In terms of activation mechanism, transition metal compounds react with PS to produce a large amount of ·SO
4−; the reaction equation follows:
As can be seen from the above reaction, metal ions are in a free state dispersed in the solution during the reaction process. Although the wastewater can be degraded by the activation persulfate mechanism, it belongs to homogeneous catalysis; metal ions will be dissolved in the aqueous solution, which causes difficult separation from solution. Therefore, the production cost is greatly increased due to its difficult recycling nature, and it is easy to cause secondary pollution to the environment. Therefore, MeFe
2O
4 with a low metal leaching rate has become a new research direction. Through PS/PMS [
36] heterogeneous catalytic technology, these problems can be effectively solved [
22,
37,
38].
At present, there are several common methods for preparing iron-based catalysts: hydrothermal, solvothermal, sol–gel preparation, and coprecipitation methods.
In the hydrothermal method, the solute is dispersed into the solution, stirred, and heated in the reactor, and finally washed and dried to obtain the required product [
39].
Similar to the hydrothermal method, the solvothermal method changes water into an organic solvent. By dissolving one or more precursors in a nonaqueous solvent, the reaction occurs in liquid phase or supercritical conditions [
40].
The sol–gel method is to dissolve the metal alkoxides in organic solvents, form homogeneous solutions, add other components, react at a certain temperature to form gels, and finally make products by drying [
41].
Coprecipitation is an important method to prepare composite oxide ultrafine powder containing a large variety of metal elements [
42].
The electron transfer between transition metal oxides is much higher [
43] than that between single transition metal oxides. Generally, AB
2O
4 [
44,
45] structure is referred to as spinel structure. CuFe
2O
4 is a typical spinel ferrite with a magnetic structure, which has high chemical stability and low metal leaching rate. Taking CuFe
2O
4 as an example, compared with single transition metal oxides, Fe and Cu elements can play a role in the reaction; respectively, they can also activate PS to produce ·OH and ·SO
4−.
G. Xian et al. [
46] comprehensively compared the catalytic degradation effects of CoFe
2O
4, CuFe
2O
4, MnFe
2O
4, and ZnFe
2O
4. In detail, CuFe
2O
4 presented the best and fastest catalytic performance in organics removal. Almost 87.6% azo dye acid orange 7 (AO7) was removed in PS solution coupled with CuFe
2O
4 [
46]. Additionally, it was known that CuFe
2O
4 had the best catalytic effect. Moreover, through the quenching experiment, it was not ·OH but ·SO
4− that played a major role in the reaction.
Table 1 shows the degradation effects of some different MeFe
2O
4-activated PS/PMS on different kinds of wastewater. It can be seen from the table that the iron-based catalyst with spinel structure mainly acts on ·SO
4− in the mechanism of activation persulfate; the effect of ·OH is slightly worse [
47]. Of course, there are also some nonfree radical pathways, which degrade pollutants in water by generating singlet oxygen
1O
2 [
48,
49,
50].
Table 1. Effect of Different MeFe
2O
4-activated PMS on degradation of different wastewater [
39,
40,
41,
42,
47,
51,
52,
53].
| Catalyst |
Pollution |
Main Mechanism |
Pollutant Concentration |
Catalyst Concentration |
Oxidant |
Oxidation Concentration |
T/min |
Degradation Rate/% |
Number of Cycles |
Synthesis
Techniques |
Ref. |
| PbFe2O4 |
Thionine |
1O2 |
10 μM |
0.4 g/L |
PMS |
400 μM |
20 |
100 |
Not mentioned |
Solution combustion |
[51] |
| CoFe2O4–loaded quartz sand |
Sulfachloropyridazine
sodium |
·SO4−
·OH |
2 g/L |
10 g |
PMS |
75 mg/L |
150 |
90 |
Not mentioned |
Citrate combustion |
[52] |
| CoFe2O4-SAC |
Norfloxacin (NOF) |
·SO4−
·OH |
10 mg/L |
0.1 g/L |
PMS |
0.15 g/L |
120 |
TOC reduction
81 |
5
(>80%) |
Hydrothermal |
[47] |
| The biochar loaded with CoFe2O4 nanoparticles |
Bisphenol A
(BPA) |
·SO4−
·OH |
10 mg/L |
0.05 g/L |
PMS |
0.5 g/L |
8 |
93 |
Not mentioned |
Hydrothermal |
[39] |
| C3N4@MnFe2O4-graphene |
Metronidazole |
·SO4−
·OH |
20 mg/L |
1.0 g/L |
PS |
0.01 M |
90 |
94.5 |
5
(>80%) |
Solvothermal |
[40] |
| Zn0.8Cu0.2Fe2O4 |
Atrazine |
·SO4− |
4.4 μM |
200 mg/L |
PS |
0.5 mM |
30 |
95 |
Not mentioned |
Sol–gel |
[41] |
| CuFe2O4/O3 |
2,4-Dichlorophenoxyacetic acid
(2,4-D) |
Not mentioned |
20 mg/L |
0.20 g/L |
PMS
O3 |
PMS 2.0 mM;
O3 16.0 mg/L; |
40 |
88.9 |
5
(>80%) |
Coprecipitation |
[42] |
| CoFe2O4 |
Atrazine
(ATZ) |
·SO4− |
10 mg/L |
0.4 g/L |
PMS |
0.8 mM |
30 |
>99 |
5
(>60%) |
Hydrothermal |
[53] |
2. MeFe2O4 Combined with the Carrier
The carrier recombination method can increase the specific surface area and increase the contact of chemical sites [
54], thus greatly improving the rate of chemical reaction. At present, SiO
2 [
54,
55], black phosphorus [
56,
57], and rGO [
58,
59] (reduced graphene oxide) are commonly used as carriers. After compositing with the carrier, it is closely combined with the carrier by van der Waals force [
58] or electrostatic interaction [
60], making it difficult to fall off the surface of the carrier.
Pure graphene is a benzene-ring-like two-dimensional nanomaterial consisting of sp2 hybrid orbitals. However, its high production cost limits its large-scale application. Afterward, by improving Hummer’s method, a large number of oxygen-containing functional groups were linked at the edge of the plane by a strong oxidant, hence the name GO (graphene oxide) (Figure 1); rGO (Figure 2) was obtained by sodium borohydride and other means of reduction, which has low synthesis cost and is suitable for use as a good carrier of catalysis.
Figure 1. Plane structure (left) and solid structure (right) of GO (bond line type).
Figure 2. Plane structure (left) and solid structure (right) of rGO (bond line type).
Taking CuFe
2O
4, a representative of MeFe
2O
4, as an example, by comparing the effect of pure CuFe
2O
4 with that of CuFe
2O
4 combined with the carrier, it can be seen that the latter has a stronger catalytic effect under acidic and photoinduced conditions [
61]. CuFe
2O
4 in CuFe
2O
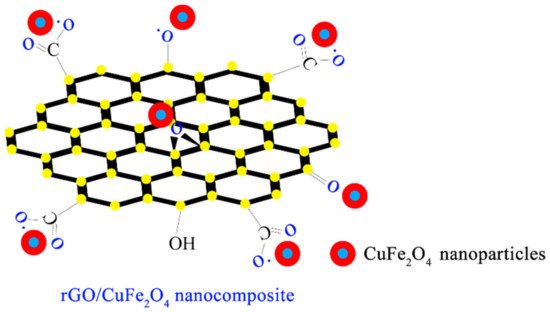
4–rGO is closely combined with the oxygen-containing groups on rGO through electrostatic interaction, as shown in
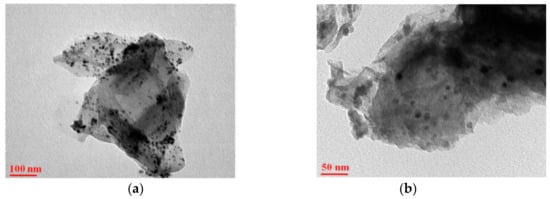
Figure 3. Images from a scanning electron microscope are shown in
Figure 4.
Figure 3. Chemical structural formula of CuFe
2O
4-rGO [
60].
Figure 4. TEM images of (
a,
b) rGO/CuFe
2O
4 nanostructures under different magnifications [
60].
Table 2 shows the degradation effects of some CuFe
2O
4 and rGO composite materials on different kinds of wastewater. It can be seen from the table that the composite catalyst can still produce good effects even without the presence of PS. Not only the Cu, Fe, and other elements in the catalyst can produce pure chemical catalytic effect, but the carrier rGO can produce electron transition under the light condition, promoting the transfer of electrons, and plays a part of the photocatalytic effect [
62,
63].
Table 2 contains some other carriers, which can also greatly influence degradation of different kinds of wastewater.
Table 2. Effects of partial MeFe
2O
4 and carrier composite materials on degradation of different kinds of wastewater [
61,
64,
65,
66,
67,
68,
69,
70].
| Catalyst |
Pollution |
Main Mechanism |
Pollutant Concentration |
Catalyst Concentration |
Oxidant |
Oxidation Concentration |
T/min |
Degradation Rate /% |
Number of Cycles |
Synthesis
Techniques |
Ref. |
CuFe2O4-
20%rGO |
Methylparaben |
SO4−·
·OH |
10 mg/L |
0.2 mg/L |
PS |
5 mM |
120 |
96 |
Not mentioned |
Sol-gel |
[64] |
CuFe2O4-
1% (w/w)
rGO |
Phenol |
·OH |
20 ppm |
5 mL |
30%
H2O2 |
6 mg/L |
240 |
100 |
Not mentioned |
Coprecipitation |
[61] |
| CuFe2O4/g-C3N4 |
Propranolol |
SO4−· |
0.02 mM |
1 g/L |
PS |
1 mM |
120 |
82.2 |
Not mentioned |
Sol-gel |
[65] |
| CoFe2O4/CCNF |
Dimethyl phthalate |
SO4−· |
0.05 mM |
0.5 g/L |
PMS |
1.5 mM |
60 |
>90 |
5
(>90%) |
Sol-gel |
[66] |
| TiO2@CuFe2O4/UV |
2,4-D |
SO4−· |
20 mg/L |
0.1 g/L |
PMS |
0.3 mM |
60 |
97.2 |
5
(>90%) |
Sol-gel |
[67] |
| ZnS-ZnFe2O4 |
Rhodamine B |
SO4−· |
20 mg/L |
20 mg |
PS |
5 mg |
90 |
97.67 |
3
(>95%) |
Hydrothermal |
[68] |
| Fe2O3@CoFe2O4 |
NOF |
SO4−·
·OH |
15 μM |
0.3 g/L |
PMS |
0.4 mM |
25 |
89.8 |
4
(90%) |
Hydrothermal |
[69] |
| Nitrogen and sulfur codoped CNTs-COOH loaded CuFe2O4 |
2-Phenylbenzimidazole-5-sulfonic acid |
SO4−· |
5 mg/L |
50 mg/L |
PMS |
1:100 (molar ratio) |
40 |
98 |
5
(>95%) |
Coprecipitation |
[70] |
3. Activation Persulfate by Fe0
In recent years, activation persulfate based on Fe
0 (zero-valent iron, ZVI) have been widely used in chemical production and environmental remediation [
71,
72]. As mentioned above, the activation persulfate/Fe (II) mechanism can cause secondary pollution to water, so ZVI/PS [
73,
74] is used instead to reduce a series of problems caused by the reduction of Fe
2+ content due to the change of pH and other factors in water [
71].
ZVI/PS system has strong reducibility (Fe
0,E
0 = −0.44 V) [
75]. Compared with CuFe
2O
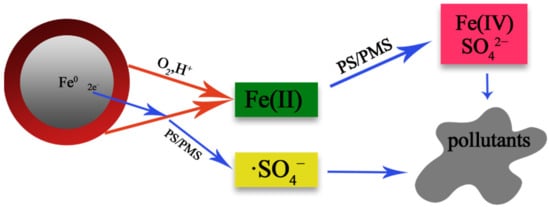
4, its reaction process is more complex, as shown in
Figure 5. Fe
0 is first converted to Fe
2+ in the presence of acid and oxidant, then further oxidized to Fe
3+ by Fe
2+, and finally to Fe(IV) [
76,
77]. The reaction mechanism follows [
78]: According to the reaction equation, the reaction is easily affected by pH, and the reaction will gradually slow with the increase of pH. Weng et al. [
79] point out that the Fe
0/PS system exhibits two-stage kinetics. The kinetic first stage is mostly attributed to a heterogeneous reaction occurring on the surface of the Fe
0 aggregate. As the reaction proceeds, decolorization shifts from the slow kinetic first stage to the fast kinetic second stage when sufficient Fe
2+ ions are maintained in the system [
80].
Figure 5. Schematic of the formation of ·SO
4− and Fe(IV) in nZVI/persulfate systems containing methyl phenyl sulfoxide [
81].
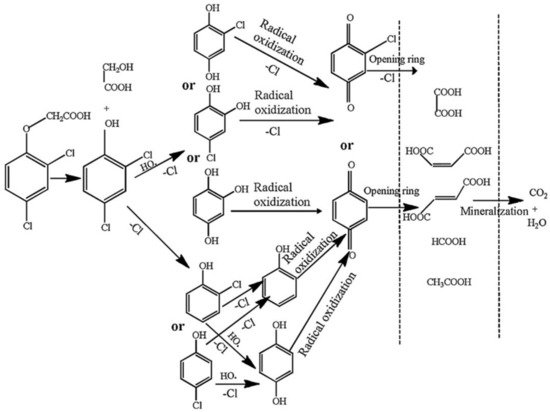
Figure 6 shows the proposed degradation pathway of 2,4-D [
82]. By examining
Figure 6, it can further confirm that macromolecular organic matter is decomposed into small molecular organic matter, which is gradually mineralized.
Figure 6. The proposed degradation pathway of 2,4-D [
82].
Table 3 shows the degradation effects of various types of polluted water bodies activated by PS/PMS based on elemental iron. Usually, an appropriate amount of H
2O
2 [
83] will be added to the water when PS is activated by Fe
0, so as to reduce the cost of oxidant. Through the analysis of the table, it can be seen that the effect of ZVI when used alone [
84] is worse than when it is combined with the carrier or when other conditions exist.
Table 3. Degradation effect of different kinds of wastewater based on PS/PMS activated by different kinds of iron [
85,
86,
87,
88,
89,
90,
91,
92].
| Catalyst |
Pollution |
Main Mechanism |
Pollutant Concentration |
Catalyst Concentration |
Oxidant |
Oxidation Concentration |
T/min |
Degradation Rate /% |
Number of Cycles |
Synthesis
Techniques |
Ref. |
| nZVI |
Sulfamethazine |
·OH
·SO4− |
50 mg/L |
2 mM |
PS
H2O2 |
1 mM
0.5 mM |
30 |
96 |
Not mentioned |
Sol-gel |
[88] |
| CN-Fe |
Sulfamethazine |
·SO4−
·OH
1O2 |
50 μM |
0.5 g/L |
PMS |
1 mM |
15 |
82 |
Not mentioned |
Carbothermal |
[87] |
| Carbon-coated nZVI |
4-chlorophenol |
·SO4−
·OH |
150 μM |
0.25 g/L |
PMS |
1 mM |
120 |
96 |
Not mentioned |
Commercially available |
[86] |
| US-nZVI |
Chloramphenicol |
·SO4−
·OH |
5 mg/L |
0.5 g/L |
PMS |
1 mM |
90 |
98.1 |
Not mentioned |
Liquid phase reduction |
[85] |
| Fe0@Fe3O4 |
Dibutyl phthalate |
·OH
·SO4− |
18 μM |
0.5 g L−1 |
PS |
1.8 mM |
180 |
94.7 |
6
(>68%) |
Calcination |
[89] |
| Fe0@Fe3O4 |
Atrazine |
·OH
·SO4− |
500 μg/L |
25 mg/L |
PMS |
1 mM |
2 |
100 |
Not mentioned |
Reduction |
[90] |
| Fe@C |
Bisphenol S |
·OH
·SO4− |
5 mg/L |
0.5 g/L |
PMS |
1.0 mM |
60 |
92.8 |
Not mentioned |
Resin carbonization |
[91] |
| Fe@C/PB |
2,4-DichloroPhenol |
·OH
·SO4− |
20 mg/L |
0.6 g/L |
PMS |
2.0 g/L |
50 |
99.4 |
Not mentioned |
Calcination |
[92] |
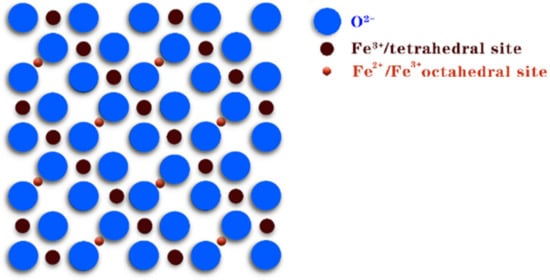
4. Fe3O4
Fe3O4 magnetite, also known as magnetic iron oxide, is a black crystal with a rotating spinel structure (Figure 7). In magnetite, Fe2+ and Fe3+ are disordered on the ferrite octahedron, so electrons can transfer rapidly between Fe2+ and Fe3+; thus, reversible redox reactions can occur at the same position on the octahedron.
Figure 7. Crystal structure of Fe3O4.
However, since Fe
3O
4 is easy to accumulate in solution and contact sites are reduced after agglomeration, single Fe
3O
4 is rarely used. Using the composite carrier method [
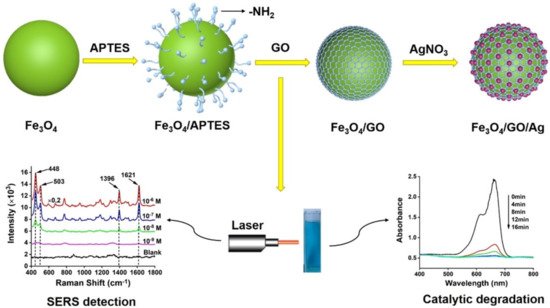
93] can not only solve these problems, but also speeds the reaction rate, making it more cost effective when applied in industrial production. He et al. [
94] pointed out that the Fe
3O
4/GO/Ag composite microspheres are formed using magnetic Fe
3O
4 as cores, followed by coating an internal layer of GO and an outer layer of Ag nanoparticles, as
Figure 8 shows. The synthesized Fe
3O
4/GO/Ag composite catalyst under the action of NaBH
4, methylene blue, and ciprofloxacin can be completely degraded within 12 min.
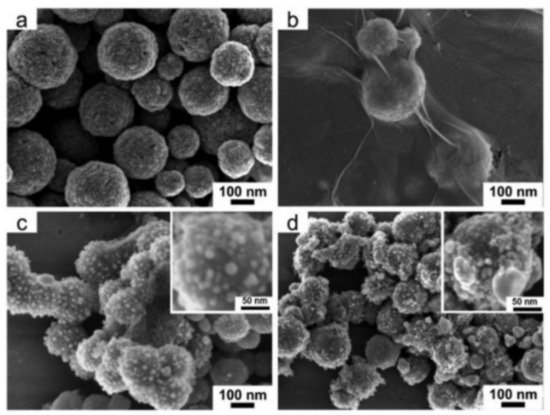
Figure 8 shows SEM images of Fe
3O
4/GO/Ag composite catalyst. In
Figure 9, we can clearly observe that Ag has been completely attached to the Fe
3O
4/GO surface, which can increase the specific surface area and improve the chemical reaction rate.
Figure 8. Illustration of the fabrication of Fe
3O
4/GO/Ag composite microspheres [
94].
Figure 9. Typical FESEM images of (
a) Fe
3O
4, (
b) Fe
3O
4/GO, (
c) Fe
3O
4/GO/Ag, and (
d) Fe
3O
4/Ag microspheres. Inserts are magnified FESEM images of Fe
3O
4/GO/Ag and Fe
3O
4/Ag microspheres [
94].
Table 4 shows the research progress of Fe3O4 and its composite materials on the degradation of different pollutants reported at present. According to the data in the table, when Fe3O4 is compounded with the carrier, the catalytic performance is greatly improved.
Table 4. Effects of Fe
3O
4 and its composite-material-activated PS/PMS on degradation of different kinds of wastewater [
95,
96,
97,
98,
99,
100,
101].