
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hiroyuki Naruse | + 1267 word(s) | 1267 | 2021-11-25 08:02:49 | | | |
| 2 | Beatrix Zheng | Meta information modification | 1267 | 2021-11-30 07:10:19 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 1267 | 2021-11-30 07:11:18 | | |
Video Upload Options
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a widespread impact on health, including a substantial mortality among patients with various pre-existing health conditions. Patients with cardiovascular disease (CVD) are more susceptible to the development of severe COVID-19 infection. The incidence of mechanical complications of acute coronary syndrome (ACS) increased fivefold after the declaration of a state of emergency in Japan. Therefore, vaccination against SARS-CoV-2 is generally recommended in patients with CVD, as is vaccination against other infectious agents. The BNT162b2 mRNA COVID-19 vaccine has shown promising efficacy and safety, mainly in people without apparent pre-existing comorbidities. A nationwide mass vaccination study focused on the estimated vaccine effectiveness of patients with various comorbidities such as heart disease. No data, however, are available regarding the vaccine effectiveness in patients with CVD alone. So it's necessary to investigate the humoral response of patients with CVD to the BNT162b2 mRNA COVID-19 vaccine compared to that in healthcare workers (HCWs).
1. Analysis on Results
1.1. Baseline Characteristics of the Study Participants
| Patients (n = 85) | HCWs (n = 179) | |
|---|---|---|
| Age, y | 74 (68–77) | 49 (41–55) |
| Male | 67 (79) | 58 (32) |
| Hypertension | 56 (66) | 16 (9) |
| Dyslipidemia | 58 (68) | 5 (3) |
| Diabetes | 26 (31) | 1 (1) |
| Allergic disease | 8 (9) | 86 (48) |
| Diagnosis | ||
| Coronary artery disease | 53 (63) | NA |
| Arrhythmia | 9 (11) | |
| Hypertensive heart disease | 10 (12) | |
| Cardiomyopathy | 6 (7) | |
| Aortic dissection or aneurysm | 4 (5) | |
| Valvular disease | 3 (4) | |
| Previous myocardial infarction | 26 (31) | |
| Previous coronary revascularization | 38 (45) | |
| Paroxysmal or persistent AF | 12 (14) | |
| Medications | ||
| RAAS inhibitors | 41 (48) | NA |
| Beta-blockers | 34 (40) | |
| Diuretics | 8 (9) | |
| Statins | 46 (54) | |
| Antiplatelet drugs | 37 (44) | |
| Anticoagulant drugs | 16 (19) | |
| Intervals between the first vaccination and sampling, day | 14.7 ± 1.9 | 14.7 ± 1.7 |
| Intervals between the second vaccination and sampling, day | 14.9 ± 1.7 | 14.3 ± 1.6 |
1.2. Seropositivity after Vaccination
| Variables | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value | |
| Patients with CVD (vs. HCWs) | 0.01 | 0.01 to 0.03 | <0.001 | 0.08 | 0.02 to 0.33 | <0.001 |
| Age (per 1 year increment) | 0.86 | 0.82 to 0.89 | <0.001 | 0.95 | 0.90 to 0.99 | 0.02 |
| Male | 0.19 | 0.10 to 0.34 | <0.001 | 0.78 | 0.30 to 2.00 | 0.61 |
| Allergic disease | 5.33 | 2.58 to 11.0 | <0.001 | 1.16 | 0.39 to 3.45 | 0.79 |
| Hypertension | 0.09 | 0.05 to 0.16 | <0.001 | 0.60 | 0.21 to 1.74 | 0.35 |
| Dyslipidemia | 0.05 | 0.03 to 0.10 | <0.001 | 0.88 | 0.26 to 2.95 | 0.84 |
| Diabetes | 0.07 | 0.02 to 0.19 | <0.001 | 0.63 | 0.19 to 2.10 | 0.45 |
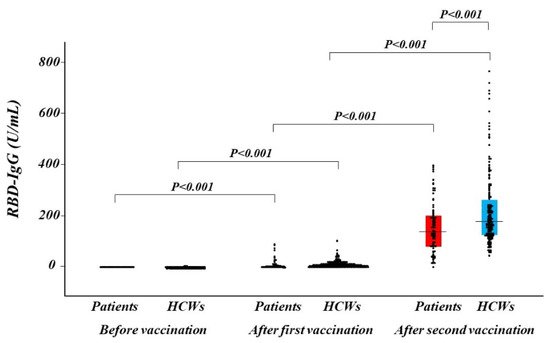
1.3. Antibody Titers after Vaccination
| Variables | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| β Coefficient | 95% CI | p Value | β Coefficient | 95% CI | p Value | |
| Patients with CVD (vs. HCWs) | −0.22 | −0.34 to −0.10 | <0.001 | −0.32 | −0.60 to −0.04 | 0.02 |
| Age (per 1 year increment) | −0.14 | −0.27 to −0.02 | 0.03 | 0.12 | −0.08 to 0.31 | 0.25 |
| Male | −0.22 | −0.34 to −0.10 | <0.001 | −0.17 | −0.30 to −0.03 | 0.02 |
| Hypertension | −0.21 | −0.33 to −0.08 | 0.001 | −0.17 | −0.34 to 0.01 | 0.06 |
| Dyslipidemia | −0.14 | −0.26 to −0.01 | 0.03 | 0.17 | −0.03 to 0.38 | 0.10 |
| Diabetes | −0.14 | −0.26 to −0.01 | 0.03 | −0.04 | −0.18 to 0.10 | 0.60 |
2. Current Insights on BNT162b2 mRNA COVID-19 Vaccine
References
- Barrière, J.; Chamorey, E.; Adjtoutah, Z.; Castelnau, O.; Mahamat, A.; Marco, S.; Petit, E.; Leysalle, A.; Raimondi, V.; Carles, M. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 2021, 32, 1053–1055.
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J. Hepatol. 2021, 75, 435–438.
- Schramm, R.; Costard-Jäckle, A.; Rivinius, R.; Fischer, B.; Müller, B.; Boeken, U.; Haneya, A.; Provaznik, Z.; Knabbe, C.; Gummert, J. Poor humoral and T-cell response to two-dose SARS-CoV-2 messenger RNA vaccine BNT162b2 in cardiothoracic transplant recipients. Clin. Res. Cardiol. 2021, 110, 1142–1149.
- Korth, J.; Jahn, M.; Dorsch, O.; Anastasiou, O.E.; Sorge-Hädicke, B.; Eisenberger, U.; Gäckler, A.; Dittmer, U.; Witzke, O.; Wilde, B.; et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 2021, 13, 756.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhartet, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
- Baden, L.R.; Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416.
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katzet, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA COVID-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423.
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2021, 21, 00460-6.
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246.
- Terpos, E.; Trougakos, I.P.; Apostolakou, F.; Charitaki, I.; Sklirou, A.D.; Mavrianou, N.; Papanagnou, E.D.; Liacos, C.I.; Gumeni, S.; Rentziou, G.; et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am. J. Hematol. 2021, 96, E257–E259.
- Bayart, J.L.; Morimont, L.; Closset, M.; Wieërs, G.; Roy, T.; Gerin, V.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Ausselet, N.; et al. Confounding Factors Influencing the Kinetics and Magnitude of Serological Response Following Administration of BNT162b2. Microorganisms 2021, 9, 1340.
- Salvagno, G.L.; Henry, B.M.; Di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.L.; Lippi, G. Anti-SARS-CoV-2 Receptor-Binding Domain Total Antibodies Response in Seropositive and Seronegative Healthcare Workers Undergoing COVID-19 mRNA BNT162b2 Vaccination. Diagnostics 2021, 11, 832.
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushita, K.; Hanaoka, H.; Nakada, T.A.; et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin. Microbiol. Infect. 2021, 21, 00437-7.
- Zhang, Y.N.; Paynter, J.; Sou, C.; Fourfouris, T.; Wang, Y.; Abraham, C.; Ngo, T.; Zhang, Y.; He, L.; Zhu, J. Mechanism of a COVID-19 nanoparticle vaccine candidate that elicits a broadly neutralizing antibody response to SARS-CoV-2 variants. Sci. Adv. 2021, 7, eabj3107.
- Tegally, H.; Wilkinson, E.; Giovanetti, M.; Iranzadeh, A.; Fonseca, V.; Giandhari, J.; Doolabh, D.; Pillay, S.; San, E.J.; Msomi, N.; et al. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 2021, 592, 438–443.
- Cherian, S.; Potdar, V.; Jadhav, S.; Yadav, P.; Gupta, N.; Das, M.; Rakshit, P.; Singh, S.; Abraham, P.; Panda, S.; et al. SARS-CoV-2 Spike Mutations, L452R, T478K, E484Q and P681R, in the Second Wave of COVID-19 in Maharashtra, India. Microorganisms 2021, 9, 1542.





