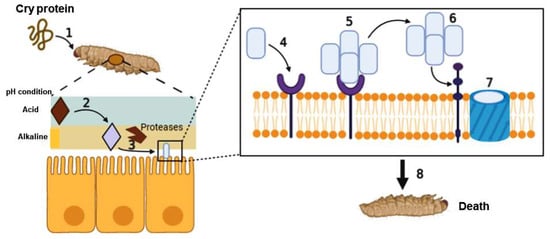
A hallmark of
Bacillus thuringiensis bacteria is the formation of one or more parasporal crystal (Cry) proteins during sporulation. The toxicity of these proteins is highly specific to insect larvae, exerting lethal effects in different insect species but not in humans or other mammals.
B. thuringiensis was isolated for the first time in 1902 by the Japanese scientist Ishiwata, who was studying the cause of mortality in silkworm larvae; thus, this disease was also called Soto disease. Ishiwata initially named this bacterium
Bacillus sotto [
6]. A few years later, in 1911, a German scientist, Ernst Berliner, isolated a bacterial strain in dead moth larvae in Mediterranean flour, located in a flour mill in the German state of Thuringia. For this reason, Ernst named this Bacillus
B. thuringiensis (Bt) [
7]. Subsequently, the probable mechanism of cytotoxic action of particular Bt inclusions, called parasporal, was shown in silkworm larvae (
Bombyx mori). Changes in the permeability of the intestinal walls of the insect were observed, consequently causing its death. These results showed that the parasporal inclusions contained crystals of δ-endotoxin, which was the cause of the larva deaths [
8,
9]. The successful use of Bt in agriculture lies in the production of crystal proteins called Cry, which have specific cytotoxic activity against different insect orders, such as Lepidoptera, Diptera, Coleoptera, Hymenoptera, Homoptera, Orthoptera, and Mallophaga [
10,
11,
12]. In 1989, a nomenclature was proposed to classify proteins according to their sequence and specificity. In this initial nomenclature, there were only four classes. The first class included proteins with action against Lepidoptera with a size of approximately 130–140 kDa. The second class included smaller proteins (65 kDa) with activity against Lepidoptera and Diptera; this class included only two members: CryIIA and CryIIB. The third class constituted the active toxin against Coleoptera, CryIIIA. The last class was Cry1A, the members of which were closely related: they were called Cry1Aa, Cry1Ab, and Cry1Ac [
14]. The activity of Cry proteins as bioinsecticides has been widely studied [
51]; however, their other activities, such as their potential adjuvant function, have been inadequately explored. Although some experiments have confirmed their potential action as immunopotentiators [
92,
99,
101], there are many Cry proteins that have not been evaluated yet. Furthermore, in different studies, the lack of toxicity of these proteins has been demonstrated in mammals, especially humans, and it has even been shown that these proteins can generate an immune response mediated by antibodies [
108]. However, it is crucial to determine the mechanism by which Cry proteins are activated to induce an immune response, as well as to evaluate the possible risks that they could pose in the short and long term, such as allergies or other immune alterations.