.; Ma, Y. Activation of persulfate by molecularly imprinted 2 for the targeted degradation of dimethyl phthalate: Effects of operating parameters and chlorine. Chem. Eng. J. 2021, 422, 130406.
- Wang, L.; Guan, R.; Qi, Y.; Zhang, F.; Li, P.; Wang, J.; Qu, P.; Zhou, G.; Shi, W. Constructing Zn-P charge transfer bridge over ZnFe2O4-black phosphorus 3D microcavity structure: Efficient photocatalyst design in visible-near-infrared region. J. Colloid Interface Sci. 2021, 600, 463–472.
- Shao, S.; Deng, J.; Lv, X.; Ji, H.; Xiao, Y.; Zhu, X.; Feng, K.; Xu, H.; Zhong, J. Black phosphorus nanoflakes decorated hematite photoanode with functional phosphate bridges for enhanced water oxidation. Chem. Eng. J. 2021, 425, 131500.
- Askari, M.B.; Salarizadeh, P.; Beitollahi, H.; Tajik, S.; Eshghi, A.; Azizi, S. Electro-oxidation of hydrazine on NiFe2O4-rGO as a high-performance nano-electrocatalyst in alkaline media. Mater. Chem. Phys. 2022, 275, 125313.
- Wang, X.; Wang, D.; Ma, C.; Yang, Z.; Yue, H.; Zhang, D.; Sun, Z. Conductive Fe2N/N-rGO composite boosts electrochemical redox reactions in wide temperature accommodating lithium-sulfur batteries. Chem. Eng. J. 2022, 427, 131622.
- Karthikeyan, C.; Ramachandran, K.; Sheet, S.; Yoo, D.J.; Lee, Y.S.; Satish kumar, Y.; Kim, A.R.; Gnana kumar, G. Pigeon-Excreta-Mediated Synthesis of Reduced Graphene Oxide (rGO)/CuFe2O4 Nanocomposite and Its Catalytic Activity toward Sensitive and Selective Hydrogen Peroxide Detection. ACS Sustain. Chem. Eng. 2017, 5, 4897–4905.
- Othman, I.; Abu Haija, M.; Ismail, I.; Zain, J.H.; Banat, F. Preparation and catalytic performance of CuFe2O4 nanoparticles supported on reduced graphene oxide (CuFe2O4/rGO) for phenol degradation. Mater. Chem. Phys. 2019, 238.
- Wang, W.; Yu, J.C.; Xia, D.; Wong, P.K.; Li, Y. Graphene and g-C3N4 nanosheets cowrapped elemental alpha-sulfur as a novel metal-free heterojunction photocatalyst for bacterial inactivation under visible-light. Environ. Sci. Technol. 2013, 47, 8724–8732.
- Sun, B.; Ma, W.; Wang, N.; Xu, P.; Zhang, L.; Wang, B.; Zhao, H.; Lin, K.A.; Du, Y. Retraction of “Polyaniline: A New Metal-Free Catalyst for Peroxymonosulfate Activation with Highly Efficient and Durable Removal of Organic Pollutants”. Environ. Sci. Technol. 2021, 55, 3451.
- Hao, P.; Hu, M.; Xing, R.; Zhou, W. Synergistic degradation of methylparaben on CuFe2O4-rGO composite by persulfate activation. J. Alloys Compd. 2020, 823, 153757.
- Li, R.; Cai, M.; Xie, Z.; Zhang, Q.; Zeng, Y.; Liu, H.; Liu, G.; Lv, W. Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation. Appl. Catal. B Environ. 2019, 244, 974–982.
- Gan, L.; Zhong, Q.; Geng, A.; Wang, L.; Song, C.; Han, S.; Cui, J.; Xu, L. Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Sci. Total Environ. 2019, 694, 133705.
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337.
- Zhu, B.; Cheng, H.; Ma, J.; Kong, Y.; Komarneni, S. Efficient degradation of rhodamine B by magnetically separable ZnS–ZnFe2O4 composite with the synergistic effect from persulfate. Chemosphere 2019, 237, 124547.
- Kohantorabi, M.; Hosseinifard, M.; Kazemzadeh, A. Catalytic activity of a magnetic Fe2O3@CoFe2O4 nanocomposite in peroxymonosulfate activation for norfloxacin removal. N. J. Chem. 2020, 44, 4185–4198.
- Zhang, X.; Feng, M.; Wang, L.; Qu, R.; Wang, Z. Catalytic degradation of 2-phenylbenzimidazole-5-sulfonic acid by peroxymonosulfate activated with nitrogen and sulfur co-doped CNTs-COOH loaded CuFe2O4. Chem. Eng. J. 2017, 307, 95–104.
- Cuervo Lumbaque, E.; Lopes Tiburtius, E.R.; Barreto-Rodrigues, M.; Sirtori, C. Current trends in the use of zero-valent iron (Fe0) for degradation of pharmaceuticals present in different water matrices. Trends Environ. Anal. Chem. 2019, 24, e00069.
- Hussain, I.; Zhang, Y.; Huang, S.; Gao, Q. Degradation of p-chloroaniline by FeO3−xH3−2x/Fe0 in the presence of persulfate in aqueous solution. RSC Adv. 2015, 5, 41079–41087.
- Rodriguez, S.; Vasquez, L.; Romero, A.; Santos, A. Dye Oxidation in Aqueous Phase by Using Zero-Valent Iron as Persulfate Activator: Kinetic Model and Effect of Particle Size. Ind. Eng. Chem. Res. 2014, 53, 12288–12294.
- Li, J.; Zhang, X.; Sun, Y.; Liang, L.; Pan, B.; Zhang, W.; Guan, X. Advances in Sulfidation of Zerovalent Iron for Water Decontamination. Environ. Sci. Technol. 2017, 51, 13533–13544.
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265.
- Wang, Z.; Qiu, W.; Pang, S.-Y.; Zhou, Y.; Gao, Y.; Guan, C.; Jiang, J. Further understanding the involvement of Fe(IV) in peroxydisulfate and peroxymonosulfate activation by Fe(II) for oxidative water treatment. Chem. Eng. J. 2019, 371, 842–847.
- Huang, J.; Zhang, H. Mn-based catalysts for sulfate radical-based advanced oxidation processes: A review. Environ. Int. 2019, 133, 105141.
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323.
- Weng, C.H.; Ding, F.; Lin, Y.T.; Liu, N. Effective decolorization of polyazo direct dye Sirius Red F3B using persulfate activated with Fe-0 aggregate. Sep. Purif. Technol. 2015, 147, 147–155.
- Li, H.; Wan, J.; Ma, Y.; Wang, Y.; Huang, M. Influence of particle size of zero-valent iron and dissolved silica on the reactivity of activated persulfate for degradation of acid orange 7. Chem. Eng. J. 2014, 237, 487–496.
- Wang, Z.; Qiu, W.; Pang, S.; Gao, Y.; Zhou, Y.; Cao, Y.; Jiang, J. Relative contribution of ferryl ion species (Fe(IV)) and sulfate radical formed in nanoscale zero valent iron activated peroxydisulfate and peroxymonosulfate processes. Water Res. 2020, 172, 115504.
- Li, X.; Zhou, M.; Pan, Y. Enhanced degradation of 2,4-dichlorophenoxyacetic acid by pre-magnetization Fe-C activated persulfate: Influential factors, mechanism and degradation pathway. J. Hazard. Mater. 2018, 353, 454–465.
- Ye, C.; Liu, P.; Ma, Z.; Xue, C.; Zhang, C.; Zhang, Y.; Liu, J.; Liu, C.; Sun, X.; Mu, Y. High H2O2 Concentrations Observed during Haze Periods during the Winter in Beijing: Importance of H2O2 Oxidation in Sulfate Formation. Environ. Sci. Technol. Lett. 2018, 5, 757–763.
- Peng, X.; Xi, B.; Zhao, Y.; Shi, Q.; Meng, X.; Mao, X.; Jiang, Y.; Ma, Z.; Tan, W.; Liu, H.; et al. Effect of Arsenic on the Formation and Adsorption Property of Ferric Hydroxide Precipitates in ZVI Treatment. Environ. Sci. Technol. 2017, 51, 10100–10108.
- Wu, J.; Wang, B.; Cagnetta, G.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation. Sep. Purif. Technol. 2020, 239, 116534.
- Zhang, T.; Yang, Y.; Gao, J.; Li, X.; Yu, H.; Wang, N.; Du, P.; Yu, R.; Li, H.; Fan, X.; et al. Synergistic degradation of chloramphenicol by ultrasound-enhanced nanoscale zero-valent iron/persulfate treatment. Sep. Purif. Technol. 2020, 240, 116575.
- Chen, L.; Huang, Y.; Zhou, M.; Xing, K.; Lv, W.; Wang, W.; Chen, H.; Yao, Y. Nitrogen-doped porous carbon encapsulating iron nanoparticles for enhanced sulfathiazole removal via peroxymonosulfate activation. Chemosphere 2020, 250, 126300.
- Li, S.; Tang, J.; Liu, Q.; Liu, X.; Gao, B. A novel stabilized carbon-coated nZVI as heterogeneous persulfate catalyst for enhanced degradation of 4-chlorophenol. Environ. Int. 2020, 138, 105639.
- Li, H.; Wan, J.; Ma, Y.; Wang, Y. Synthesis of novel core–shell Fe0@Fe3O4 as heterogeneous activator of persulfate for oxidation of dibutyl phthalate under neutral conditions. Chem. Eng. J. 2016, 301, 315–324.
- Feng, Y.; Zhong, J.; Zhang, L.; Fan, Y.; Yang, Z.; Shih, K.; Li, H.; Wu, D.; Yan, B. Activation of peroxymonosulfate by Fe0@Fe3O4 core-shell nanowires for sulfate radical generation: Electron transfer and transformation products. Sep. Purif. Technol. 2020, 247, 116942.
- Liu, Y.; Guo, H.; Zhang, Y.; Cheng, X.; Zhou, P.; Wang, J.; Li, W. carbonized resin for peroxymonosulfate activation and bisphenol S degradation. Environ. Pollut. 2019, 252, 1042–1050.
- Guo, F.; Wang, K.; Lu, J.; Chen, J.; Dong, X.; Xia, D.; Zhang, A.; Wang, Q. Activation of peroxymonosulfate by magnetic carbon supported Prussian blue nanocomposite for the degradation of organic contaminants with singlet oxygen and superoxide radicals. Chemosphere 2019, 218, 1071–1081.
- Wang, H.; Zhang, C.; Zhang, X.; Wang, S.; Xia, Z.; Zeng, G.; Ding, J.; Ren, N. Construction of Fe3O4@β-CD/g-C3N4 nanocomposite catalyst for degradation of PCBs in wastewater through photodegradation and heterogeneous Fenton oxidation. Chem. Eng. J. 2022, 429, 132445.
- He, J.; Song, G.; Wang, X.; Zhou, L.; Li, J. Multifunctional magnetic Fe3O4/GO/Ag composite microspheres for SERS detection and catalytic degradation of methylene blue and ciprofloxacin. J. Alloys Compd. 2022, 893, 162226.
- Zhen, J.; Zhang, S.; Zhuang, X.; Ahmad, S.; Lee, T.; Si, H.; Cao, C.; Ni, S.-Q. Sulfate radicals based heterogeneous peroxymonosulfate system catalyzed by CuO-Fe3O4-Biochar nanocomposite for bisphenol A degradation. J. Water Process Eng. 2021, 41, 102078.
- Yin, F.; Wang, C.; Lin, K.-Y.A.; Tong, S. Persulfate activation for efficient degradation of norfloxacin by a rGO-Fe3O4 composite. J. Taiwan Inst. Chem. Eng. 2019, 102, 163–169.
- Yan, J.; Lei, M.; Zhu, L.; Anjum, M.N.; Zou, J.; Tang, H. Degradation of sulfamonomethoxine with Fe3O4 magnetic nanoparticles as heterogeneous activator of persulfate. J. Hazard. Mater. 2011, 186, 1398–1404.
- Wu, Z.; Wang, Y.; Xiong, Z.; Ao, Z.; Pu, S.; Yao, G.; Lai, B. Core-shell magnetic Fe3O4@Zn/Co-ZIFs to activate peroxymonosulfate for highly efficient degradation of carbamazepine. Appl. Catal. B Environ. 2020, 277, 119136.
- Zhao, G.; Zou, J.; Chen, X.; Liu, L.; Wang, Y.; Zhou, S.; Long, X.; Yu, J.; Jiao, F. Iron-based catalysts for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Chem. Eng. J. 2021, 421, 127845.
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980.
- Lu, J.-D. The effect of two ferromagnetic metal stripes on valley polarization of electrons in a graphene. Phys. Lett. A 2020, 384, 126402.
More
Information
Subjects:
Chemistry, Applied
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
24 Jan 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




