
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alejandro Carabarin-Lima | + 2781 word(s) | 2781 | 2021-10-18 06:27:03 | | | |
| 2 | Dean Liu | Meta information modification | 2781 | 2021-10-19 02:36:45 | | |
Video Upload Options
A hallmark of Bacillus thuringiensis bacteria is the formation of one or more parasporal crystal (Cry) proteins during sporulation. The toxicity of these proteins is highly specific to insect larvae, exerting lethal effects in different insect species but not in humans or other mammals. In 1989, a nomenclature was proposed to classify proteins according to their sequence and specificity. In this initial nomenclature, there were only four classes. The first class included proteins with action against Lepidoptera with a size of approximately 130–140 kDa. The second class included smaller proteins (65 kDa) with activity against Lepidoptera and Diptera; this class included only two members: CryIIA and CryIIB. The third class constituted the active toxin against Coleoptera, CryIIIA. The last class was Cry1A, the members of which were closely related: they were called Cry1Aa, Cry1Ab, and Cry1Ac.
1. Introduction
2. Action Mechanism of Cry as a Bioinsecticide
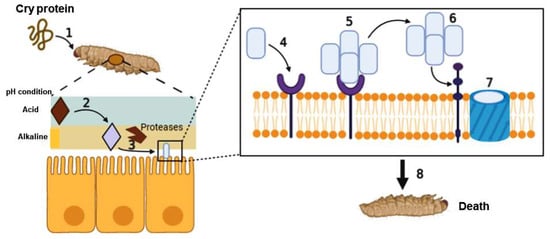
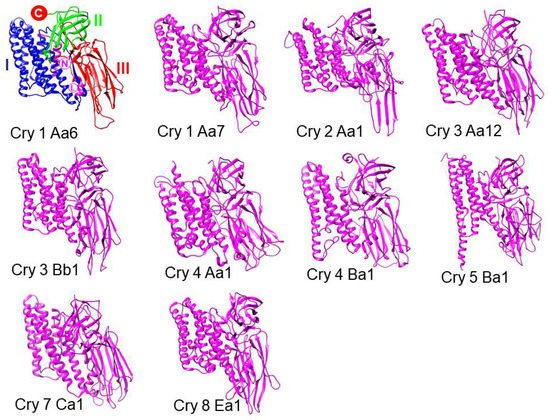
3. Cry Proteins
4. An Overview of Cry as a Natural Adjuvant
References
- Ibrahim, M.A.; Griko, N.; Junker, M.; Bulla, L.A. Bacillus thuringiensis: A genomics and proteomics perspective. Bioeng. Bugs 2010, 1, 31–50.
- de Barjac, H.; Bonnefoi, A. A classification of strains of Bacillus thuringiensis Berliner with a key to their differentiation. J. Invertebr. Pathol. 1968, 11, 335–347.
- Fast, P.G.; Angus, T.A. Effects of Parasporal Inclusions of Bacillus Thuringiensis Var. Sotto Ishiwata on the Permeability of the Gut Wall of Bompyx Mori (Linnaeus) Larvae. J. Invertebr. Pathol. 1965, 20, 29–32.
- Fast, P.G.; Donaghue, T.P. The delta-endotoxin of Bacillus thuringiensis. II. On the mode of action. J. Invertebr. Pathol. 1971, 18, 135–138.
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711.
- Schnepf, E.; Crickmore, N.; Van Rie, J.; Lereclus, D.; Baum, J.; Feitelson, J.; Zeigler, D.R.; Dean, D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 775–806.
- Wei, J.Z.; Hale, K.; Carta, L.; Platzer, E.; Wong, C.; Fang, S.C.; Aroian, R.V. Bacillus thuringiensis crystal proteins that target nematodes. Proc. Natl. Acad. Sci. USA 2003, 100, 2760–2765.
- Raymond, B. The biology, ecology and taxonomy of Bacillus thuringiensis and related bacteria. In Bacillus thuringiensis and Lysinibacillus sphaericus; Springer: Berlin/Heidelberg, Germany, 2017; pp. 19–39.
- Palma, L.; Munoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325.
- Moreno-Fierros, L.; Garcia, N.; Gutierrez, R.; Lopez-Revilla, R.; Vazquez-Padron, R.I. Intranasal, rectal and intraperitoneal immunization with protoxin Cry1Ac from Bacillus thuringiensis induces compartmentalized serum, intestinal, vaginal and pulmonary immune responses in BALB/c mice. Microbes. Infect. 2000, 2, 885–890.
- Torres-Martinez, M.; Rubio-Infante, N.; Garcia-Hernandez, A.L.; Nava-Acosta, R.; Ilhuicatzi-Alvarado, D.; Moreno-Fierros, L. Cry1Ac toxin induces macrophage activation via ERK1/2, JNK and p38 mitogen-activated protein kinases. Int. J. Biochem. Cell Biol. 2016, 78, 106–115.
- Ibarra-Moreno, S.; Garcia-Hernandez, A.L.; Moreno-Fierros, L. Coadministration of protoxin Cry1Ac from Bacillus thuringiensis with metacestode extract confers protective immunity to murine cysticercosis. Parasite Immunol. 2014, 36, 266–270.
- Rubio-Infante, N.; Moreno-Fierros, L. An overview of the safety and biological effects of Bacillus thuringiensis Cry toxins in mammals. J. Appl. Toxicol. 2016, 36, 630–648.
- Jurat-Fuentes, J.L.; Crickmore, N. Specificity determinants for Cry insecticidal proteins: Insights from their mode of action. J. Invertebr. Pathol. 2017, 142, 5–10.
- Walters, F.S.; Stacy, C.M.; Lee, M.K.; Palekar, N.; Chen, J.S. An engineered chymotrypsin/cathepsin G site in domain I renders Bacillus thuringiensis Cry3A active against Western corn rootworm larvae. Appl. Environ. Microbiol. 2008, 74, 367–374.
- Du, C.; Martin, P.A.; Nickerson, K.W. Comparison of Disulfide Contents and Solubility at Alkaline pH of Insecticidal and Noninsecticidal Bacillus thuringiensis Protein Crystals. Appl. Environ. Microbiol. 1994, 60, 3847–3853.
- Melo, A.L.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2016, 36, 317–326.
- Pigott, C.R.; Ellar, D.J. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281.
- Tay, W.T.; Mahon, R.J.; Heckel, D.G.; Walsh, T.K.; Downes, S.; James, W.J.; Lee, S.F.; Reineke, A.; Williams, A.K.; Gordon, K.H. Insect Resistance to Bacillus thuringiensis Toxin Cry2Ab Is Conferred by Mutations in an ABC Transporter Subfamily A Protein. PLoS Genet. 2015, 11, e1005534.
- Vachon, V.; Paradis, M.J.; Marsolais, M.; Schwartz, J.L.; Laprade, R. Ionic permeabilities induced by Bacillus thuringiensis in Sf9 cells. J. Membr. Biol. 1995, 148, 57–63.
- Soberon, M.; Portugal, L.; Garcia-Gomez, B.I.; Sanchez, J.; Onofre, J.; Gomez, I.; Pacheco, S.; Bravo, A. Cell lines as models for the study of Cry toxins from Bacillus thuringiensis. Insect. Biochem. Mol. Biol. 2018, 93, 66–78.
- Guihard, G.; Vachon, V.; Laprade, R.; Schwartz, J.L. Kinetic properties of the channels formed by the bacillus thuringiensis insecticidal crystal protein Cry1C in the plasma membrane of Sf9 cells. J. Membr. Biol. 2000, 175, 115–122.
- Portugal, L.; Munoz-Garay, C.; Martinez de Castro, D.L.; Soberon, M.; Bravo, A. Toxicity of Cry1A toxins from Bacillus thuringiensis to CF1 cells does not involve activation of adenylate cyclase/PKA signaling pathway. Insect. Biochem. Mol. Biol. 2017, 80, 21–31.
- Bravo, A.; Gill, S.S.; Soberon, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435.
- Hofmann, C.; Luthy, P.; Hutter, R.; Pliska, V. Binding of the delta endotoxin from Bacillus thuringiensis to brush-border membrane vesicles of the cabbage butterfly (Pieris brassicae). Eur. J. Biochem. 1988, 173, 85–91.
- Hofmann, C.; Vanderbruggen, H.; Hofte, H.; Van Rie, J.; Jansens, S.; Van Mellaert, H. Specificity of Bacillus thuringiensis delta-endotoxins is correlated with the presence of high-affinity binding sites in the brush border membrane of target insect midguts. Proc. Natl. Acad. Sci. USA 1988, 85, 7844–7848.
- Nishiguchi, S.; Yagi, A.; Sakai, N.; Oda, H. Divergence of structural strategies for homophilic E-cadherin binding among bilaterians. J. Cell Sci. 2016, 129, 3309–3319.
- Boonserm, P.; Mo, M.; Angsuthanasombat, C.; Lescar, J. Structure of the functional form of the mosquito larvicidal Cry4Aa toxin from Bacillus thuringiensis at a 2.8-angstrom resolution. J. Bacteriol. 2006, 188, 3391–3401.
- Li, J.D.; Carroll, J.; Ellar, D.J. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature 1991, 353, 815–821.
- Grochulski, P.; Masson, L.; Borisova, S.; Pusztai-Carey, M.; Schwartz, J.L.; Brousseau, R.; Cygler, M. Bacillus thuringiensis CryIA(a) insecticidal toxin: Crystal structure and channel formation. J. Mol. Biol. 1995, 254, 447–464.
- Galitsky, N.; Cody, V.; Wojtczak, A.; Ghosh, D.; Luft, J.R.; Pangborn, W.; English, L. Structure of the insecticidal bacterial delta-endotoxin Cry3Bb1 of Bacillus thuringiensis. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 1101–1109.
- Boonserm, P.; Davis, P.; Ellar, D.J.; Li, J. Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications. J. Mol. Biol. 2005, 348, 363–382.
- Guo, S.; Ye, S.; Liu, Y.; Wei, L.; Xue, J.; Wu, H.; Song, F.; Zhang, J.; Wu, X.; Huang, D.; et al. Crystal structure of Bacillus thuringiensis Cry8Ea1: An insecticidal toxin toxic to underground pests, the larvae of Holotrichia parallela. J. Struct. Biol. 2009, 168, 259–266.
- Hui, F.; Scheib, U.; Hu, Y.; Sommer, R.J.; Aroian, R.V.; Ghosh, P. Structure and glycolipid binding properties of the nematicidal protein Cry5B. Biochemistry 2012, 51, 9911–9921.
- Derbyshire, D.J.; Ellar, D.J.; Li, J. Crystallization of the Bacillus thuringiensis toxin Cry1Ac and its complex with the receptor ligand N-acetyl-D-galactosamine. Acta Crystallogr. D Biol. Crystallogr. 2001, 57, 1938–1944.
- Morse, R.J.; Yamamoto, T.; Stroud, R.M. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure 2001, 9, 409–417.
- Sawaya, M.R.; Cascio, D.; Gingery, M.; Rodriguez, J.; Goldschmidt, L.; Colletier, J.P.; Messerschmidt, M.M.; Boutet, S.; Koglin, J.E.; Williams, G.J.; et al. Protein crystal structure obtained at 2.9 A resolution from injecting bacterial cells into an X-ray free-electron laser beam. Proc. Natl. Acad. Sci. USA 2014, 111, 12769–12774.
- Jing, X.; Yuan, Y.; Wu, Y.; Wu, D.; Gong, P.; Gao, M. Crystal structure of Bacillus thuringiensis Cry7Ca1 toxin active against Locusta migratoria manilensis. Protein Sci. 2019, 28, 609–619.
- Evdokimov, A.G.; Moshiri, F.; Sturman, E.J.; Rydel, T.J.; Zheng, M.; Seale, J.W.; Franklin, S. Structure of the full-length insecticidal protein Cry1Ac reveals intriguing details of toxin packaging into in vivo formed crystals. Protein Sci. 2014, 23, 1491–1497.
- Fernandez, L.E.; Perez, C.; Segovia, L.; Rodriguez, M.H.; Gill, S.S.; Bravo, A.; Soberon, M. Cry11Aa toxin from Bacillus thuringiensis binds its receptor in Aedes aegypti mosquito larvae through loop alpha-8 of domain II. FEBS Lett. 2005, 579, 3508–3514.
- Parenti, M.D.; Santoro, A.; Del Rio, A.; Franceschi, C. Literature review in support of adjuvanticity/immunogenicity assessment of proteins. EFSA Supporting Publ. 2019, 16, 1551E.
- Lycke, N. Recent progress in mucosal vaccine development: Potential and limitations. Nat. Rev. Immunol. 2012, 12, 592–605.
- Bomsel, M.; Tudor, D.; Drillet, A.S.; Alfsen, A.; Ganor, Y.; Roger, M.G.; Mouz, N.; Amacker, M.; Chalifour, A.; Diomede, L.; et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 2011, 34, 269–280.
- Guimaraes, V.; Drumare, M.F.; Lereclus, D.; Gohar, M.; Lamourette, P.; Nevers, M.C.; Vaisanen-Tunkelrott, M.L.; Bernard, H.; Guillon, B.; Creminon, C.; et al. In vitro digestion of Cry1Ab proteins and analysis of the impact on their immunoreactivity. J Agric. Food Chem. 2010, 58, 3222–3231.
- Moreno-Fierros, L.; Ruiz-Medina, E.J.; Esquivel, R.; Lopez-Revilla, R.; Pina-Cruz, S. Intranasal Cry1Ac protoxin is an effective mucosal and systemic carrier and adjuvant of Streptococcus pneumoniae polysaccharides in mice. Scand. J. Immunol. 2003, 57, 45–55.
- Rojas-Hernandez, S.; Rodriguez-Monroy, M.A.; Lopez-Revilla, R.; Resendiz-Albor, A.A.; Moreno-Fierros, L. Intranasal coadministration of the Cry1Ac protoxin with amoebal lysates increases protection against Naegleria fowleri meningoencephalitis. Infect. Immun. 2004, 72, 4368–4375.
- Joshi, S.S.; Barnett, B.; Doerrer, N.G.; Glenn, K.; Herman, R.A.; Herouet-Guicheney, C.; Hunst, P.; Kough, J.; Ladics, G.S.; McClain, S.; et al. Assessment of potential adjuvanticity of Cry proteins. Regul. Toxicol. Pharmacol. 2016, 79, 149–155.
- Gonzalez-Gonzalez, E.; Garcia-Hernandez, A.L.; Flores-Mejia, R.; Lopez-Santiago, R.; Moreno-Fierros, L. The protoxin Cry1Ac of Bacillus thuringiensis improves the protection conferred by intranasal immunization with Brucella abortus RB51 in a mouse model. Vet. Microbiol. 2015, 175, 382–388.
- Legorreta-Herrera, M.; Meza, R.O.; Moreno-Fierros, L. Pretreatment with Cry1Ac protoxin modulates the immune response, and increases the survival of Plasmodium-infected CBA/Ca mice. J. Biomed. Biotechnol. 2010, 2010, 198921.
- Vazquez-Padron, R.I.; Gonzales-Cabrera, J.; Garcia-Tovar, C.; Neri-Bazan, L.; Lopez-Revilla, R.; Hernandez, M.; Moreno-Fierro, L.; de la Riva, G.A. Cry1Ac protoxin from Bacillus thuringiensis sp. kurstaki HD73 binds to surface proteins in the mouse small intestine. Biochem. Biophys. Res. Commun. 2000, 271, 54–58.
- Guerrero, G.G.; Dean, D.H.; Moreno-Fierros, L. Structural implication of the induced immune response by Bacillus thuringiensis Cry proteins: Role of the N-terminal region. Mol. Immunol. 2004, 41, 1177–1183.
- Adel-Patient, K.; Guimaraes, V.D.; Paris, A.; Drumare, M.F.; Ah-Leung, S.; Lamourette, P.; Nevers, M.C.; Canlet, C.; Molina, J.; Bernard, H.; et al. Immunological and metabolomic impacts of administration of Cry1Ab protein and MON 810 maize in mouse. PLoS ONE 2011, 6, e16346.




