Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianhong Gu | + 4576 word(s) | 4576 | 2021-12-14 04:56:44 | | | |
| 2 | Rita Xu | -1381 word(s) | 3195 | 2021-12-15 11:41:04 | | | | |
| 3 | Rita Xu | -2325 word(s) | 2251 | 2021-12-15 11:41:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gu, J. Lα,25-(OH)2D3 on Osteoclastogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/17153 (accessed on 07 February 2026).
Gu J. Lα,25-(OH)2D3 on Osteoclastogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/17153. Accessed February 07, 2026.
Gu, Jianhong. "Lα,25-(OH)2D3 on Osteoclastogenesis" Encyclopedia, https://encyclopedia.pub/entry/17153 (accessed February 07, 2026).
Gu, J. (2021, December 15). Lα,25-(OH)2D3 on Osteoclastogenesis. In Encyclopedia. https://encyclopedia.pub/entry/17153
Gu, Jianhong. "Lα,25-(OH)2D3 on Osteoclastogenesis." Encyclopedia. Web. 15 December, 2021.
Copy Citation
The active form of vitamin D, 1α,25-(OH)2D3, not only promotes intestinal calcium absorption, but also regulates the formation of osteoclasts (OCs) and their capacity for bone mineral dissolution. Gal-3 is a newly discovered bone metabolic regulator involved in the proliferation, differentiation, and apoptosis of various cells.
galectin-3
1α
25-(OH)2D3
osteoclasts
bone resorption
1. Introduction
OCs are derived from bone marrow mononuclear macrophages (BMMs) and are the only cells capable of bone resorption in the body. They not only degrade the bone organic and inorganic matrix but also cooperate with osteoblasts (OBs) to regulate bone formation and reconstruction [1][2][3]. Overactive bone resorption by OCs in physiological processes (such as aging and menopause) [4] and pathological processes (such as bone metastasis and rheumatoid arthritis) [5][6] can lead to osteoporosis. OCPs, such as bone marrow cells, splenocytes, and RAW264.7 macrophages, co-cultured with stromal cells, OBs, or osteocytes in vitro can be induced into OCs by the parathyroid hormone (PTH), dexamethasone, tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and 1α,25-(OH)2D3, which regulate the expression of membrane-bound RANKL in OBs, stromal cells, and osteocytes [1][7]. BMMs could also be induced into OCs directly by M-CSF and RANKL [8]. M-CSF mainly promotes the proliferation and differentiation of OCPs, which further differentiate into OCs under the action of RANKL [9][10].
The physiologically active form of vitamin D, 1α,25-(OH)2D3, regulates intestinal calcium absorption and acts on bone cells directly and a series of cytokines or signaling pathways in bone [11][12][13]. OB-lineage cells express vitamin D receptor (VDR) [14], and 1α,25-(OH)2D3 promotes OBs’ maturation and bone mineralization in vitro and in vivo via VDR and reduces the formation of unmineralized osteoids [15]. However, bone tissue is in a state of dynamic equilibrium, and over-mineralization or absorption is not conducive to bone health. To prevent excessive bone mineralization, 1α,25-(OH)2D3 can enhance OC formation indirectly by promoting the expression of RANKL in a concentration-dependent manner [15][16]. OC formation can also be directly regulated by 1α,25-(OH)2D3. Although mature OCs do not express VDR, OCPs do [17][18][19]. The mechanism by which it regulates OC formation needs to be further clarified.
Gal-3 is a 29–35 kDa protein expressed in a variety of tissues and is a member of the β-galactosyl-binding protein family [20]. It is a marker of chondrocyte and OB lineages in bone and is also present in OCs and BMMs [21][22]. In proteomic studies, we found that 1α,25-(OH)2D3 activates gal-3 expression during OC formation in vitro [23][24]. Simon et al. have also shown that gal-3 is a novel regulator of bone homeostasis directly or indirectly by regulating the association between OBs and OCs. Accordingly, gal-3 plays an important role in bone biology and is expected to be a potential target for the prevention of bone diseases. However, its role in the regulation of OC formation by 1α,25-(OH)2D3 needs to be further elucidated.
2. 1α,25-(OH)2D3 Had No Effect on Osteoclast Precursor Viability
We confirmed that 1α,25-(OH)2D3 upregulates VDR mRNA and protein expression in OCPs [25]. In this study, we observed that adding 0.1, 1, and 10 nmol/L 1α,25-(OH)2D3 to the medium had no effect on OCPs’ viability (Figure 1A). RANKL significantly inhibited cell proliferation during OCs formation (p < 0.01). However, 1α,25-(OH)2D3 had no significant effect on OCPs’ viability in the absence or presence of RANKL (Figure 1B).
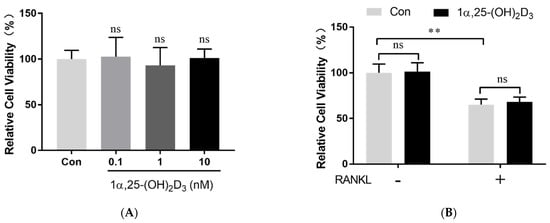
Figure 1. OCP viability was not affected by 1α,25-(OH)2D3. (A) Cell viability detected by CCK-8 24 h after treatment with different concentrations of 1α,25-(OH)2D3. (B) Cell viability detected by CCK-8 24 h after treatment with 10 nmol/L 1α,25-(OH)2D3 in the absence or presence of 50 ng/mL RANKL. Data are shown as means ± SD. n = 6, ns, p > 0.05; ** p < 0.01.
3. 1α,25-(OH)2D3 Promoted Gal-3 Expression
To elucidate the effect of 1α,25-(OH)2D3 on gal-3 protein expression, 0.1, 1, and 10 nmol/L 1α,25-(OH)2D3 were added to the culture medium during OC formation induced by 25 ng/mL M-CSF and 50 ng/mL RANKL for 3 days. 1α,25-(OH)2D3 upregulated gal-3 protein expression in a dose-dependent manner (Figure 2). The 10 nmol/L 1α,25-(OH)2D3 group had a higher level of gal-3 protein expression than that in the other groups.
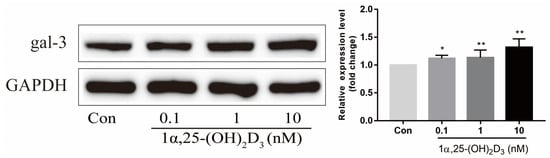
Figure 2. The expression of gal-3 protein was upregulated by 1α,25-(OH)2D3 dose-dependently on day 3 as determined by Western blotting. Histograms show gray values of gal-3 protein. Data are shown as means ± SD. n = 5, * p < 0.05, ** p < 0.01.
To confirm the effect of 1α,25-(OH)2D3 on gal-3 protein expression at different time points, OCPs induced by 25 ng/mL M-CSF and 50 ng/mL RANKL were treated with 10 nmol/L 1α,25-(OH)2D3 for 0, 1, 3, and 5 days. Anhydrous ethanol was used as a control. Compared with the level in the control group, 1α,25-(OH)2D3 significantly increased gal-3 protein expression on days 3 and 5 (p < 0.01) (Figure 3). No significant difference was observed between the control group and the 1α,25-(OH)2D3 group on day 1 (p > 0.05). Compared with day 1, 10 nmol/L 1α,25-(OH)2D3 significantly increased gal-3 protein expression on days 3 and 5 (p < 0.01). However, there was no significant change between days 3 and 5 1α,25-(OH)2D3 groups (p > 0.05). These data indicated that 1α,25-(OH)2D3 promoted the protein expression of gal-3 at the same cultivation time.
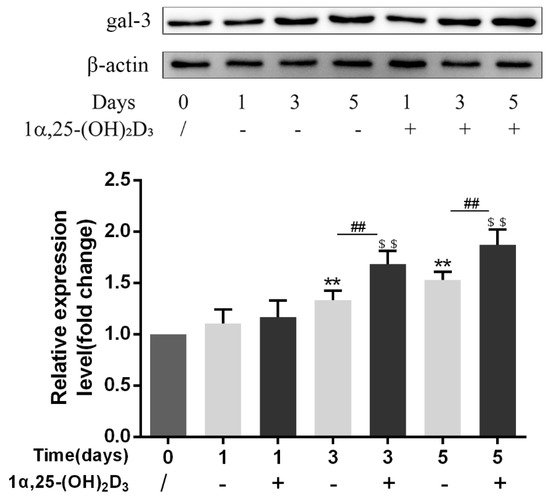
Figure 3. Gal-3 protein expression was upregulated by 1α,25-(OH)2D3 at the same cultivation time. Histograms show relative expression levels of gal-3 protein. Data are shown as means ± SD. n = 3, ** p < 0.01 vs. the 0 d group; $$ p < 0.01 vs. the 1α,25-(OH)2D3 treatment group on day 1; ## p < 0.01 vs. different groups on the same day.
To further confirm the effect of 1α,25-(OH)2D3 on gal-3 protein distribution, an immunofluorescence assay was performed. The gal-3 protein was visualized by green fluorescence, while F-actin was visualized in red on day 6 after treatment with 10 nmol/L 1α,25-(OH)2D3. Anhydrous ethanol was used as a control. Gal-3 mainly distributed in the nuclei (cyan) and cell membranes (yellow) of OCs (large cells with more than three nuclei marked by white triangles) and in the whole OCPs (small cells with one nucleus marked by white arrow) (Figure 4). Compared with OCs, OCPs had a wider green fluorescence distribution of gal-3. These data confirmed that 1α,25-(OH)2D3 changed the protein distribution of gal-3.
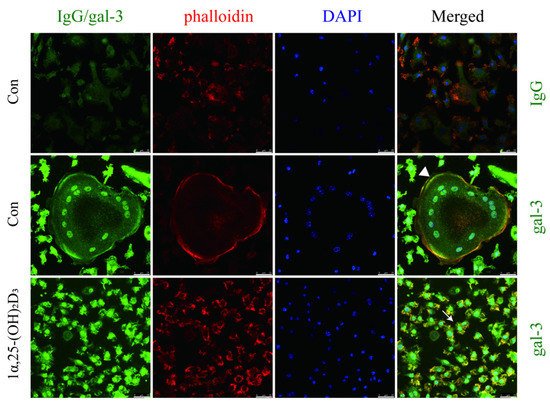
Figure 4. Immunofluorescence showed the distribution and expression of gal-3 after the treatment of 1α,25-(OH)2D3. Gal-3 mainly distributed in the nuclei (cyan) and cell membranes (yellow) of OCs (marked by white triangle) and in the whole cell of OCPs (white arrows). Gal-3 protein distribution was regulated by 1α,25-(OH)2D3. In non-merged images, green indicates gal-3, red indicates F-actin, and blue indicates nuclei. Bars = 25 μm.
To elucidate the effect of 1α,25-(OH)2D3 on the expression of Lgals3, which encodes the gal-3 protein, 10 nmol/L 1α,25-(OH)2D3 was added to the culture medium during OC formation induced by 25 ng/mL M-CSF and 50 ng/mL RANKL for 0, 1, 3, and 5 days. Anhydrous ethanol was used as a control. The expression of Lgals3 first increased and then decreased over time. Compared with control groups (without 1α,25-(OH)2D3), 10 nmol/L 1α,25-(OH)2D3 significantly increased Lgals3 expression on days 3 and 5 (p < 0.01). Compared with day 1, 10 nmol/L 1α,25-(OH)2D3 significantly increased Lgals3 expression on days 3 and 5 (p < 0.01). However, Lgals3 expression on day 5 was lower than day 3 in the groups with or without 1α,25-(OH)2D3 (Figure 5).
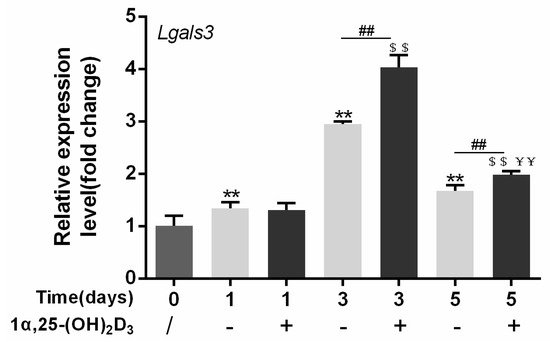
Figure 5. Lgals3 expression is upregulated by 1α,25-(OH)2D3. Data are shown as means ± SD. n = 3, ** p < 0.01 vs. the 0 d group; $$ p < 0.01 vs. the 1α,25-(OH)2D3 treatment group on day 1; ## p < 0.01 vs. different groups on the same day; ¥¥ p < 0.01 vs. the 1α,25-(OH)2D3 treatment group on day 3.
4. Gal-3 Contributed to Osteoclasts Formation and Activation Regulated by 1α,25-(OH)2D3
We found that 1α,25-(OH)2D3 increased gal-3 expression at the mRNA and protein levels. To confirm the role of gal-3 in 1α,25-(OH)2D3-mediated OC formation and bone resorption, we constructed stable Lgals3 knockdown OCPs using gal-3 siRNA. Negative control (NC) siRNA was used as the control. These OCPs were treated with 10 nM 1α,25-(OH)2D3 in the presence of 25 ng/mL M-CSF and 50 ng/mL RANKL. Anhydrous ethanol was used as a control.
First, OC formation was detected by TRAP staining on day 6 after the treatment of 1α,25-(OH)2D3. In all groups, large cells with wine-red granules regarded as OCs were found. In the NC group, the volume of OCs treated with 1α,25-(OH)2D3 and the number and the size of OCs decreased significantly (p < 0.01). In the gal-3 knockdown group, 1α,25-(OH)2D3 had no significant effect on OC formation, but significantly decreased the size of OCs. These data confirmed that gal-3 contributes to the regulation of OC formation by 1α,25-(OH)2D3. Additionally, gal-3 knockdown significantly promoted OC formation and average size (p < 0.01) (Figure 6A–C). This suggests that gal-3 is a negative regulator of OC formation and average size.
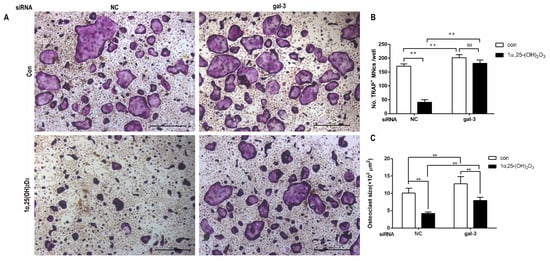
Figure 6. Gal-3 knockdown attenuated the inhibitory effect of 1α,25-(OH)2D3 on OC formation. (A) TRAP staining. Large multinuclear cells (MNCs) with wine-red granules were regarded as OCs (yellow arrows). Bars = 400 μm. (B) Quantitative analysis of OC quantity. (C) Quantitative analysis of OC size. Data are shown as means ± SD. n = 3, ** p < 0.01, ns means p > 0.05.
Gal-3 and OC-related proteins (NFATc1 and MMP-9) were investigated by Western blotting on day 3 after treatment with 1α,25-(OH)2D3. Compared to the NC group, cells with Lgals3 knockdown exhibited a significant decrease in gal-3 protein level (p < 0.01) (Figure 7). In the NC group, the expression of NFATc1 and MMP-9 proteins were significantly inhibited by 1α,25-(OH)2D3 (p < 0.01). In gal-3 knockdown groups, 1α,25-(OH)2D3 had no significant effect on the expression of NFATc1 and MMP-9 proteins. These data confirmed that gal-3 contributed to OC-related protein expression regulated by 1α,25-(OH)2D3. Gal-3 knockdown significantly increased OC-related protein expression levels (p < 0.01) (Figure 7). These findings further suggest that gal-3 is a negative regulator of OC formation.
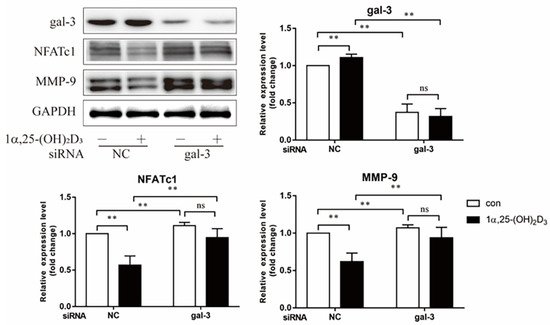
Figure 7. Gal-3 knockdown attenuated the inhibitory effect of 1α,25-(OH)2D3 on OC-related protein expression. The histograms show the relative expression level of proteins. Data are shown as means ± SD. n =3, ** p < 0.01, ns means p > 0.05.
mRNA expression levels of OC-related genes, Ctsk, and Mmp-9 were evaluated by qPCR on day 3 after treatment with 1α,25-(OH)2D3. In the NC group, Ctsk and MMP-9 levels were significantly inhibited by 1α,25-(OH)2D3 (p < 0.01). In the gal-3 knockdown groups, 1α,25-(OH)2D3 also inhibited Ctsk and MMP-9 expression. However, compared to levels in the NC groups, the inhibitory effects of 1α,25-(OH)2D3 on Ctsk and MMP-9 were significantly attenuated by gal-3 knockdown (p < 0.01). Additionally, gal-3 knockdown significantly increased OC-related gene expression levels (p< 0.01) (Figure 8). These findings were consistent with TRAP-positive OC formation and OC-related protein expression results.
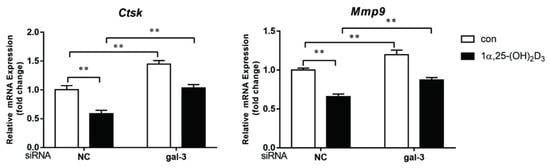
Figure 8. Gal-3 knockdown attenuated the inhibitory effect of 1α,25-(OH)2D3 on OC-related gene expression. Histograms show relative expression levels of genes. Data are shown as means ± SD. n = 3, ** p < 0.01.
To evaluate the effects of gal-3 on bone resorption regulated by 1α,25-(OH)2D3, equal number of BMMs were cultured on an osteoassay surface multiple-well plate for each group. Bone resorption lacunae were observed using an inverted microscope on day 6 after the treatment with 1α,25-(OH)2D3. We observed bone resorption lacunae in each group (Figure 9A, black arrow). Based on the area of bone resorption lacunae, in the NC group, bone resorption was significantly inhibited by 1α,25-(OH)2D3 (p < 0.01). In the gal-3 knockdown groups, 1α,25-(OH)2D3 had no effect on bone resorption activity. Gal-3 knockdown significantly attenuated the inhibitory effect of 1α,25-(OH)2D3 on bone resorption (p < 0.01). Additionally, gal-3 knockdown significantly increased OC bone resorption (p < 0.01) (Figure 9B). These data confirmed that gal-3 is a negative regulator of OC bone resorption and contributes to the inhibitory effect of 1α,25-(OH)2D3 on OC bone resorption.
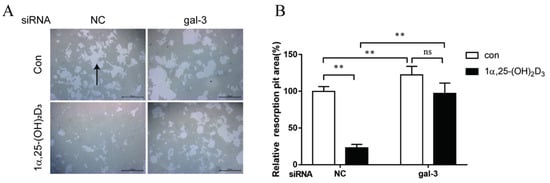
Figure 9. Gal-3 knockdown attenuated the inhibitory effect on bone resorption by 1α,25-(OH)2D3. (A) Bone resorption lacunae (marked by black arrows) observed by inverted microscopy. Bars = 400 μm. (B) Statistical analysis of the area of bone resorption lacunae. Data are shown as means ± SD. n = 3, ** p < 0.01, ns means p > 0.05.
5. Interaction between Gal-3 and VDR
To verify the relationship between gal-3 and VDR proteins, they were evaluated by co-immunoprecipitation and immunofluorescence double staining. The expression of VDR and gal-3 could be detected in the input group (Figure 10A). The expression of VDR and gal-3 was also detected in the protein samples precipitated by the anti-VDR antibody (Figure 10A). These results suggest that there is an interaction between gal-3 and the VDR proteins.
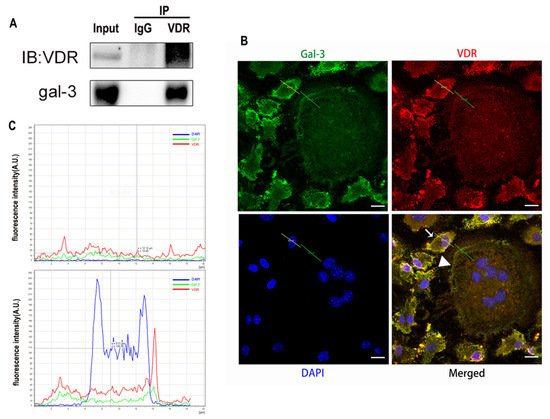
Figure 10. Images showing co-localization and possible interactions between gal-3 and VDR proteins. (A) Interaction between gal-3 and VDR proteins confirmed by co-immunoprecipitation. (B,C) Co-localization of gal-3 and VDR proteins detected by immunofluorescence. In non-merged images, gal-3 is green, VDR is red, and nuclei are blue. Bars = 10 μm.
The expression of gal-3 (green) and VDR (red) protein was also observed by confocal fluorescence microscopy. OCPs (small cells marked by white arrows in Figure 10B) showed high expression levels of gal-3 and VDR, while gal-3 and VDR expression levels were low in OCs (large cells with multiple nuclei, marked by white triangles). Gal-3 and VDR proteins were mainly co-localized (yellow) in the cell membrane (Figure 10B). The red and green curves change in the same way, which suggests that gal-3 and VDR are co-located. (Figure 10C). These results further supported the co-localization and possible interaction between gal-3 and VDR.
References
- William J. Boyle; W. Scott Simonet; David L. Lacey; Osteoclast differentiation and activation. Nature 2003, 423, 337-342, 10.1038/nature01658.
- Megan M. Weivoda; Chee Kian Chew; David G. Monroe; Joshua N. Farr; Elizabeth J. Atkinson; Jennifer R. Geske; Brittany Eckhardt; Brianne Thicke; Ming Ruan; Amanda J. Tweed; et al.Louise K. McCreadyRobert A. RizzaAleksey MatveyenkoMoustapha KassemThomas Levin AndersenAdrian VellaMatthew T. DrakeBart L. ClarkeMerry Jo OurslerSundeep Khosla Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nature Communications 2020, 11, 1-13, 10.1038/s41467-019-14003-6.
- Brendan F. Boyce; Advances in osteoclast biology reveal potential new drug targets and new roles for osteoclasts. Journal of Bone and Mineral Research 2013, 28, 711-722, 10.1002/jbmr.1885.
- Anaïs Marie Julie Møller; Jean-Marie Delaissé; Jacob Bastholm Olesen; Jonna Skov Madsen; Luisa Matos Canto; Troels Bechmann; Silvia Regina Rogatto; Kent Søe; Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Research 2020, 8, 1-11, 10.1038/s41413-020-0102-7.
- K P McHugh; Z. Shen; T N Crotti; M R Flannery; R P O'sullivan; P E Purdue; S R Goldring; The role of cell-substrate interaction in regulating osteoclast activation: potential implications in targeting bone loss in rheumatoid arthritis. Annals of the Rheumatic Diseases 2009, 69, i83-i85, 10.1136/ard.2009.120188.
- Jianjiao Ni; Xiaofei Zhang; Juan Li; Zhiqin Zheng; Junhua Zhang; Weixin Zhao; Liang Liu; Tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell Death & Disease 2021, 12, 1-10, 10.1038/s41419-021-03928-w.
- Aseel Marahleh; Hideki Kitaura; Fumitoshi Ohori; Akiko Kishikawa; Saika Ogawa; Wei-Ren Shen; Jiawei Qi; Takahiro Noguchi; Yasuhiko Nara; Itaru Mizoguchi; et al. TNF-α Directly Enhances Osteocyte RANKL Expression and Promotes Osteoclast Formation. Frontiers in Immunology 2019, 10, 2925, 10.3389/fimmu.2019.02925.
- Cheng-Ming Wei; Yi-Ji Su; Xiong Qin; Jia-Xin Ding; Qian Liu; Fang-Ming Song; Shao-Hui Zong; Jiake Xu; Bo Zhou; Jin-Min Zhao; et al. Monocrotaline Suppresses RANKL-Induced Osteoclastogenesis In Vitro and Prevents LPS-Induced Bone Loss In Vivo. Cellular Physiology and Biochemistry 2018, 48, 644-656, 10.1159/000491892.
- Steven Teitelbaum; F. Patrick Ross; Genetic regulation of osteoclast development and function. Nature Reviews Genetics 2003, 4, 638-649, 10.1038/nrg1122.
- Chengchao Song; Xiaobin Yang; Yongsheng Lei; Zhen Zhang; Wanli Smith; Jinglong Yan; Lingbo Kong; Evaluation of efficacy on RANKL induced osteoclast from RAW264.7 cells. Journal of Cellular Physiology 2018, 234, 11969-11975, 10.1002/jcp.27852.
- Sylvia Christakos; Puneet Dhawan; Angela Porta; Leila J. Mady; Tanya Seth; Vitamin D and intestinal calcium absorption. Molecular and Cellular Endocrinology 2011, 347, 25-29, 10.1016/j.mce.2011.05.038.
- Jianhong Gu; Xi-Shuai Tong; Guo-Hong Chen; Dong Wang; Yang Chen; Yan Yuan; Xue-Zhong Liu; Jian-Chun Bian; Zong-Ping Liu; Effects of 1α,25-(OH)2D3 on the formation and activity of osteoclasts in RAW264.7 cells. The Journal of Steroid Biochemistry and Molecular Biology 2015, 152, 25-33, 10.1016/j.jsbmb.2015.04.003.
- J. Wesley Pike; Sylvia Christakos; Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinology and Metabolism Clinics of North America 2017, 46, 815-843, 10.1016/j.ecl.2017.07.001.
- Tomoki Mori; Kanji Horibe; Masanori Koide; Shunsuke Uehara; Yoko Yamamoto; Shigeaki Kato; Hisataka Yasuda; Naoyuki Takahashi; Nobuyuki Udagawa; Yuko Nakamichi; et al. The Vitamin D Receptor in Osteoblast-Lineage Cells Is Essential for the Proresorptive Activity of 1α,25(OH)2D3 In Vivo. Endocrinology 2020, 161, bqaa178, 10.1210/endocr/bqaa178.
- Renata C. Pereira; Isidro B. Salusky; Richard E. Bowen; Earl G. Freymiller; Katherine Wesseling-Perry; Vitamin D sterols increase FGF23 expression by stimulating osteoblast and osteocyte maturation in CKD bone. Bone 2019, 127, 626-634, 10.1016/j.bone.2019.07.026.
- Naoyuki Takahashi; Nobuyuki Udagawa; Tatsuo Suda; Vitamin D endocrine system and osteoclasts. BoneKEy Reports 2014, 3, 495, 10.1038/bonekey.2013.229.
- Jianhong Gu; Xishuai Tong; Yang Chen; Chuang Zhang; Tianhong Ma; Saihui Li; Wenyan Min; Yan Yuan; Xuezhong Liu; Jianchun Bian; et al.Zongping Liu Vitamin D Inhibition of TRPV5 Expression During Osteoclast Differentiation. International Journal of Endocrinology and Metabolism 2019, 17, e91583, 10.5812/ijem.91583.
- Jumpei Teramachi; Yuko Hiruma; Seiichi Ishizuka; Hisako Ishizuka; Jacques P. Brown; Laëtitia Michou; Huiling Cao; Deborah L. Galson; Mark A Subler; Hua Zhou; et al.David W DempsterJolene J WindleG. David RoodmanNoriyoshi Kurihara Role of ATF7-TAF12 interactions in the vitamin D response hypersensitivity of osteoclast precursors in Paget's disease. Journal of Bone and Mineral Research 2013, 28, 1489-1500, 10.1002/jbmr.1884.
- M. Kogawa; D.M. Findlay; P.H. Anderson; G.J. Atkins; Modulation of osteoclastic migration by metabolism of 25(OH)-vitamin D3. The Journal of Steroid Biochemistry and Molecular Biology 2013, 136, 59-61, 10.1016/j.jsbmb.2012.09.008.
- Colnot, C.; Sidhu, S.S.; Poirier, F.; Balmain, N; Cellular and subcellular distribution of galectin-3 in the epiphyseal cartilage and bone of fetal and neonatal mice. Cell Mol Biol 1999, 45, 1191-1202.
- Jane E. Aubin; F. Liu; Luc Malaval; A.K. Gupta; Osteoblast and chondroblast differentiation. Bone 1995, 17, S77-S83, 10.1016/8756-3282(95)00183-e.
- Kosei Nakajima; Dhong Hyo Kho; Takashi Yanagawa; Yosuke Harazono; Victor Hogan; Wei Chen; Rouba Ali-Fehmi; Rohit Mehra; Avraham Raz; Galectin-3 Cleavage Alters Bone Remodeling: Different Outcomes in Breast and Prostate Cancer Skeletal Metastasis.. Cancer Research 2016, 76, 1391-402, 10.1158/0008-5472.CAN-15-1793.
- Gu, J.; Kong, Q.; Wang, D.; Tong, X.; Bian, J.; Liu, X.; Yuan, Y.; Liu, Z; Deferential protein expressions and bioinformatic analysis of OC fromation regulated by vitamin D. Chinese Journal of Veterinary Science (in chinese) 2019, 39, 2207-2214.
- Dominic Simon; Anja Derer; Fabian T. Andes; Patrick Lezuo; Aline Bozec; Georg Schett; Martin Herrmann; Ulrike Harre; Galectin-3 as a novel regulator of osteoblast-osteoclast interaction and bone homeostasis. Bone 2017, 105, 35-41, 10.1016/j.bone.2017.08.013.
- Gu, J.; Zhang, C.; Min, W.; ZHao, Y.; Li, S.; Liu, Z; Effects of 1α,25-(OH)2D3 on osteoclastogenesis and activity in mice. Chinese Journal of Veterinary Science (in chinese) 2019, 49, 593-600.
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al.et al Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165-176.
- Paola Spessotto; Francesca Maria Rossi; Massimo Degan; Raffaele Di Francia; Roberto Perris; Alfonso Colombatti; Valter Gattei; Hyaluronan–CD44 interaction hampers migration of osteoclast-like cells by down-regulating MMP-9. Journal of Cell Biology 2002, 158, 1133-1144, 10.1083/jcb.200202120.
- Saki Nakagawa; Kazuhiro Omori; Masaaki Nakayama; Hiroki Mandai; Satoshi Yamamoto; Hiroya Kobayashi; Hidefumi Sako; Kyosuke Sakaida; Hiroshi Yoshimura; Satoki Ishii; et al.Soichiro IbaragiKimito HiraiKeisuke YamashiroTadashi YamamotoSeiji SugaShogo Takashiba The fungal metabolite (+)-terrein abrogates osteoclast differentiation via suppression of the RANKL signaling pathway through NFATc1. International Immunopharmacology 2020, 83, 106429, 10.1016/j.intimp.2020.106429.
- Takayanagi, H.; Kim, S.; Koga, T.; Nishina, H.; Isshiki, M.; Yoshida, H.; Saiura, A.; Isobe, M.; Yokochi, T.; Inoue, J.; et al.et al Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 2002, 3, 889-901.
- Masakazu Kogawa; Koji Hisatake; Gerald Atkins; David M. Findlay; Yuichiro Enoki; Tsuyoshi Sato; Peter C. Gray; Yukiko Kanesaki-Yatsuka; Paul Anderson; Seiki Wada; et al.Naoki KatoAya FukudaShigehiro KatayamaMasafumi TsujimotoTetsuya YodaTatsuo SudaYasushi OkazakiMasahito Matsumoto The Paired-box Homeodomain Transcription Factor Pax6 Binds to the Upstream Region of the TRAP Gene Promoter and Suppresses Receptor Activator of NF-κB Ligand (RANKL)-induced Osteoclast Differentiation. Journal of Biological Chemistry 2013, 288, 31299-31312, 10.1074/jbc.m113.461848.
- Denis Duplat; Marlène Gallet; Sophie Berland; Arul Marie; Lionel Dubost; Marthe Rousseau; Said Kamel; Christian Milet; Michel Brazier; Evelyne Lopez; et al.Laurent Bedouet The effect of molecules in mother-of-pearl on the decrease in bone resorption through the inhibition of osteoclast cathepsin K. Biomaterials 2007, 28, 4769-4778, 10.1016/j.biomaterials.2007.07.036.
- Vaishali Veldurthy; Ran Wei; Leyla Oz; Puneet Dhawan; Yong Heui Jeon; Sylvia Christakos; Vitamin D, calcium homeostasis and aging. Bone Research 2016, 4, 16041, 10.1038/boneres.2016.41.
- Riko Kitazawa; Kiyoshi Mori; Akira Yamaguchi; Takeshi Kondo; Sohei Kitazawa; Modulation of mouse RANKL gene expression by Runx2 and vitamin D3. Journal of Cellular Biochemistry 2008, 105, 1289-1297, 10.1002/jcb.21929.
- Dong Wang; Jian-Hong Gu; Yang Chen; Hong-Yan Zhao; Wei Liu; Rui-Long Song; Jian-Chun Bian; Xue-Zhong Liu; Yan Yuan; Zong-Ping Liu; et al. 1α,25-Dihydroxyvitamin D3 inhibits the differentiation and bone resorption by osteoclasts generated from Wistar rat bone marrow-derived macrophages. Experimental and Therapeutic Medicine 2015, 10, 1039-1044, 10.3892/etm.2015.2632.
- Sadaoki Sakai; Hironari Takaishi; Kenichiro Matsuzaki; Hironori Kaneko; Mitsuru Furukawa; Yoshiteru Miyauchi; Ayako Shiraishi; Keiji Saito; Akio Tanaka; Tadatsugu Taniguchi; et al.Toshio SudaTakeshi MiyamotoYoshiaki Toyama 1-Alpha, 25-dihydroxy vitamin D3 inhibits osteoclastogenesis through IFN-beta-dependent NFATc1 suppression. Journal of Bone and Mineral Metabolism 2009, 27, 643-652, 10.1007/s00774-009-0084-4.
- Junichi Kikuta; Shunsuke Kawamura; Fumie Okiji; Mai Shirazaki; Sadaoki Sakai; Hitoshi Saito; Masaru Ishii; Sphingosine-1-phosphate-mediated osteoclast precursor monocyte migration is a critical point of control in antibone-resorptive action of active vitamin D. Proceedings of the National Academy of Sciences 2013, 110, 7009-7013, 10.1073/pnas.1218799110.
- Gong, H.C.; Honjo, Y.; Nangia-Makker, P.; Hogan, V.; Mazurak, N.; Bresalier, R.S.; Raz, A; The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res 1999, 59, 6239-6245.
- Milica Velickovic; Aleksandar Arsenijevic; Aleksandar Acovic; Dragana Arsenijevic; Jelena Milovanovic; Jelena Dimitrijevic; Zeljko Todorovic; Marija Milovanovic; Tatjana Kanjevac; Nebojsa Arsenijevic; et al. Galectin-3, Possible Role in Pathogenesis of Periodontal Diseases and Potential Therapeutic Target. Frontiers in Pharmacology 2021, 12, 638258, 10.3389/fphar.2021.638258.
- Huajun Tang; Peiyue Zhang; Lianlin Zeng; Yu Zhao; Libo Xie; Bo Chen; Mesenchymal stem cells ameliorate renal fibrosis by galectin-3/Akt/GSK3β/Snail signaling pathway in adenine-induced nephropathy rat. Stem Cell Research & Therapy 2021, 12, 1-22, 10.1186/s13287-021-02429-z.
- Guodong Fu; Olena Polyakova; Ronald Chazen; Jeremy Freeman; Ian Witterick; Diagnostic Value of Galectin-3 in Distinguishing Invasive Encapsulated Carcinoma from Noninvasive Follicular Thyroid Neoplasms with Papillary-Like Nuclear Features (NIFTP). Cancers 2021, 13, 2988, 10.3390/cancers13122988.
- Weizhen Jia; Lingyu Kong; Hiroyasu Kidoya; Hisamichi Naito; Fumitaka Muramatsu; Yumiko Hayashi; Han-Yun Hsieh; Daishi Yamakawa; Daniel K. Hsu; Fu-Tong Liu; et al.Nobuyuki Takakura Indispensable role of Galectin-3 in promoting quiescence of hematopoietic stem cells. Nature Communications 2021, 12, 1-17, 10.1038/s41467-021-22346-2.
- Carla Iacobini; Claudia Blasetti Fantauzzi; Rossella Bedini; Raffaella Pecci; Armando Bartolazzi; Bruno Amadio; Carlo Pesce; Giuseppe Pugliese; Stefano Menini; Galectin-3 is essential for proper bone cell differentiation and activity, bone remodeling and biomechanical competence in mice. Metabolism 2018, 83, 149-158, 10.1016/j.metabol.2018.02.001.
- Adriana Lepur; Michael C. Carlsson; Ruđer Novak; Jerka Dumić; Ulf J. Nilsson; Hakon Leffler; Galectin-3 endocytosis by carbohydrate independent and dependent pathways in different macrophage like cell types. Biochimica et Biophysica Acta (BBA) - General Subjects 2012, 1820, 804-818, 10.1016/j.bbagen.2012.02.018.
- Wei Liu; Chung Chi Le; Dong Wang; Di Ran; Yi Wang; Hongyan Zhao; Jianhong Gu; Hui Zou; Yan Yuan; Jianchun Bian; et al.Zongping Liu Ca2+/CaM/CaMK signaling is involved in cadmium-induced osteoclast differentiation. Toxicology 2020, 441, 152520, 10.1016/j.tox.2020.152520.
- Xishuai Tong; Chuang Zhang; Dong Wang; Ruilong Song; Yonggang Ma; Ying Cao; Hongyan Zhao; Jianchun Bian; Jianhong Gu; Zongping Liu; et al. Suppression of AMP‐activated protein kinase reverses osteoprotegerin‐induced inhibition of osteoclast differentiation by reducing autophagy. Cell Proliferation 2019, 53, e12714, 10.1111/cpr.12714.
- Xishuai Tong; Jianhong Gu; Miaomiao Chen; Tao Wang; Hui Zou; Ruilong Song; Hongyan Zhao; Jianchun Bian; Zongping Liu; p53 positively regulates osteoprotegerin-mediated inhibition of osteoclastogenesis by downregulating TSC2-induced autophagy in vitro. Differentiation 2020, 114, 58-66, 10.1016/j.diff.2020.06.002.
- Ahmed Hasbi; Melissa L. Perreault; Maurice Y. F. Shen; Theresa Fan; Tuan Nguyen; Mohammed Alijaniaram; Tomek J. Banasikowski; Anthony A. Grace; Brian F. O'dowd; Paul Fletcher; et al.Susan R. George Activation of Dopamine D1-D2 Receptor Complex Attenuates Cocaine Reward and Reinstatement of Cocaine-Seeking through Inhibition of DARPP-32, ERK, and ΔFosB. Frontiers in Pharmacology 2018, 8, 924, 10.3389/fphar.2017.00924.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
635
Revisions:
3 times
(View History)
Update Date:
29 Mar 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




