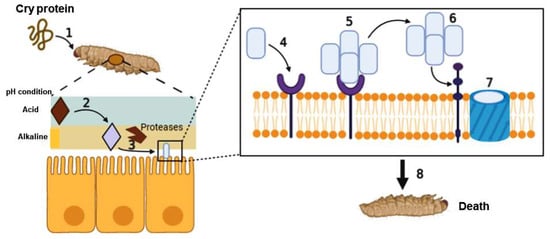
A strategy used in the design of vaccines to enhance their immunogenicity includes the co-administration of adjuvants that stimulate and improve immunity. Cry proteins have been described as possible adjuvants for their resistance and stability in highly alkaline environments. The structural characteristics of proteins allow them to modulate the immune response and function as adjuvants. For this reason, several proteins have been studied in the context of therapeutic proteins
[41][94]. Importantly, a wide variety of proteins have been used as adjuvants to immunize animals intranasally, which can stimulate a protective immune response in the lungs and upper respiratory tract and possibly at distant sites, such as the gastric and genital mucosa
[42][95].
With respect to mucosal vaccines, when rhesus macaques were vaccinated intranasally with a trimeric gp41 protein coupled to virosomes to evaluate an HIV-1 vaccine, IgA antibodies increased in the genital tract, and immunization also prevented transmission of infection
[43][96]. Studies have been conducted to identify proteins able to induce a mucosal immune response, which was initially realized by Guimares et al. when they demonstrated the immunogenicity of the Cry1Ab protein of
Bacillus thuringiensis. When Cry1Ab was exposed to different pH values, the results showed that the protein was only slightly degraded at pH 2.0 and, most importantly, it maintained its immunoreactivity
[44][97]. Subsequently, Cry proteins were used as adjuvants for their resistance and stability. This was demonstrated by immunizing BALB/c mice intranasally, which is an alkaline environment. After immunization, the mice were protected when they were infected by the bacterium
Streptococcus pneumoniae [45][93] or the parasite
Naegleria fowleri [46][98].
Because of these characteristics, several research groups have studied the use of Cry as a potential adjuvant
[45][46][11][47][93,98,99,100]. The role of Cry proteins as vaccine adjuvants was initially observed with the Cry1Ac protein, which was administered by different routes, including intragastric, intraperitoneal (i.p.), and intranasal immunization
[10][92]. The last two are the most efficient due to their ability to induce isotypes of IgA and IgG antibodies in the murine model. These antibodies demonstrated protection in animal models when they were infected with the bacterium
Brucella abortus or with parasites
Naegleria fowleri,
Plasmodium chabaudi,
Plasmodium berghei, and
Taenia crassiceps [10][46][12][48][49][92,98,101,102,103]. In the case of
B. abortus, an intracellular bacterium, the use of Cry1Ac with the RB51
B. abortus strain conferred protection against an intranasal challenge with the virulent strain
B. abortus 2308 in BALB/c mice. The results showed that the vaccine conferred immunoprotection, as evidenced by a decrease in the splenic bacterial load in immunized animals. The proliferation of cytotoxic TCD8+ cells increased the production of TNF-α and IFN-γ, and the generation of an IgG2a antibody response was also observed. These results indicate that the use of the Cry1Ac protein as a mucosal adjuvant via the intranasal route may be a promising strategy for developing a vaccine against brucellosis
[48][102].
When the Cry1Ac protein was administered together with total extracts of the free-living amoeba
N. fowleri, the immunized animals had 100% protection against the development of meningoencephalitis; however, when the animals were immunized with the Cry1Ac protein alone, only 60% of infected mice survived
[46][98].
On the other hand, mice previously treated with Cry1Ac and infected with
P. chabaudi (considered non-lethal) had 100% survival compared to mice previously treated with PBS, which demonstrated 80% survival. Furthermore, mice previously treated with Cry1Ac and subsequently infected with
P. berghei (lethal parasite) survived longer (12 days) than control mice previously treated with PBS, which died on day 9 post-infection. Regarding the induced immune response, an increase in IFN-γ and TGF-β cytokines was demonstrated, in addition to an increase in the levels of IgG and IgM immunoglobulins, in animals treated and infected with two types of Plasmodium
[49][103].
Conversely, when mice were immunized with the Cry1Ac protein and total lysates of
T. crassiceps, only 40% protection was observed in the experimentally infected animal model, and mice immunized only with Cry1Ac did not survive
[10][46][11][47][12][92,98,99,100,101]. These results demonstrate that Cry1Ac alone is not able to generate a specific immune response; however, when it is used in the company of a specific antigen, it potentiates the immune response and can function as an adjuvant. At present, the immunological mechanism underlying the effects of the Cry1Ac protein is not entirely understood, but in macrophages this protein can stimulate the overexpression of surface glycoproteins CD80 and CD86, which stimulate the secretion of TNF-α and IL-6 cytokines, allowing activation of an immune response that promotes the survival of animal models against some experimental infections
[50][104].
Another protein used as a potential adjuvant is Cry1Ab (a protein with 86% homology at the amino acid level with Cry1Ac). When administered intranasally, this protein was not able to generate serum and mucosal IgG antibody responses. It was only able to elicit a low level of IgM and SIgA. In contrast, when Cry1Ab was administered by the intraperitoneal route, it was able to induce high levels of IgG and IgM antibodies, similar to the effects of Cry1Aa and Cry1Ac
[51][105]. In another study, the administration of Cry1Ab (1 µg) to BALB/c mice by the i.p. route induced a mixed Th1-Th2 immune response. No evidence of allergenicity has been observed with the administration of Cry proteins
[52][106], though the levels of leukotrienes, cytokines, and eosinophils have notably increased. However, another study conducted with the direct consumption of Bt corn with Cry1Ab protein showed that the inclusion of Cry1Ab in the diet did not affect the severity of asthma or allergic inflammation induced by ovalbumin
[50][104].