
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yingyi Zhang | + 2093 word(s) | 2093 | 2021-08-05 08:12:15 | | | |
| 2 | Catherine Yang | Meta information modification | 2093 | 2021-11-23 02:57:59 | | |
Video Upload Options
Niobium (Nb)-based alloys have been extensively used in the aerospace field owing to their excellent high-temperature mechanical properties. However, the inferior oxidation resistance severely limits the application of Nb-based alloys in a high-temperature, oxygen-enriched environment. Related scholars have extensively studied the oxidation protection of niobium alloy and pointed out that surface coating technology is ideal for solving this problem.
1. Introduction
With the human need for space exploration, the development of hypersonic vehicles has attracted wide attention worldwide [1][2]. Due to the nature of long-term hypersonic cruises and the flight of hypersonic aircraft back and forth between the atmosphere and atmospheric reentry [3], the aircraft must face extremely harsh environments, producing high dynamic pressure and aerodynamic heating effects [4][5]. The high-temperature structural materials need to withstand extreme thermal and mechanical loads [6], resulting in large temperature gradients and thermal stresses inside the material, thereby significantly reducing the cycle life of the components. Especially critical parts or components include aircraft nose cones, sharp leading edges, nozzle openings, hot ends of engines [7], etc. Accordingly, the thermal development and high-temperature oxidation resistance of high-temperature structural materials are increasingly required. Traditional steel materials, aluminum alloys, and titanium alloys can no longer meet the extreme environmental requirements of hypersonic aircraft [8]. Niobium and its alloys have become a critical applicant material for high-temperature structural parts in the aerospace and nuclear industries due to their high melting point [9], moderate density, excellent high-temperature strength, and good processability. Niobium-based alloys are expected to replace nickel-based materials and become critical structural materials in the aerospace field by the end of the 21st century [10][11]. Nonetheless, the oxidation resistance of niobium-based alloys is lacking [12], and severe pulverization will occur when exposed to air above 500 °C for a short time, severely restricting its application in high-temperature, oxygen-rich environments. At present, the commonly used methods to inhibit the occurrence of this kind of oxidation include alloying and surface coating technology [13]. Although alloying can improve the corrosion resistance of niobium-based alloys in high temperature and oxygen-enriched environments to a certain extent, this measure often seriously affects the physical properties of the base alloy itself. Surface coating technology is the most effective method for enhancing the oxidation resistance of niobium-based alloys while ensuring the substrate’s physical properties [13]. The arrangement of high-temperature, oxidation-resistant defensive coatings on the surface of niobium alloys has developed into a current research hotspot.
At present, there are many reports on the surface coating and oxidation protection of niobium and its alloys, but there are few studies on the growth mechanism and oxidation behavior of the surface coating. This article reviews the main preparation methods of niobium and its alloy surface coatings in recent years (such as the slurry sintering method (SS), suspension plasma spraying method (SPS), halide activated filling cementing method (HAPC), etc.). The latest research status of high-temperature, oxidation-resistant coatings on niobium-based alloys is discussed, and the advantages and disadvantages of various preparation methods are analyzed and summarized. The microscopic morphology, phase composition, and oxidation resistance of coatings prepared by different methods are compared and analyzed. The growth mechanism and oxidation behavior of various coatings are analyzed and summarized. At the same time, the future development direction of niobium and its alloy surface coatings is put forward with the purpose of adding valuable summaries for researchers on this ground.
2. Anti-Oxidation Coating on Niobium Alloys
The comprehensive properties of niobium alloy surface coatings are different due to the different preparation methods. In the following summary, different methods for preparing niobium alloy surface coatings will be described in detail, and the oxidation resistance performance of coatings prepared by different methods will be comprehensively compared.
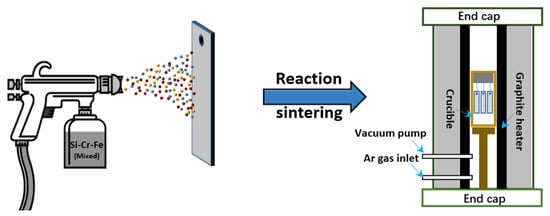
The slurry sintering method is the most commonly used coating preparation method on the surface of niobium alloys, and the process flow is shown in Figure 1 . Firstly, the coating slurry is uniformly mixed with the components of the coating and the binder in proportion, to prepare the coating slurry [14][15], and the slurry is applied to the surface of the substrate by brushing, dipping, or spraying. Then it is solidified by pressurization and heating, and sintered in a vacuum or atmosphere furnace. Finally, a coating is formed on the face of the substrate. The process conditions, composition thickness [16], and the oxidation characteristics of the SS coatings on Nb-based alloys are summarized in Table 1 . It can be perceived that the thickness of SS coatings was between 150 and 300 μm under the sintering temperature of 1200 to 1500 °C for 1–4 h in a vacuum or Ar atmosphere.
| Substrate | Slurry Composition (wt.%) |
Process Conditions | Coating Composition and Thickness (μm) | Oxidation System | Oxidation Products | Quality Change (mg/cm2) |
References | ||
|---|---|---|---|---|---|---|---|---|---|
| Outer Layer | Interface Layer | Oxidation Temperature (°C) |
Oxidation Time (h) |
||||||
| C-103 | 60Si-15Fe-20Cr-5NaF | 1400 °C/2 h vacuum |
NbSi2 Cr3Si Fe3Si2 (200) |
Nb5Si3 (30) |
1300 | 2.5 | Nb2O5 SiO2 Fe2O3 |
1.5 | [17] |
| R512E | 20Si-35Fe-35Cr-10NaF | 1250 °C/4 h vacuum |
MoSi2 M5Si3 (135) |
Nb5Si3 (15) |
1200 | 2 | Nb2O5 Cr2O3 SiO2 CrNbO4 |
1.8 | [18] |
| Nb521 | 45Mo-45Si-10NaF | 1500 °C/1 h Ar |
NbSi2 MoSi2 (110) |
Nb5Si3 (25) |
1700 | 25 | SiO2 MoSi2 |
−9.5 | [19] |
| Nb-Si | 60Si-15Fe-20Cr-5NaF | 1400 °C/2 h vacuum |
NbSi2 Nb4Si5CrFe3 Fe4Nb4Si7 (250) |
Nb5Si3 (50) |
1400 | 6.5 | Nb2O5 SiO2 |
3.5 | [20] |
| Nb-Si-Ti | 20Fe-20Cr-50Si-10NaF | 1400 °C/2 h vacuum |
NbSi2 (Fe, Cr)3Si2 (170) |
Nb5Si3 (20) |
1400 | 7 | Nb2O5 SiO2 |
1.9 | [21] |
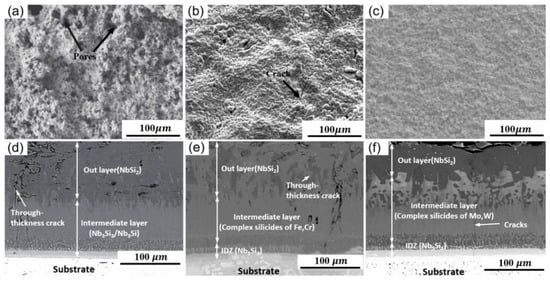
The typical surface and corresponding cross-sectional morphologies of SS coatings are shown in Figure 2 . It can be noticed that the coating surface was relatively rough with dozens of holes and cracks. This was caused by the considerable particle size of the mixture, uneven mixing, and volatilization of the solvent and binder during the sintering process, as shown in Figure 2 a,b. It is worth noting that Xiao et al. used smaller material particles for sintering and obtained a lower surface roughness, and the coating surface was relatively uniform and dense without apparent defects, as shown in Figure 2 c. It can be observed from the cross-sectional images that the coating consisted of the outermost layer (NbSi 2), the intermediate layer, and the internal interdiffusion layer (IZD). In addition, as there was a mismatch of thermal expansion coefficient between the substrate and the coating during the sintering process, a small number of longitudinal cracks were observed inside the coating [22]. However, the IZD area, where the coating and the substrate were connected, was heavy and uniform, revealing that an excellent metallurgical bond was achieved between the coating and the substrate [23], as shown in Figure 2 d–f. Han et al. [24] systematically explained the delamination phenomenon of such coatings. They believed that the growth process of the coating was firstly combined by chemical adsorption and physical adsorption, and then polar groups and substrates diffused between them at high temperatures. Finally, a firm and dense interwoven network coating layer was formed at the interface.
Summary for preparation and oxidation resistance of SPS coatings on Nb-based alloys.
3. Oxidation Mechanism and Failure Behavior of Coating
According to the summary of the oxidation characteristics of Nb alloys surface coatings, the oxidation behavior and failure mechanism are summarized, as shown in Figure 3 . It can be noticed that oxidation can be divided into two stages. The inner and outer layers of the coating are composed of the Nb 5Si 3 layer and NbSi 2 layer, respectively, as shown in Figure 3 a. In the initial stage of oxidation, the oxygen-philic compounds on the coating surface are rapidly oxidized. The oxidation reaction is more severe at weak areas such as cracks, gaps, etc., and the generated oxides such as Nb 2O 5 and SiO 2 are transformed into defects, start to grow, and gradually spread to the entire surface. As the reaction progresses, Nb 2O 5, NbO 2, etc., gradually volatilize, leaving many holes on the surface. In addition, due to the release of thermal stress and the mismatch of thermal expansion coefficients between systems, many cracks sprout on the surface of the coating. At the same time, the addition of some modifying elements (X) improves the fluidity of SiO 2, fills up these defects to a certain extent, and forms an Nb 2O 5-SiO 2-X 2O 3 protective film system on its surface, as shown in Figure 3 b. At last, the oxide layer gradually thickens, the NbSi 2 layer as the central part of the coating is gradually consumed, and the self-healing ability of the coating gradually deteriorates. However, the Nb 5Si 3 layer with poor oxidation resistance gradually becomes thicker. With the oxidation process, the low oxidation resistance Nb 5Si 3 layer is gradually destroyed, resulting in the oxidation failure of the coating, and a large number of holes and cracks are observed, as shown in Figure 3 c.

4. Conclusions and Prospects
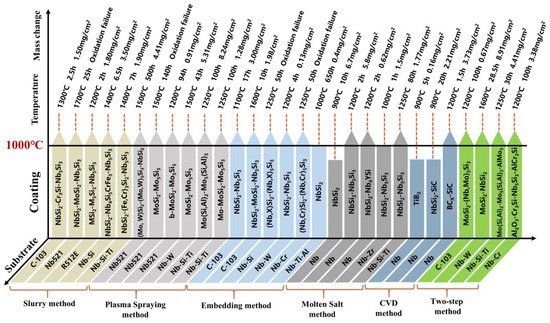
In this work, the preparation methods of anti-oxidation coatings on Nb-based alloys are reviewed, and the structure and anti-oxidation performance of coatings obtained by different methods are summarized, as shown in Figure 4 . Overall, the high-temperature oxidation resistance of Nb-based alloys has been significantly enhanced by surface coating technology. Through in-depth comparison and analysis of various methods, it can be known that the volatilization of solvents and cement, and the uneven particle size of the mixture during the sintering process, result in poor surface quality and high porosity of the coating prepared by SS. Although the two processes of HAPC and CVD have no volatilization phenomenon and are not limited by the shape of the substrate, their lower deposition temperature makes the growth of the coating slower, and the preparation cycle is longer. In contrast, due to its high diffusion temperature, SPS can deposit coatings of tens to hundreds of microns in a short time. However, due to the uneven melting of the spray paint and a small amount of gas during the spraying process, the porosity of the coating is higher, and the bond with the substrate is poor. In addition, although the two-step coating has a relatively excellent structure, its process is complicated, and the coating preparation efficiency is low. As a new coating preparation process, HDS technology dominates due to short deposition time, high coating preparation productivity, smooth and dense coating surface, etc., and it is expected to protect Nb-based alloys at high temperatures.
Summarizing the anti-oxidation mechanism of the coating prepared by the above method, it can be found that the outer protective scale of the coating with better anti-oxidation performance is generally composed of SiO 2 and other inert melt film shielding substances. However, inert molten films such as SiO 2 are formed after oxidation tests, and the oxidation process is challenging to control. Therefore, in order to further improve the high-temperature oxidation resistance of the coating, some beneficial elements are usually added in an appropriate amount during the coating preparation process. Among them, the “selective oxidation type” alloy element X (X = A1, Cr, Mo, Ti, etc.) is added to make it preferentially combine with the O element to form an oxide during the high-temperature oxidation process, setting a dense isolated layer on the surface of the substrate. This blocks the inward diffusion of oxygen atoms, inhibits the formation of Nb 2O 5, and reduces the oxidation rate. The addition of element B and mullite can improve the fluidity of SiO 2 and promote the formation of a uniform and thick oxide film on the coating surface. The addition of Y and Ce elements can refine the coating grains, optimize the coating structure, and significantly improve the strength of the coating at high temperatures so that it can maintain a good shape during the oxidation process. The addition of W and Ge can constrain the diffusion of Si elements into the substrate, slow down the generation of Nb 5Si 3 with poor oxidation resistance, and lengthen the oxidation service life of the coating. The introduction of a proper amount of mullite can fill the pores inside and on the coating surface, optimize the coating structure, increase the density of the coating, inhibit the recrystallization of SiO 2, and promote the thick oxide film on the surface of the coating. In addition, optimizing the coating preparation process and structure can significantly reduce defects produced by thermal expansion coefficient mismatch between coating and substrate, which also plays a crucial role in improving the high-temperature oxidation resistance of the coating.
References
- Subramanian, P.; Mendiratta, M.; Dimiduk, D. The development of Nb-based advanced intermetallic alloys for structural applications. JOM 1996, 48, 33–38.
- Wadsworth, J.; Froes, F. Developments in metallic materials for aerospace applications. JOM 1989, 41, 12–19.
- Zhu, Y.; Peng, W.; Xu, R.; Jiang, P. Review on active thermal protection and its heat transfer for airbreathing hypersonic vehicles. Chin. J. Aeronaut. 2018, 31, 1929–1953.
- Ji, T.; Zhang, R.; Sunden, B.; Xie, G. Investigation on thermal performance of high temperature multilayer insulations for hypersonic vehicles under aerodynamic heating condition. Appl. Therm. Eng. 2014, 70, 957–965.
- Jian, D.; Qiuru, Z. Key technologies for thermodynamic cycle of precooled engines: A review. Acta Astronaut. 2020, 177, 299–312.
- Yuan, B.; Harvey, C.M.; Thomson, R.C.; Critchlow, G.W.; Wang, S. A new spallation mechanism of thermal barrier coatings on aero-engine turbine blades. Theor. Appl. Mech. Lett. 2018, 8, 7–11.
- Prasad, V.S.; Baligidad, R.; Gokhale, A.A. Niobium and other high temperature refractory metals for aerospace applications. In Aerospace Materials and Material Technologies; Springer: Cham, Switzerland, 2017; pp. 267–288.
- Inouye, H. Niobium in high temperature applications. In Proceedings of the Niobium-Proceedings of the International Symposium; Metallurgical Society of AIME: Warrendale, PA, USA, 1984.
- Eckert, J. Niobium compounds and alloys. Int. J. Refract. Met. Hard Mater. 1993, 12, 335–340.
- Wojcik, C.C. Processing, properties and applications of high-temperature niobium alloys. Mrs Online Proc. Libr. (OPL) 1993, 322, 519.
- Wadsworth, J.; Nieh, T.; Stephens, J. Recent advances in aerospace refractory metal alloys. Int. Mater. Rev. 1988, 33, 131–150.
- Zhang, S.; Li, X.; Zuo, J.; Qin, J.; Cheng, K.; Feng, Y.; Bao, W. Research progress on active thermal protection for hypersonic vehicles. Prog. Aerosp. Sci. 2020, 119, 100646.
- Wojcik, C.C.; Chang, W. Thermomechanical processing and properties of niobium alloys. In Proceedings of the International Symposium on Niobium 2001, Orlando, FL, USA, 2–5 December 2001; pp. 163–173.
- Packer, C.M.; Perkins, R.A. Development of a fused slurry silicide coating for the protection of tantalum alloys. J. Less Common Met. 1974, 37, 361–378.
- Streiff, R. Protection of materials by advanced high temperature coatings. J. Phys. IV 1993, 3, C9–C17.
- Kumawat, M.K.; Alam, M.Z.; Kumar, A.; Gopinath, K.; Saha, S.; Singh, V.; Srinivas, V.; Das, D.K. Tensile behavior of a slurry Fe-Cr-Si coated Nb-alloy evaluated by Gleeble testing. Surf. Coat. Technol. 2018, 349, 695–706.
- Alam, M.Z.; Sarin, S.; Kumawat, M.K.; Das, D.K. Microstructure and oxidation behaviour of Fe–Cr–silicide coating on a niobium alloy. Mater. Sci. Technol. 2016, 32, 1826–1837.
- Novak, M.D.; Levi, C.G. Oxidation and Volatilization of Silicide Coatings for Refractory Niobium Alloys. In Proceedings of the ASME 2007 International Mechanical Engineering Congress and Exposition, Seattle, WA, USA, 11–15 November 2007; pp. 261–267.
- Pan, Y.; Guan, W.M. The hydrogenation mechanism of PtAl and IrAl thermal barrier coatings from first-principles investigations. Int. J. Hydrog. Energy 2020, 45, 20032–20041.
- Geethasree, K.; Satya Prasad, V.V.; Brahma Raju, G.; Alam, M.Z. Cyclic oxidation behavior of Fe-Cr modified slurry silicide coated Nb-18.7Si alloyed with Ti and Zr. Corros. Sci. 2019, 148, 293–306.
- Geethasree, K.; Alam, M.Z.; Raju, G.B.; Prasad, V.V.S. Microstructure and mechanical properties of uncoated Nb-18.7Si and Nb-18.7Si-5Ti alloys and their improved oxidation resistance after application of silicide coating. Mater. Today Proc. 2019, 15, 36–43.
- Li, Y.; Lin, X.; Hu, Y.; Gao, X.; Yu, J.; Qian, M.; Dong, H.; Huang, W. Microstructure and isothermal oxidation behavior of Nb-Ti-Si-based alloy additively manufactured by powder-feeding laser directed energy deposition. Corros. Sci. 2020, 173, 108757.
- Pan, Y.; Pu, D.L.; Yu, E.D. Structural, electronic, mechanical and thermodynamic properties of Cr–Si binary silicides from first-principles investigations. Vacuum 2021, 185, 110024.
- Han, J.; Su, B.; Meng, J.; Zhang, A.; Wu, Y. Microstructure and composition evolution of a fused slurry silicide coating on MoNbTaTiW refractory high-entropy alloy in high-temperature oxidation environment. Materials 2020, 13, 3592.




