Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Danielle Gelardi | + 6765 word(s) | 6765 | 2021-09-10 05:56:13 | | | |
| 2 | Vivi Li | Meta information modification | 6765 | 2021-10-18 05:15:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gelardi, D. Optimizing Sustainability Opportunities for Biochar. Encyclopedia. Available online: https://encyclopedia.pub/entry/15070 (accessed on 08 February 2026).
Gelardi D. Optimizing Sustainability Opportunities for Biochar. Encyclopedia. Available at: https://encyclopedia.pub/entry/15070. Accessed February 08, 2026.
Gelardi, Danielle. "Optimizing Sustainability Opportunities for Biochar" Encyclopedia, https://encyclopedia.pub/entry/15070 (accessed February 08, 2026).
Gelardi, D. (2021, October 15). Optimizing Sustainability Opportunities for Biochar. In Encyclopedia. https://encyclopedia.pub/entry/15070
Gelardi, Danielle. "Optimizing Sustainability Opportunities for Biochar." Encyclopedia. Web. 15 October, 2021.
Copy Citation
Biochar is most commonly considered for its use as a soil amendment, where it has gained attention for its potential to improve agricultural production and soil health. Twenty years of near exponential growth in investigation has demonstrated that biochar does not consistently deliver these benefits, due to variables in biochar, soil, climate, and cropping systems. While biochar can provide agronomic improvements in marginal soils, it is less likely to do so in temperate climates and fertile soils. Here, biochar and its coproducts may be better utilized for contaminant remediation or the substitution of nonrenewable or mining-intensive materials.
biochar
soil
agriculture
climate change
remediation
bioenergy
coproducts
sustainability
carbon sequestration
perspective
1. Introduction
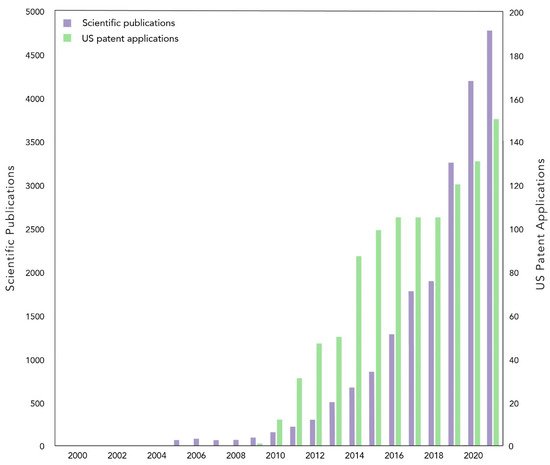
The rise in popularity of biochar, or pyrolyzed biomass, can be traced through multiple sectors across the globe. In the scientific community, articles mentioning the word biochar are published at a near exponential rate [1], and in the private industry, United States patent applications mentioning biochar increase nearly every year [2] (Figure 1). Surrounding this scientific and entrepreneurial activity are the many associations dedicated to biochar, from governmental groups at the federal, state, and local level, to nonprofits and trade groups. In 2021, the International Biochar Initiative (IBI) reported a 30% increase in membership revenues from 2020, comprised of both corporate and individual memberships, and a greater than 60% increase in newsletter subscribers from 2018 [3]. Perhaps most surprising is the rise in interest from the general public. “How to make biochar” workshops abound, as do YouTube videos with presentations on the “exceptional results” of biochar in home gardens, and impassioned descriptions of how biochar is the future of sustainable agriculture.
In this regard, the study of biochar is not dissimilar to that of soil health or soil carbon sequestration, with interest spreading beyond scientific and agricultural communities and its merit debated in the public arena [4][5]. While claims that adding biochar to soils can increase soil health and sustainability are commonplace [6][7][8], the scientific community has yet to reach consensus on whether biochar can consistently deliver these benefits, or if it has merely fallen victim to hyperbole [9]. In agriculture, biochar is proposed as a soil amendment that can increase crop yield, soil fertility, and water holding capacity [10][11][12][13]. However, a growing number of meta-analyses and literature reviews demonstrate that these benefits are not consistently realized [13][14][15]. Divergent results are in part due to the fact that “biochar” is an umbrella term for many diverse carbonaceous products, created from different feedstocks at different temperatures using different methods. Once biochar has been produced, still more variables are introduced. Is biochar being added to the soil? If so, which biochar, to what soil texture, at what rate, in what climate, to what system, and for what purpose? Is biochar being used for remediation? For what contaminant and in what medium? The decision tree that accompanies the production and use of biochar can generate a myriad of possible outcomes, which will not equally deliver agricultural, ecological, environmental, or climate change mitigation benefits. This poses numerous challenges to making comparisons between biochar studies, and to creating unified recommendations for the effective and sustainable use of biochar.
Nevertheless, the idea of adding biochar to working lands is garnering attention in the policy arena. In 2018, the Intergovernmental Panel on Climate Change (IPCC) described biochar as a leading natural climate solution [6]. In 2020, the National Resource Conservation Service included biochar alongside compost and whole orchard recycling in their Soil Carbon Amendment, as a practice that can deliver a suite of agronomic and environmental benefits [16]. With the public movement for biochar surging ahead, it is essential that the scientific community begins a new era of self-awareness concerning biochar research. We must ask ourselves: (1) What do we really know about biochar? (2) What are the critical gaps in knowledge? (3) How can we best move forward? Answers to these questions can improve our work in biochar research to produce deeper, more mechanistic results, and to provide stakeholders with smart and efficient uses of biochar that optimize environmental and agronomic benefits. The enthusiasm that surrounds biochar may be useful for raising awareness, stimulating scientific dialogue, and leveraging research funding. However, the scientific community must prevent biochar from being stigmatized as a trend at best, or snake oil at worst. Instead, we can assist in the evaluation of biochars for benefits they are likely to deliver, those they are unlikely to, and the production and use parameters which optimize intended benefits for specific conditions.

2. What Do We Really Know about Biochar?
Biochar is a category of heterogenous materials which possess unique chemical and physical properties. These properties are a function of production variables such as the method (i.e., gasification, pyrolysis, flow-through, batch), production temperature, and feedstock. Hassan et al. (2020) reviewed 533 datasets to determine that increased production temperature resulted in an overall increase in pH, surface area, pore size, ash content, hydrophobicity, and biochar stability, as determined by the oxygen to carbon vs. hydrogen to carbon ratios [17]. They also determined that biochars derived from hardwood and softwood have greater surface area and carbon content than those from manure or grass, while manure and grass biochars have more abundant oxygen-containing functional groups and mineral constituents. While there is clearly high variation amongst biochars, they are overall characterized by stable carbon (C), high surface area, reactive surface functional groups, low bulk density, and neutral to basic pH [18]. These properties endow biochar with the potential to deliver a broad range of benefits.
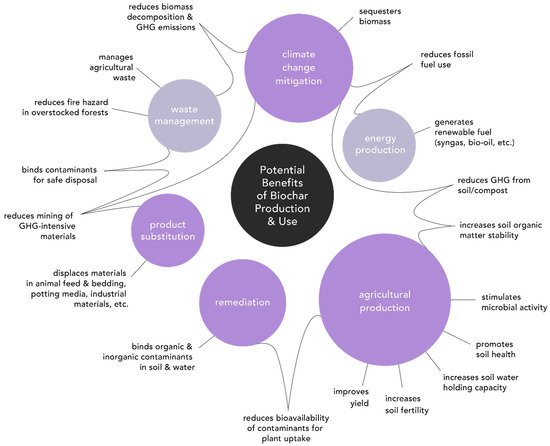
For the purposes of this discussion, benefits will be grouped into the following categories, though we do not claim the categories are entirely comprehensive or mutually exclusive: agricultural production, climate change mitigation, contaminant remediation, product substitution, waste management, and renewable fuel production (Figure 2). The first three categories, in order, dominate scientific investigation [15], while product substitution is an emerging but less studied use. The categories of waste management and renewable fuel production also receive less scientific investigation, but may be considered nontarget or co-benefits resulting from the production and use of biochar. Despite less research into non-soil applications, they may deliver increased environmental and economic advantages. Further investigation into strategies for optimizing co-benefits, and into new and innovative uses for biochar, is required.

Figure 2. Conceptual diagram of the potential benefits of biochar production and use. Primary benefits (bold purple) are sized according to the number of related publications [15]. Co-benefits are rendered in light purple. Benefits included here are not exhaustive or mutually exclusive, nor will they be consistently realized depending on variables in the biochar and in the system in which they are utilized.
2.1. Agricultural Production
Adding biochar to agricultural land is its most commonly investigated use [15]. While application rates vary widely, one meta-analysis concluded that the greatest benefits were observed in studies utilizing 100 t ha−1 [19]. This unrealistically high rate poses obvious practical and economic challenges. More recently, Oladele (2019) developed a soil quality index using data from a three-year field trial, which concluded that a biochar application rate of 6–12 t ha−1 was optimal, though results were constrained to acidic alfisols (USDA Soil Taxonomy) [20]. Pandit et al. (2018) conducted an economic analysis which included payments for C sequestration, to determine an optimal rate of 15 t ha−1 [21]. Guo (2020) made even more specific recommendations based on the results of a literature review and from greenhouse trials, concluding that biochar should be applied at a concentration of 2–5% by weight for wood- and crop residue-derived biochars, and 1–3% for manure-derived biochars [22]. While biochar is typically amended to soils by broadcasting it on the soil surface and tilling it in, subsurface banding may be a more efficient method for concentrating biochar in the crop rhizosphere and for minimizing dust emissions [23]. Unlike other soil amendments, biochar is persistent in the soil and does not need to be applied every year. As biochar will weather over time, it has been suggested it should be applied every three years to optimize potential benefits [20]. This recommendation should be considered with caution, as there have been no long-term studies which analyze the effects of biochar application repeated every three years.
Biochar has been reported to deliver a suite of agronomic benefits when added to agricultural soils, including increased crop yields [10], soil fertility [11], soil water holding capacity [12][13], microbial biomass and activity [24][25], a shift towards more fungal-dominated microbial community composition [25], and the suppression of soil borne pathogens and disease [26]. While many studies demonstrate increased crop yield following biochar addition [21][27][28], others show little to no effect [29][30][31], and in some cases, negative effects have been reported [32]. The efficacy of biochar to deliver agronomic outcomes is dependent on the myriad of variables previously discussed. This has resulted in multiple researchers concluding that location- and material-specific consideration is required to optimize agronomic benefits for a particular system [14][15][19].
Biochar amendment can increase soil fertility, which in turn may increase crop yield [21][27][28], or lead to minor [30][33], temporary [31], or soil texture-specific yield increases [34][35][36]. Increased soil fertility is often attributed to the typically high cation exchange capacity of biochars (CEC), which can result in increased exchangeable cations such as magnesium (Mg2+), potassium (K+), calcium (Ca2+) [21][34], and ammonium (NH4+) [37] in the soil. Biochar amendment has also been reported to benefit saline and sodic soils, through the sorption of sodium (Na+) on exchange sites, or by releasing non-sodium base cations to decrease exchangeable sodium percentage [38][39][40]. The retention of anions such as nitrate (NO3−) is typically hindered due to unfavorable electrostatic interactions [41]; however, pores within biochar may retard nitrate leaching and make it more available for plant uptake [42][43][44]. Additionally, modification of biochar to increase the anion exchange capacity has been shown effective for nitrate retention [41]. Although biochars are not considered fertilizing materials, there can be temporary changes in soil fertility from the release of nutrients within biochar, particularly for animal manure-based biochars. Regardless, the use of synthetic fertilizers, compost, or manure is recommended for use in conjunction with biochar amendment [45][46][47][48].
The potential impact of biochar on soil water dynamics has led to many investigations of the material as a strategy for increasing crop water use efficiency and mitigating the vulnerability of soils to drought conditions. Recent meta-analyses concluded that biochar substantially increased soil water content at field capacity and permanent wilting point in coarse textured soils only [12][13]. Despite these observed trends, benefits have also been observed in fine textured soils, including reduced crop water stress, increased yield [49][50], and reduced crop loss during deficit irrigation [51]. Other authors have reported little to no effect, or transient effects, of biochar on soil water dynamics in both fine and coarse textured soils [29][30][52].
The conditions in which biochar appears to deliver the most consistent agronomic benefits are those in which soils require conditioning or remediation for the successful growth of crops, including low pH and sandy soils. A global meta-analysis concluded that biochar boosts yields in the tropics by 25%, but overall has no effect on yield in temperate latitudes [14]. Arable tropical soils are typically characterized by acidity, low fertility, and receive limited fertilizer inputs, and therefore may have the most to gain from the addition of biochar. Since most biochars are alkaline within a pH range of 8 to 10, they can increase soil pH to reduce aluminum toxicity and increase phosphorous availability [53][54]. However, it has been observed that up to 50 t of biochar per hectare may be required to match the liming effect of just 3 t per hectare of dolomite [55]. Biochar can also immobilize heavy metals or organic pollutants, thereby increasing yields and decreasing the concentration of contaminants in crop biomass [56][57]. Collectively, this research suggests biochar has a promising role in remediating or conditioning soils that may otherwise pose challenges for agricultural production. There appear to be fewer agronomic benefits from applying biochar to temperate and fertile cropping systems [14].
Current research regarding the use of biochar in soils demonstrates mixed results, with studies indicating that cropping systems in sandy or highly weathered soils are most likely to benefit. Soil health and sustainability benefits are less widely studied; however, results also appear dependent on soil texture, with increased aggregation more likely to occur in fine soils [58][59], and microbial benefits in coarse soils [24]. Mixed results emphasize the need for a prescribed approach to amending agricultural soils with biochar, in which the right biochar is added, at the right rate, in the right form, in the right context to increase the probability of tangible benefits.
2.2. Climate Change Mitigation
The policy interest in biochar is driven primarily by climate change mitigation goals, as the production and use of biochar may reduce global warming potential (GWP) through multiple mechanisms. Biochar is produced through the thermochemical conversion of organic waste materials such as wood from forest thinning operations, manure, and crop residues such as corn stalks, rice hulls, and grape pomace. This process has clear waste management benefits. Depending on production parameters, energy coproducts can also be generated. Gasification and slow pyrolysis are two common strategies for the thermochemical conversion of biomass. Each process transfers the heating value from carbonaceous materials into syngas, bio-oil, and biochar [60]. Syngas and bio-oil can be utilized to displace the onsite use of regional electricity grids and therefore reduce the dependence on fossil-based fuels. Biochar can mitigate greenhouse gas (GHG) emissions by fixing C that would otherwise be emitted as biogenic carbon dioxide during biomass decomposition, into recalcitrant aromatic ring structures [61]. While biochar is commonly cited as a source of persistent C, biochar stability varies as a function of its chemistry, with lower oxygen (O) to C ratios (O/C) linked with greater stability [62]. A meta-analysis based on 128 observations estimated that 3% of biochar C has a mean residence time (MRT) of only 108 days, while 97% has an MRT of 556 years [61].
While similar coproducts are generated from gasification and slow pyrolysis, each process results in different quantities of syngas and biochar. Because gasification involves partial oxidation, less C is fixed into biochar resulting in lower biochar yields and higher syngas yields [63]. Slow pyrolysis, by contrast, occurs in low or no-oxygen conditions and optimizes for the production of biochar rather than syngas. Multiple life cycle assessments (LCAs) have been conducted to evaluate slow pyrolysis and gasification waste management systems in terms of their GHG balance and economic feasibility [63][64][65][66][67][68]. Studies frequently conclude that gasification is a more favorable waste management strategy in terms of economic viability, as syngas has a higher monetary value than biochar and is not dependent on the cost of C offset incentive programs. Because of the higher biochar yield, however, slow pyrolysis has been shown to deliver greater benefits in terms of C abatement and an overall reduction in GWP [63]. A recent literature review concluded that biochar LCAs collectively demonstrate “significant” and “obvious” climate change mitigation benefits [69], most of which are the result of direct C sequestration rather than energy production. Because of the uncertainty in the literature regarding benefits and drawbacks of biochar as a soil amendment, most LCAs do not model the performance of biochar once it has been applied to soil. This uncertainty is reflected in policy arenas as well, such as in the IPCC 2018 Special Report, which included biochar production as a negative emissions technology due to C fixation, but excluded secondary GHG benefits of biochar in the soil [6].
The purported secondary GHG benefits of adding biochar to soil are due in part due to the potential for biochar to promote the formation and preservation of soil organic C. Since biochar itself is primarily comprised of C [18], it is not surprising that many authors report increased total C following biochar addition [10][20][24][70]. Simply put, adding C to the soil increases C in the soil. Due to challenges in methods, few authors distinguish between added biochar C and secondary soil C formation or preservation that may occur due to the addition of biochar. However, methods such as dichromate oxidation [71][72] and the use of molecular markers via the benzene polycarboxylic acid (BPCA) method [73][74] are available to make these distinctions.
Biochar has been observed to increase microbial biomass C by altering the soil chemical environment through increased nutrients or changes in pH, or the soil physical environment by providing additional habitat via increased surface area or porosity [25][75]. Biochar may also shift the soil microbial community composition towards increased ratios of fungal to bacterial phospholipid fatty acids (PLFAs), and Gram positive to Gram negative PLFAs [25]. These shifts are often interpreted to indicate a bacterial community more effective at utilizing recalcitrant C or enduring soil nutrient and water shortages [25][76]. Multiple authors have observed that biochar amendments can lead to increased soil aggregate size and stability [58][59][77], which may indicate an increased ability to store and protect soil C [78]. Two proposed mechanisms for this are an increase in multivalent cations which bridge organic colloids and clays, and the increased presence of bacterial mucilage and fungal hyphae [79]. Finally, biochar has been observed to protect soil C through negative priming of soil organic matter [61]. As with other effects, the ability of biochar to influence soil aggregation or the microbial community appears dependent on a suite of biochar properties, such as pH, nutrient content, and labile C, as well as the physiochemical properties of the soil [25][58][75][80]. Furthermore, benefits may not persist over time [81], or may be overridden quickly by shifts in management practices [82].
Another potential climate benefit resulting from the use of biochar is its ability to reduce GHG emissions from the matrix it is added to. A meta-analysis of 608 observations from 88 studies concluded that, overall, biochar reduced nitrous oxide (N2O) emissions by 32% when added to the soil, though effects were negligible after one year [83]. Results were greatest in paddy soils and sandy soils, but were not observed in grasslands or perennial crops. Reductions in soil ammonia (NH3) volatilization have also been observed, although a meta-analysis of 144 observations concluded there was minimal response overall [84]. Biochar application to acidic soil was observed to increase the emission of NH3, while combining biochar with urea or organic fertilizer, or using wood-based or acidified biochar, was observed to reduce NH3 emissions [84]. Mechanisms for biochar associated GHG reductions are poorly understood, though hypotheses frequently include the ability of biochar to alter pH or soil water and aeration dynamics, chemically or physically retain nitrate or ammonium, increase pH buffering capacity, and serve as an electron shuttle for microbes involved in the nitrogen cycle [83][84][85][86]. These hypotheses have been used to explain reductions in the emissions of N2O, NH3, methane (CH4) [46][87], and volatile organic compounds (VOCs) [88] from green waste and manure composting process, as well. Finally, biochar has been observed to reduce the GHG impacts of livestock production when added to animal feed. A literature review of 112 publications determined that in most studies, and for all investigated livestock species, adding biochar to animal feed resulted in reduced GHG emissions, as well as positive effects on parameters such as toxin adsorption, digestion, blood values, feed efficiency, and meat quality [89].
The climate change mitigation benefits of biochar can be optimized by ensuring the production and distribution of biochars with low O to C ratios for maximum stability [62]. The widespread adoption of policies such as California’s AB-2511, which requires biochars to be a minimum of 60% C [90], could assist in this goal. However, this definition of biochar excludes materials with less than 60% C, and may reduce the availability and use of these potentially beneficial materials. Nuanced categories based on specific criteria may be more useful to ensuring the sustainability benefits of biochar. It is also essential to avoid generating additional GHG emissions during the biochar life cycle, by optimizing systems to minimize feedstock and biochar transportation, energy inputs, and the use of non-waste biomass products [63][64][65][66][68]. Finally, careful regional analyses should be conducted on the existing uses of feedstocks, to avoid utilizing biomass from domains where it is already providing ecosystem services, such as the return of crop residues for increased soil health or erosion control.
2.3. Environmental Remediation
Though biochar is primarily considered an agricultural soil amendment, there is great promise for its use in soil remediation. Biochars possess a range of unique chemical and physical properties which can make them highly effective materials for facilitating contaminant binding, bioremediation, and phytostabilization. Similar to activated carbon, which is commonly used in remediation, biochars typically have high surface area, aromaticity, and cation exchange capacity, making them favorable absorbents for a range of heavy metals [91][92], pesticides [93][94], and organic compounds [95][96]. In many cases, tailoring the biochar feedstock and pyrolysis conditions for specific contaminants is key for remediation efficiency [41][97]. As the costs of soil remediation are typically high, biochar can serve as a low-cost and sustainable alternative in certain scenarios.
The high affinity of organic hydrocarbons to activated carbon and charcoal has long been established. It is, therefore, not surprising that biochar can serve as a sink for a wide range of organic contaminants, with affinity typically increasing with increased hydrophobicity and aromaticity [17][56][98][99]. Thus, biochar can be utilized to immobilize pharmaceuticals in animal wastes [100], wastewater [101], and biosolids [102], reduce the mobility of pesticides [103], or serve as a sink for petroleum-based compounds [104]. Through these mechanisms, the bioavailability of contaminants to surrounding organisms can be reduced via sorption to biochar [105][106][107]. There is also a growing body of evidence which demonstrates that biochar can facilitate biodegradation of organic contaminants in impacted soils [108][109][110]. This may have enormous implications for the cost and effectiveness of organic remediation efforts. However, for these technologies to be fully developed, further investigation is required into the physical and chemical parameters of biochars which best facilitate specific contaminant binding and bioremediation of organic compounds.
Remediation of metal(oid)-contaminated soils is particularly challenging as metal(oid)s do not degrade. Numerous studies demonstrate that biochars can favorably bind heavy metals [17][111][112], due to their high surface area and cation exchange capacity, through chemisorption and electrostatic interactions. As a result, biochars applied to heavy metal-contaminated soils can reduce metal bioavailability and phytotoxicity [113][114], thus facilitating phytostabilization via establishment of plant cover and, subsequently, additional contaminant immobilization through plant uptake or through increased exudates and organic matter production [115][116][117][118]. While metal retention can contribute to enhanced food safety, it is not a perfect solution, as metals will remain in the soil and may be released as biochars age. A novel approach to remediate metal(oid)-contaminated soils is via magnetic biochars that bind metal(oid)s, and can subsequently be recovered to remove bound contaminants [119][120][121][122]. However, this approach is likely best suited for aquatic environments, as magnetic biochar is challenging to recover from drier soils.
Wastewater, stormwater runoff, and various aquatic environments can offer ideal systems in which to utilize biochar for the removal of nutrients and contaminants [123][124][125], especially where biochars have been modified to carry out this task [41][123][126]. Biochars are frequently modified with the aim to increase positive charge on the biochar surface, through the addition of iron [127], magnesium [128], and cationic polymers [129]; through its incorporation into layered double hydroxides [130]; and by adding oxidants, acids, or alkalis [126][131]. In scenarios where biochar remediates excess nutrients from aqueous environments, the nutrient-loaded biochar can be reutilized as a fertilizing material in agriculture [123]. Additionally, sewage sludge from aquatic systems can be converted into biochar [132] for carbon stabilization and as a potential source of nutrients.
The ability of modified and unmodified biochars to bind both organic and inorganic contaminants makes their use in remediation highly effective. Research is ongoing into successful and cost-effective strategies for tailoring biochars for bioremediation, phytostabilization, contaminant removal, and nutrient recovery. These uses likely provide greater economic and environmental benefits than its use as a soil amendment in agriculture. In summary, biochar offers a low-tech and cost-efficient option for addressing many environmental challenges, with the potential co-benefits of biomass waste management, carbon sequestration, and renewable energy production.
2.4. Product Substitution
The primary carbon sequestration benefits of biochar are achieved merely through its production, and do not necessitate that biochar be added to the soil. Perhaps the least studied but most promising uses of biochar are those that employ it as a substitute for materials otherwise requiring GHG-intensive processing. In an idealized system, biochar can be created from waste biomass as a coproduct of energy production, and then utilized as a substitute for materials that would otherwise be extracted, mined, or refined.
Biochar is increasingly being studied as a substitute for the porous and low-weight materials in potting media [133]. Headlee et al. (2014) observed a biochar produced from red oak at 500 °C to completely replace vermiculite, a nonrenewable and mining-intensive material, with no negative effect to the growth or nutrient uptake of poplar trees [134]. Margenot et al. (2018) illustrated that a softwood biochar produced at 800 °C could replace 100% of the peat in a potting mix, with no negative effect on marigold growth or quality [135]. Yan et al. (2020) demonstrated no negative effects on mint yield or quality when 80% of the peat was substituted with a hardwood biochar produced from fast pyrolysis [136]. Data from these and other studies, including an LCA [137], have enormous implications for the nursery industry. Currently, potting media rely heavily on the GHG-intensive mining of peat bogs, which collectively serve as a global C sink and are part of irreplaceable wetland ecosystems [138][139][140]. Biochars produced from softwood and hardwood at multiple temperatures have also been observed to replace peat as an inoculum carrier in seed coatings, with increased corn yield and germination rates [141].
Because biochars are typically highly porous, have low bulk density, and contain reactive surface functional groups, it has been observed that biochar can serve as an odor control additive to various matrices [142]. Vaughn et al. (2020) determined that biochar produced from Eastern Redwood Cedar at 928 °C could replace mining-intensive clays in a low dust, odor-controlling, biodegradable cat litter formulation [143]. Flores et al. (2021) observed that biochar produced from Miscanthus grass at 400 °C could be added to turkey bedding at a rate of 20%, to reduce reliance on increasingly scarce pine shavings [144]. Biochar in this study resulted in increased hygienic conditions in the bedding matrix, reduced incidence of animal stress, and an increase in the growth rate and overall body weight of turkeys. When added to animal feed, biochar has been observed to reduce odor-causing NH3 and CH4 [89]. While biochar cannot completely displace ingredients in livestock feed, it can be added to dilute the matrix and reduce the use of non-waste ingredients overall.
Coproducts generated from the biochar production process may also serve to displace GHG-intensive materials. As described earlier, the use of syngas and bio-oil can reduce dependence on fuel mixes—fossil or otherwise—from regional utility grids. Pyroligneous acid (PA)—an organic liquid consisting of aliphatic, aromatic, and naphthenic hydrocarbons and other oxygenated compounds such as alcohols, aldehydes, ketones, furans, acids, phenols, and ethers—may also be generated through pyrolysis [145]. The use of PA in agriculture long predates the petrochemical industry, with most contemporary scientific investigation carried out in Asia. Grewal et al. (2019) produced a detailed review of studies which have demonstrated the ability of PA to deliver benefits such as enhanced plant growth and development, use as an antifungal and insecticide, and the ability to replace synthetic chemical herbicides [145].
There is also vast potential for biochar to be used in industrial applications, though this has been minimally studied. Its low thermal conductivity and water absorption properties may make it effective at regulating temperature and humidity in building applications, where it can replace non-biodegradable Styrofoam, or dilute the use of nonrenewable clay, lime, sand, and cement mortar [146]. Bartoli et al. (2020) authored a detailed review of the potential for biochar to aid in energy storage applications, by serving as anodic material in batteries, as fuel in direct carbon fuel cells, and, through chemical or physical activation, as an elevated surface for the double ionic layer in supercapacitors [147]. Furthermore, biochar has also been observed to reinforce plastics, epoxies, and cements when mixed into the material matrix [147]. Recent focus on the environmental challenges associated with the use of micro-plastics in cosmetics has led scientists and entrepreneurs to search for low-cost and sustainable alternatives [148]. As biochar already has a long history in the beauty industry as a detoxicant in soaps and homeopathic salves [146], it may be a promising replacement for the plastic beads in skin and teeth exfoliants.
The substantial potential of biochar to be used in the substitution of materials with high GHG emissions provides further opportunities for biochar to “close the loop” and address global sustainability. While biochar is a form of sequestered carbon, its ability to replace products that generate carbon dioxide emissions makes it a highly attractive climate change mitigation tool. Additionally, biochar represents a low-cost option as a “green” technology to replace other products which are harmful to the environment.
2.5. Potential Consequences to the Widespread Use of Biochar
Though biochar LCAs illustrate clear climate change mitigation benefits, authors of two separate analyses warn that biochar-related pollution may contribute to a larger negative effect over its life cycle, due to the potential adverse human health impacts [63][65]. Potential consequences remain critically understudied [149]. While a low bulk density and high surface area can lead to benefits such as water and nutrient retention, they also render biochar susceptible to off-site transport via surface runoff, or airborne release as particulate matter less than 2.5 or 10 µm in diameter (PM2.5 or PM10, respectively) [150][151]. This can occur during low-tech biochar production, during biochar application to the soil, or after it has been incorporated as the result of mechanical tillage or wind- or water-driven erosion. Agricultural dust is a major contributor to PM10, particularly in intensively farmed regions [152][153][154]. Exposure to both the organic and inorganic components from agricultural PM10 have been linked with adverse health effects in farmworkers, including increased chronic respiratory symptoms and the worsening of lung and heart disease [155][156][157][158]. With the growing interest in biochar as a soil amendment comes an imperative to better understand potential consequences for water and air quality, and how these might affect the health of agricultural workers, neighboring farm communities, livestock, and wildlife.
The organic and inorganic chemical constituents of biochar may also present a human health risk [159][160]. Polycyclic aromatic hydrocarbons (PAHs), for example, are known to form during the pyrolysis of biomass, with concentrations heavily dependent on production methods, feedstock, and temperature [161][162][163]. While most biochars contain PAH concentrations well below environmental quality standards [161][164][165], others have values well beyond [161][166][167][168][169]. Other native toxicants formed or concentrated during biochar production may include heavy metals, volatile organic compounds (VOCs), dioxins, furans, and polychlorinated biphenyl (PCBs), with some biochars showing endocrine disrupting potential [170]. Heavy metals occur naturally in biomass feedstocks and are concentrated in biochar through the production process [165]. As with PAHs, many studies demonstrate biochars to have metal concentrations well below most environmental quality standards [164][165][167][171]. Biochar copper and zinc levels, however, have been observed to have phytotoxic effects in cucumber, cress, and sorghum [172]. Similarly, high levels of VOCs have been detected in biochar and observed to cause phytotoxicity in cress [173]. In contrast, observed levels of total dioxins, PCBs and furans in biochar are often very low, with bioavailable fractions below analytical detection limits [161][174].
As this is an emerging field of research, few studies have investigated potential ecological and human health consequences. While rare, the potentially toxic properties in biochar have been observed to cause phytotoxicity, cytotoxicity, and additional adverse effects such as extracellular enzyme inhibition or earthworm avoidance [175]. There are also studies which suggest biochar PAHs may be carcinogenic if ingested via vegetables grown in biochar-amended soils [176][177]. By contrast, Liu et al. (2019) determined there was little to no release of PAHs from biochar in simulated lung fluids, even under “worst case scenario” exposure [178]. This may indicate that biochar PAHs are not readily bioavailable through inhalation pathways. Additionally, the concentration, and therefore, potential toxicity, of PAHs has been demonstrated to decrease as biochar ages [179][180], indicating that potential threats may be short-term and easily managed. Fortunately, there are a growing number of strategies for mitigating the potential consequences of the production and use of biochar.
2.6. Strategies for Mitigating Consequences
During the biochar production process, steps can be taken to create a safer, more effective soil amendment. As heavy metals and metalloids are concentrated in biochar through thermal conversion [165], the use of treated feedstocks such as chromated copper arsenate (CCA)-pressure treated wood should be avoided, along with materials from construction and demolition, and feedstocks of unknown origin. Research suggests that slow pyrolysis may minimize biochar PAH content compared to gasification, and that increased residence time [161] and rate of carrier gas flow [163][181][182] can offset PAH formation under high temperatures. There may also exist simple pre- and post- production modifications that reduce levels of contaminants. Studies indicate that drying feedstock biomass prior to pyrolysis may reduce production-related PM10 emissions, as well as PM-bound PAHs, as the presence of moisture encourages the incomplete combustion of volatile compounds formed during pyrolysis [183]. Research has also demonstrated that biochars can be dried at temperatures between 100 and 300 °C, effectively removing PAHs through thermal desorption within 24 h [184]. Efforts have also been made to improve the physical properties of biochar during its production. An increasingly popular technique is to pelletize biochars to increase resistance to abrasion [185]. Addition of binders during pelletization, such as lignin and Ca(OH)2, can further enhance the mechanical strength of biochars [186]. With increased mechanical strength and abrasion resistance, biochar may emit less dust compared to those that have not been compressed.
Major (2010) summarizes various biochar application methods and recommends combining biochar amendment with other on-farm processes to reduce costs and to minimize the potential for dust emissions [23]. Suggestions include adding biochar to compost or liquid fertilizer. To reduce dust emissions, recommendations for applying biochar with high moisture content or in liquid slurries are common, as are warnings to avoid application on windy days [159][187][188]. While most biochar field trials utilize a broadcasting application technique (Figure 3d), Major (2010) suggests that subsurface banding (Figure 3a–c) may have the greatest potential to reduce wind and rain-driven biochar losses. Regardless of application method, the use of appropriate respirators, eye protection, gloves, long sleeves, and pants is recommended for farm operators and workers while handling biochar [189].

Figure 3. Practices that are recommended (left) and not recommended (right) for the application of biochar to agricultural lands. On the left, a biochar is applied to the field at 40% moisture content (a), using a subsurface banding technique (b) in which biochar is immediately covered by soil (c) so it is less likely to contaminate air or water as the result of wind- or rain-driven erosion. On the right, a biochar is applied at 0% moisture content (d), on a windy day (e), and left on the soil surface where it became airborne (f). Photo credit for (a–c) to Sanjai J. Parikh, (d) to Brian Kozlowski at the University of Tennessee, and (e,f) to Iris Holzer at the University of California, Davis.
Governments can play a key role in developing and enforcing policies that ensure the safe production and handling of biochar. The United States has not yet adopted required regulatory standards for biochar contaminant levels (e.g., PAHs, heavy metals), though maximum threshold values for a limited number of toxicants have been established in frameworks proposed by the European Biochar Certificate (EBC) [187] and the International Biochar Initiative (IBI) [190]. As of June 2021, IBI lists four biochars certified to meet their safety and quality standards, all of which are in the United States [191]. EBC lists 27 certified biochars from Austria (3), Finland (1), France (1), Germany (11), Romania (1), Serbia (1), Sweden (4), and Switzerland (5) [192]. Differences in these standards have led to inconsistencies in both scientific and legislative literature. There is a pressing need for a unified regulatory framework, which would facilitate communication in academic fields and in the emerging biochar market. Furthermore, outreach and education efforts could assist in ensuring the safe application of biochar to working lands. This may be particularly relevant when biochar is paid for by government cost-share or incentive programs as part of climate change mitigation strategies. In these cases, agencies could require producers and land managers to review simple best management practices for the safe and effective use of biochar.
3. How Can We Best Move Forward?
Scientists have a critical role in ensuring that the current boom in biochar research produces information for its safe and effective use, which optimizes benefits for humans and the environment. We propose that by working together as a scientific community, the following strategies can assist in this goal, and in doing so, increase the rate of knowledge accrual to match the rate of investigation.
1. Results and conclusions from biochar studies should be limited to the specific conditions in which results were observed. Biochar has great potential to deliver numerous agricultural and environmental benefits. However, it has many variables and will perform differently in different conditions. While the enthusiasm of the day is useful for driving research, generating awareness, and leveraging funding, scientists should be careful to manage expectations and avoid generalizing their data to indicate that results extrapolate to other biochars or conditions.
2. Researchers should strive towards standardized biochar characterization methods and increased reporting of biochar production parameters and physical and chemical properties. In order to increase the efficacy of biochar for specific applications, it is necessary that researchers and biochar producers have access to comprehensive biochar production and characterization data. This is necessary for replicability of research, to guide decisions for biochar selection based on intended use, and to assist in tailoring biochars for specific purposes.
3. Biochar must be studied in real-world conditions to evaluate its long-term effects on soil productivity and health. There is a pressing need for long-term field-scale research utilizing commercially available biochars with high consistency in composition. As in all scientific fields, the pressure to produce results within a single grant cycle or graduate student tenure is an obvious challenge. However, our current reduced rate of knowledge gain will persist if we do not push experimental designs towards real world conditions. Only then can we better understand the benefits, drawbacks, tradeoffs, and costs associated with the production and use of biochar.
4. Results from biochar studies should always be explained on a physical, chemical, or biological basis. While field-scale research is critical, it is not sufficient for scientists to present results without further investigation into their mechanistic underpinnings. In order to advance the state of knowledge about biochar, results must be supported by mechanistic experimentation or explanations supported by other evidence.
5. Soil scientists cannot ensure the sustainable and effective production and use of biochar alone. In the research arena, there should be increased collaboration with industrial and material scientists in order to link biochar production parameters with specific characteristics. Groups of collaborators could work with large-scale biochar companies to produce biochars that are tailored for specific outcomes within certain soil and climate parameters. In the policy arena, scientists can guide the safe and effective use of biochar by working with governments to develop policies that require a specific O/C ratio for maximum C sequestration benefits. Policies should also be developed which require biochars to conform to safety standards. Finally, policy could assist in outreach and education efforts, so that biochars amended to working lands are applied in ways which safeguard human and environmental health.
6. Scientists, entrepreneurs, and other stakeholders should use creativity in discovering novel and beneficial uses for biochar. While research has demonstrated that biochar can improve soil health and agricultural production under specific conditions, its use in climate change mitigation, remediation, and product substitution may provide greater environmental benefits, with fewer potential unintended consequences. We must “think outside the soil” to advance biochar research towards new frontiers.
7. Biochar investigation should prioritize maximizing its numerous potential benefits. The technologies which convert waste biomass to biochar, bioenergy, and pyroligneous acid, have great potential to operate within “closed loop” systems if optimized properly. Locally sourced feedstocks can be converted to a range of coproducts, which can then be used locally for ecological, environmental, or agricultural outcomes. In temperate climates with fertile soils, biochar may be less useful as an agricultural soil amendment, and more beneficial in the remediation of contaminated sites, in storm or wastewater management, as a substitution for mining GHG-intensive materials, or modified to retain nutrients in compost production. If produced and employed thoughtfully, biochar has a high probability of serving as a climate mitigation tool. The key is to extract as many possible benefits from biochar production systems for multiple and variable uses.
While current efforts have largely focused on the potential of biochar to increase agricultural production and soil health, biochar research need not focus on soil alone. Biochars are a category of materials that can provide numerous benefits, and should be employed in a manner where they best address global sustainability constraints including, but not limited to, climate change, soil and water contamination, and productivity in marginal lands. In order to increase the current rate of knowledge accrual, scientists, policymakers, and entrepreneurs must work together to develop new and innovative approaches to biochar research, which address both safety and sustainability.
References
- Web of Science Core Collection Database, “Biochar”. Available online: http://apps.webofknowledge.com (accessed on 1 June 2021).
- US Patent and Trademark Office Patent Application Full Text and Image Database. Available online: http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=0&f=S&l=50&TERM1=biochar&FIELD1=&co1=AND&TERM2=&FIELD2=&d=PG01 (accessed on 20 May 2021).
- International Biochar Initiative (IBI). IBI July Newsletter. Available online: https://biochar-international.org/wp-content/uploads/2021/07/2021-7-IBI-Newsletter-English.pdf (accessed on 1 June 2021).
- Bradford, M.A.; Carey, C.J.; Atwood, L.; Bossio, D.; Fenichel, E.P.; Gennet, S.; Fargione, J.; Fisher, J.R.B.; Fuller, E.; Kane, D.A.; et al. Soil carbon science for policy and practice. Nat. Sustain. 2019, 2, 1070–1072.
- Baveye, P.C. Soil health at a crossroad. Soil Use Manag. 2021, 37, 215–219.
- De Coninck, H.; Revi, A.; Babiker, M.; Bertoldi, P.; Buckeridge, M.; Cartwright, A.; Dong, W.; Ford, J.; Fuss, S.; Hourcade, J.-C.; et al. Strengthening and implementing the global response. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; IPCC: Geneva, Switzerland, 2018.
- United States Biochar Initiative (USBI) Biochar Introduction. Available online: https://biochar-us.org/biochar-introduction (accessed on 6 August 2021).
- International Biochar Initiative (IBI) About Biochar. Available online: https://biochar-international.org/biochar/ (accessed on 1 June 2021).
- Baveye, P.C. Bypass and hyperbole in soil research: Worrisome practices critically reviewed through examples. Eur. J. Soil Sci. 2021, 72, 31–34.
- MacCarthy, D.S.; Darko, E.; Nartey, E.K.; Adiku, S.G.K.; Tettey, A. Integrating biochar and inorganic fertilizer improves productivity and profitability of irrigated rice in Ghana, West Africa. Agronomy 2020, 10, 904.
- Sadowska, U.; Domagała-Świątkiewicz, I.; Żabiński, A. Biochar and Its Effects on Plant–Soil Macronutrient Cycling during a Three-Year Field Trial on Sandy Soil with Peppermint (Mentha piperita L.). Part I: Yield and Macro Element Content in Soil and Plant Biomass. Agronomy 2020, 10, 1950.
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055.
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711.
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001.
- Zhang, D.; Yan, M.; Niu, Y.; Liu, X.; van Zwieten, L.; Chen, D.; Bian, R.; Cheng, K.; Li, L.; Joseph, S.; et al. Is current biochar research addressing global soil constraints for sustainable agriculture? Agric. Ecosyst. Environ. 2016, 226, 25–32.
- United States Department of Agriculture Natural Resources Conservation Service (NRCS) Soil Carbon Amendment 808-CPS-1 (under Evaluation). Available online: https://www.nrcs.usda.gov/wps/cmis_proxy/https/ecm.nrcs.usda.gov%3A443/fncmis/resources/WEBP/ContentStream/idd_D01F2B77-0000-C211-A3DE-93A6410CE679/0/808+CPS+Soil+Carbon+Amendment+2021+-+DRAFT.pdf (accessed on 1 June 2021).
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 2020, 744, 140714.
- Downie, A.; Crosky, A.; Munroe, P. Characteristics of biochar—physical and structural properties. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 89–108.
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187.
- Oladele, S.O. Changes in physicochemical properties and quality index of an Alfisol after three years of rice husk biochar amendment in rainfed rice—Maize cropping sequence. Geoderma 2019, 353, 359–371.
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Zimmerman, A.R.; Pandit, B.H.; Cornelissen, G. Multi-year double cropping biochar field trials in Nepal: Finding the optimal biochar dose through agronomic trials and cost-benefit analysis. Sci. Total Environ. 2018, 637–638, 1333–1341.
- Guo, M. The 3R Principles for Applying Biochar to Improve Soil Health. Soil Syst. 2020, 4, 9.
- Major, J. Guidelines on Practical Aspects of Biochar Application to Field Soil in Various Soil Management Systems. Int. Biochar Initiat. 2010, 1, 1–23.
- Liu, S.; Zhang, Y.; Zong, Y.; Hu, Z.; Wu, S.; Zhou, J.; Jin, Y.; Zou, J. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. GCB Bioenergy 2016, 8, 392–406.
- Zhang, L.; Jing, Y.; Xiang, Y.; Zhang, R.; Lu, H. Responses of soil microbial community structure changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935.
- Bonanomi, G.; Ippolito, F.; Scala, F. A “black” future for plant pathology? Biochar as a new soil amendment for controlling plant diseases. J. Plant Pathol. 2015, 97, 223–234.
- Solaiman, Z.M.; Shafi, M.I.; Beamont, E.; Anawar, H.M. Poultry litter biochar increases mycorrhizal colonisation, soil fertility and cucumber yield in a fertigation system on sandy soil. Agriculture 2020, 10, 480.
- Nan, Q.; Wang, C.; Wang, H.; Yi, Q.; Liang, B.; Xu, J.; Wu, W. Biochar drives microbially-mediated rice production by increasing soil carbon. J. Hazard. Mater. 2020, 387, 121680.
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124.
- McDonald, M.R.; Bakker, C.; Motior, M.R. Evaluation of wood biochar and compost soil amendment on cabbage yield and quality. Can. J. Plant. Sci. 2019, 99, 624–638.
- Griffin, D.E.; Wang, D.; Parikh, S.J.; Scow, K.M. Short-lived effects of walnut shell biochar on soils and crop yields in a long-term field experiment. Agric. Ecosyst. Environ. 2017, 236, 21–29.
- Gajić, A.; Koch, H.-J. Sugar Beet (Beta vulgaris L.) Growth Reduction Caused by Hydrochar Is Related to Nitrogen Supply. J. Environ. Qual. 2012, 41, 1067–1075.
- Liu, X.; Zhou, J.; Chi, Z.; Zheng, J.; Li, L.; Zhang, X.; Zheng, J.; Cheng, K.; Bian, R.; Pan, G. Biochar provided limited benefits for rice yield and greenhouse gas mitigation six years following an amendment in a fertile rice paddy. Catena 2019, 179, 20–28.
- Cornelissen, G.; Martinsen, V.; Shitumbanuma, V.; Alling, V.; Breedveld, G.; Rutherford, D.; Sparrevik, M.; Hale, S.; Obia, A.; Mulder, J. Biochar Effect on Maize Yield and Soil Characteristics in Five Conservation Farming Sites in Zambia. Agronomy 2013, 3, 256–274.
- Backer, R.G.M.; Schwinghamer, T.D.; Whalen, J.K.; Seguin, P.; Smith, D.L. Crop yield and SOC responses to biochar application were dependent on soil texture and crop type in southern Quebec, Canada. J. Plant Nutr. Soil Sci. 2016, 179, 399–408.
- Omara, P.; Aula, L.; Oyebiyi, F.B.; Eickhof, E.M.; Carpenter, J.; Raun, W.R. Biochar application in combination with inorganic nitrogen improves maize grain yield, nitrogen uptake, and use efficiency in Temperate Soils. Agronomy 2020, 10, 1241.
- Hestrin, R.; Torres-Rojas, D.; Dynes, J.J.; Hook, J.M.; Regier, T.Z.; Gillespie, A.W.; Smernik, R.J.; Lehmann, J. Fire-derived organic matter retains ammonia through covalent bond formation. Nat. Commun. 2019, 10, 664.
- Sun, Y.; Yang, J.; Yao, R.; Chen, X.; Wang, X. Biochar and fulvic acid amendments mitigate negative effects of coastal saline soil and improve crop yields in a three year field trial. Sci. Rep. 2020, 10, 8946.
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar Mitigates Salinity Stress in Potato. J. Agron. Crop. Sci. 2015, 201, 368–378.
- Mahmoud, E.; El-Beshbeshy, T.; El-Kader, N.A.; El Shal, R.; Khalafallah, N. Impacts of biochar application on soil fertility, plant nutrients uptake and maize (Zea mays L.) yield in saline sodic soil. Arab. J. Geosci. 2019, 12, 719.
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Parikh, S.J.; Ok, Y.S.; Song, G.; Gelardi, D.L.; Huang, L.; et al. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, in press.
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.W.; Conte, P.; Stephen, J. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080.
- Haider, G.; Steffens, D.; Müller, C.; Kammann, C.I. Standard Extraction Methods May Underestimate Nitrate Stocks Captured by Field-Aged Biochar. J. Environ. Qual. 2016, 45, 1196–1204.
- Haider, G.; Joseph, S.; Steffens, D.; Müller, C.; Taherymoosavi, S.; Mitchell, D.; Kammann, C.I. Mineral nitrogen captured in field-aged biochar is plant-available. Sci. Rep. 2020, 10, 13816.
- Ye, L.; Camps-Arbestain, M.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar effects on crop yields with and without fertilizer: A meta-analysis of field studies using separate controls. Soil Use Manag. 2020, 36, 2–18.
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164.
- Hagemann, N.; Kammann, C.I.; Schmidt, H.P.; Kappler, A.; Behrens, S. Nitrate capture and slow release in biochar amended compost and soil. PLoS ONE 2017, 12, e0171214.
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678.
- Kerré, B.; Willaert, B.; Cornelis, Y.; Smolders, E. Long-term presence of charcoal increases maize yield in Belgium due to increased soil water availability. Eur. J. Agron. 2017, 91, 10–15.
- Nawaz, H.; Hussain, N.; Anjum, M.A.; Rehman, H.U.; Jamil, M.; Aown, M.; Raza, S.; Farooq, O. Biochar Application Improves the Wheat Productivity under Different Irrigation Water-Regimes. Int. J. Agric. Biol. 2019, 21, 936–942.
- Madari, B.E.; Silva, M.A.S.; Carvalho, M.T.M.; Maia, A.H.N.; Petter, F.A.; Santos, J.L.S.; Tsai, S.M.; Leal, W.G.O.; Zeviani, W.M. Properties of a sandy clay loam Haplic Ferralsol and soybean grain yield in a five-year field trial as affected by biochar amendment. Geoderma 2017, 305, 100–112.
- Nelissen, V.; Ruysschaert, G.; Mankaabusi, D.; D’Hose, T.; De Beuf, K.; Al-Barri, B.; Cornelis, W.; Boeckx, P. Impact of a woody biochar on properties of a sandy loam soil and spring barley during a two-year field experiment. Eur. J. Agron. 2015, 62, 65–78.
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338.
- Hale, S.E.; Nurida, N.L.; Jubaedah; Mulder, J.; Sørmo, E.; Silvani, L.; Abiven, S.; Joseph, S.; Taherymoosavi, S.; Cornelissen, G. The effect of biochar, lime and ash on maize yield in a long-term field trial in a Ultisol in the humid tropics. Sci. Total Environ. 2020, 719, 137455.
- Raboin, L.M.; Razafimahafaly, A.H.D.; Rabenjarisoa, M.B.; Rabary, B.; Dusserre, J.; Becquer, T. Improving the fertility of tropical acid soils: Liming versus biochar application? A long term comparison in the highlands of Madagascar. Field Crop. Res. 2016, 199, 99–108.
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287.
- Almaroai, Y.A.; Eissa, M.A. Effect of biochar on yield and quality of tomato grown on a metal-contaminated soil. Sci. Hortic. Amst. 2020, 265, 109210.
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117.
- Liu, X.H.; Han, F.P.; Zhang, X.C. Effect of biochar on soil aggregates in the Loess Plateau: Results from incubation experiments. Int. J. Agric. Biol. 2012, 14, 975–979.
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.W.; Kwon, E.E.; Lee, J.; Wang, C.H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253.
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523.
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303.
- Ibarrola, R.; Shackley, S.; Hammond, J. Pyrolysis biochar systems for recovering biodegradable materials: A life cycle carbon assessment. Waste Manag. 2012, 32, 859–868.
- Gaunt, J.L.; Lehmann, J. Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ. Sci. Technol. 2008, 42, 4152–4158.
- Sparrevik, M.; Field, J.L.; Martinsen, V.; Breedveld, G.D.; Cornelissen, G. Life cycle assessment to evaluate the environmental impact of biochar implementation in conservation agriculture in Zambia. Environ. Sci. Technol. 2013, 47, 1206–1215.
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life Cycle Assessment of Biochar Systems: Estimating the Energetic, Economic, and Climate Change Potential. Environ. Sci. Technol. 2010, 44, 827–833.
- Nguyen, T.L.T.; Hermansen, J.E.; Nielsen, R.G. Environmental assessment of gasification technology for biomass conversion to energy in comparison with other alternatives: The case of wheat straw. J. Clean. Prod. 2013, 53, 138–148.
- Dutta, B.; Raghavan, V. A life cycle assessment of environmental and economic balance of biochar systems in Quebec. Int. J. Energy Environ. Eng. 2014, 5, 106.
- Matuštík, J.; Hnátková, T.; Kočí, V. Life cycle assessment of biochar-to-soil systems: A review. J. Clean. Prod. 2020, 259, 120998.
- El-Naggar, A.; Awad, Y.M.; Tang, X.Y.; Liu, C.; Niazi, N.K.; Jien, S.H.; Tsang, D.C.W.; Song, H.; Ok, Y.S.; Lee, S.S. Biochar influences soil carbon pools and facilitates interactions with soil: A field investigation. Land Degrad. Dev. 2018, 29, 2162–2171.
- Hardy, B.; Dufey, J.E. The resistance of centennial soil charcoal to the “Walkley-Black” oxidation. Geoderma 2017, 303, 37–43.
- Lim, B.; Cachier, H. Determination of black carbon by chemical oxidation and thermal treatment in recent marine and lake sediments and Cretaceous-Tertiary clays. Chem. Geol. 1996, 131, 143–154.
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in soils: The use of benzenecarboxylic acids as specific markers. Org. Geochem. 1998, 29, 811–819.
- Wiedemeier, D.B.; Hilf, M.D.; Smittenberg, R.H.; Haberle, S.G.; Schmidt, M.W.I. Improved assessment of pyrogenic carbon quantity and quality in environmental samples by high-performance liquid chromatography. J. Chromatogr. A 2013, 1304, 246–250.
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22.
- Bååth, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963.
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219–220, 162–167.
- Jastrow, D. Soil Aggregate Formation and the Accrual of Particulate and Mineral-Associated Organic Matter. Soil Biol. Biochem. 1996, 28, 665–676.
- Oades, J.M. Soil organic matter and structural stability: Mechanisms and implications for management. Plant. Soil 1984, 76, 319–337.
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial utilisation of biochar-derived carbon. Sci. Total Environ. 2013, 465, 288–297.
- Ameloot, N.; Sleutel, S.; Case, S.D.C.; Alberti, G.; McNamara, N.P.; Zavalloni, C.; Vervisch, B.; delle Vedove, G.; De Neve, S. C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biol. Biochem. 2014, 78, 195–203.
- Hardy, B.; Sleutel, S.; Dufey, J.E.; Cornelis, J.-T. The Long-Term Effect of Biochar on Soil Microbial Abundance, Activity and Community Structure Is Overwritten by Land Management. Front. Environ. Sci. 2019, 7.
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364.
- Sha, Z.; Li, Q.; Lv, T.; Misselbrook, T.; Liu, X. Response of ammonia volatilization to biochar addition: A meta-analysis. Sci. Total Environ. 2019, 655, 1387–1396.
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732.
- Krause, H.-M.; Hüppi, R.; Leifeld, J.; El-Hadidi, M.; Harter, J.; Kappler, A.; Hartmann, M.; Behrens, S.; Mäder, P.; Gattinger, A. Biochar affects community composition of nitrous oxide reducers in a field experiment. Soil Biol. Biochem. 2018, 119, 143–151.
- Akdeniz, N. A systematic review of biochar use in animal waste composting. Waste Manag. 2019, 88, 291–300.
- Sánchez-Monedero, M.A.; Sánchez-García, M.; Alburquerque, J.A.; Cayuela, M.L. Biochar reduces volatile organic compounds generated during chicken manure composting. Bioresour. Technol. 2019, 288, 121584.
- Schmidt, H.P.; Hagemann, N.; Draper, K.; Kammann, C. The use of biochar in animal feeding. PeerJ 2019, 2019, e7373.
- California Food and Agricultural Code Assembly Bill No. 2511. Available online: https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=201520160AB2511 (accessed on 1 June 2021).
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826.
- Wang, Y.; Wang, H.-S.; Tang, C.-S.; Gu, K.; Shi, B. Remediation of heavy-metal-contaminated soils by biochar: A review. Environ. Geotech. 2020, 252.
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70.
- Yavari, S.; Malakahmad, A.; Sapari, N.B. Biochar efficiency in pesticides sorption as a function of production variables—A review. Environ. Sci. Pollut. Res. 2015, 22, 13824–13841.
- Zhang, H.; Lin, K.; Wang, H.; Gan, J. Effect of Pinus radiata derived biochars on soil sorption and desorption of phenanthrene. Environ. Pollut. 2010, 158, 2821–2825.
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33.
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X. The role of tailored biochar in increasing plant growth, and reducing bioavailability, phytotoxicity, and uptake of heavy metals in contaminated soil. Environ. Pollut. 2017, 230, 329–338.
- Kumari, I.; Moldrup, P.; Paradelo, M.; de Jonge, L. Phenanthrene sorption on biochar-amended soils: Application rate, aging, and physicochemical properties of soil. Water Air Soil Pollut. 2014, 225, 2105.
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27.
- Ngigi, A.N.; Ok, Y.S.; Thiele-Bruhn, S. Biochar-mediated sorption of antibiotics in pig manure. J. Hazard. Mater. 2019, 364, 663–670.
- Liyanage, A.S.; Canaday, S.; Pittman, C.U.; Mlsna, T. Rapid remediation of pharmaceuticals from wastewater using magnetic Fe3O4/Douglas fir biochar adsorbents. Chemosphere 2020, 258, 127336.
- Bair, D.A.; Mukome, F.N.D.; Popova, I.E.; Ogunyoku, T.A.; Jefferson, A.; Wang, D.; Hafner, S.C.; Young, T.M.; Parikh, S.J. Sorption of Pharmaceuticals, Heavy Metals, and Herbicides to Biochar in the Presence of Biosolids. J. Environ. Qual. 2016, 45, 1998.
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Natasha; Asif Naeem, M.; Niazi, N.K. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645.
- Nguyen, H.N.; Pignatello, J.J. Laboratory tests of biochars as absorbents for use in recovery or containment of marine crude oil spills. Environ. Eng. Sci. 2013, 30, 374–380.
- Xiang, L.; Sheng, H.; Gu, C.; Marc, R.-G.; Wang, Y.; Bian, Y.; Jiang, X.; Wang, F. Biochar combined with compost to reduce the mobility, bioavailability and plant uptake of 2,2’,4,4’-tetrabrominated diphenyl ether in soil. J. Hazard. Mater. 2019, 374, 341–348.
- Bair, D.A.; Anderson, C.G.; Chung, Y.; Scow, K.M.; Franco, R.B.; Parikh, S.J. Impact of biochar on plant growth and uptake of ciprofloxacin, triclocarban and triclosan from biosolids. J. Environ. Sci. Health Part. B Pestic. Food Contam. Agric. Wastes 2020, 55, 990–1001.
- Gomez-Eyles, J.L.; Sizmur, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. 2011, 159, 616–622.
- Qin, G.; Gong, D.; Fan, M.Y. Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int. Biodeterior. Biodegrad. 2013, 85, 150–155.
- Mukome, F.N.D.; Buelow, M.C.; Shang, J.; Peng, J.; Rodriguez, M.; Mackay, D.M.; Pignatello, J.J.; Sihota, N.; Hoelen, T.P.; Parikh, S.J. Biochar amendment as a remediation strategy for surface soils impacted by crude oil. Environ. Pollut. 2020, 265, 115006.
- Singh, C.; Tiwari, S.; Singh, J.S. Biochar: A Sustainable Tool in Soil Pollutant Bioremediation BT—Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2020; pp. 475–494. ISBN 978-981-13-3426-9.
- Wang, S.; Kwak, J.-H.; Islam, M.S.; Naeth, M.A.; Gamal El-Din, M.; Chang, S.X. Biochar surface complexation and Ni(II), Cu(II), and Cd(II) adsorption in aqueous solutions depend on feedstock type. Sci. Total Environ. 2020, 712, 136538.
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478.
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant. Soil 2011, 348, 439.
- Bandara, T.; Herath, I.; Kumarathilaka, P.; Hseu, Z.-Y.; Ok, Y.S.; Vithanage, M. Efficacy of woody biomass and biochar for alleviating heavy metal bioavailability in serpentine soil. Environ. Geochem. Health 2017, 39, 391–401.
- Visconti, D.; Álvarez-Robles, M.J.; Fiorentino, N.; Fagnano, M.; Clemente, R. Use of Brassica juncea and Dactylis glomerata for the phytostabilization of mine soils amended with compost or biochar. Chemosphere 2020, 260, 127661.
- Prapagdee, S.; Piyatiratitivorakul, S.; Petsom, A.; Tawinteung, N. Application of Biochar for Enhancing Cadmium and Zinc Phytostabilization in Vigna radiata L. Cultivation. Water Air Soil Pollut. 2014, 225, 2233.
- Lebrun, M.; Miard, F.; Nandillon, R.; Léger, J.-C.; Hattab-Hambli, N.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted phytostabilization of a multicontaminated mine technosol using biochar amendment: Early stage evaluation of biochar feedstock and particle size effects on As and Pb accumulation of two Salicaceae species (Salix viminalis and Populus euramericana). Chemosphere 2018, 194, 316–326.
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Ippolito, J.A.; Ducey, T.F.; Johnson, M.G.; Spokas, K.A. Phytostabilization of Zn and Cd in mine soil using corn in combination with biochars and manure-based compost. Environment 2019, 6, 69.
- Wan, X.; Li, C.; Parikh, S.J. Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ. Pollut. 2020, 261, 114157.
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010.
- Lu, H.P.; Li, Z.A.; Gascó, G.; Méndez, A.; Shen, Y.; Paz-Ferreiro, J. Use of magnetic biochars for the immobilization of heavy metals in a multi-contaminated soil. Sci. Total Environ. 2018, 622–623, 892–899.
- Liu, Y.; Huang, J.; Xu, H.; Zhang, Y.; Hu, T.; Chen, W.; Hu, H.; Wu, J.; Li, Y.; Jiang, G. A magnetic macro-porous biochar sphere as vehicle for the activation and removal of heavy metals from contaminated agricultural soil. Chem. Eng. J. 2020, 390, 124638.
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of biochar for resource recovery from water: A review. Chem. Eng. J. 2020, 397, 125502.
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in water and wastewater treatment—A sustainability assessment. Chem. Eng. J. 2021, 420, 129946.
- Deng, S.; Chen, J.; Chang, J. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: Performance and ecological benefits. J. Clean. Prod. 2021, 293, 126156.
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309.
- Siddiq, M.O.; Tawabini, B.; Kirmizakis, P.; Kalderis, D.; Ntarlagiannis, D.; Soupios, P. Combining geophysics and material science for environmental remediation: Real-time monitoring of Fe-biochar arsenic wastewater treatment. Chemosphere 2021, 284, 131390.
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.F.; Wang, Y.C. Application of magnesium modified corn biochar for phosphorus removal and recovery from swine wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237.
- Wang, Z.; Bakshi, S.; Li, C.; Parikh, S.J.; Hsieh, H.-S.; Pignatello, J.J. Modification of pyrogenic carbons for phosphate sorption through binding of a cationic polymer. J. Colloid Interface Sci. 2020, 579, 258–268.
- Fang, Q.; Ye, S.; Yang, H.; Yang, K.; Zhou, J.; Gao, Y.; Lin, Q.; Tan, X.; Yang, Z. Application of layered double hydroxide-biochar composites in wastewater treatment: Recent trends, modification strategies, and outlook. J. Hazard. Mater. 2021, 420, 126569.
- Vu, T.M.; Trinh, V.T.; Doan, D.P.; Van, H.T.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing ammonium from water using modified corncob-biochar. Sci. Total Environ. 2017, 579, 612–619.
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.R.; Nidheesh, P.V.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656.
- Nemati, M.R.; Simard, F.; Fortin, J.-P.; Beaudoin, J. Potential Use of Biochar in Growing Media. Vadose Zone J. 2015, 14, vzj2014.06.0074.
- Headlee, W.L.; Brewer, C.E.; Hall, R.B. Biochar as a Substitute for Vermiculite in Potting Mix for Hybrid Poplar. Bioenergy Res. 2014, 7, 120–131.
- Margenot, A.J.; Gri, D.E.; Alves, B.S.Q.; Rippner, D.A.; Li, C.; Parikh, S.J. Substitution of peat moss with softwood biochar for soil-free marigold growth. Ind. Crop. Prod. 2018, 112, 160–169.
- Yan, J.; Yu, P.; Liu, C.; Li, Q.; Gu, M. Replacing peat moss with mixed hardwood biochar as container substrates to produce five types of mint (Mentha spp.). Ind. Crop. Prod. 2020, 155, 112820.
- Fryda, L.; Visser, R.; Schmidt, J. Biochar replaces peat in horticulture: Environmental impact assessment of combined biochar & bioenergy production. Detritus 2019, 5, 132–149.
- Bridgham, S.D.; Patrick Megonigal, J.; Keller, J.K.; Bliss, N.B.; Trettin, C. The carbon balance of North American wetlands. Wetlands 2006, 26, 889–916.
- Clarkson, B.R.; Ausseil, A.E.; Gerbeaux, P. Wetland Ecosystems Services. In Ecosystem Services in New Zealand: Conditions and Trends; Dymond, J.R., Ed.; Manaaki Whenua Press, Landcare Research: Lincoln, New Zealand, 2013; pp. 192–202.
- Cleary, J.; Roulet, N.T.; Moore, T.R. Greenhouse gas emissions from Canadian peat extraction, 1990–2000: A life-cycle analysis. Ambio 2005, 34, 456–461.
- Głodowska, M.; Husk, B.; Schwinghamer, T.; Smith, D. Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad. Agron. Sustain. Dev. 2016, 36, 21.
- Kalus, K.; Koziel, J.A.; Opaliński, S. A review of biochar properties and their utilization in crop agriculture and livestock production. Appl. Sci. 2019, 9, 3494.
- Vaughn, S.F.; Winkler-Moser, J.K.; Berhow, M.A.; Byars, J.A.; Liu, S.X.; Jackson, M.A.; Peterson, S.C.; Eller, F.J. An odor-reducing, low dust-forming, clumping cat litter produced from Eastern red cedar (Juniperus virginiana L.) wood fibers and biochar1. Ind. Crop. Prod. 2020, 147, 112224.
- Flores, K.R.; Fahrenholz, A.; Grimes, J.L. Effect of pellet quality and biochar litter amendment on male turkey performance. Poult. Sci. 2021, 100, 101002.
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159.
- Schmidt, H.-P. 55 Uses of Biochar. Ithaka J. 2012, 25, 13–25.
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A review of non-soil biochar applications. Materials 2020, 13, 261.
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79.
- Genesio, L.; Vaccari, F.P.; Miglietta, F. Black carbon aerosol from biochar threats its negative emission potential. Glob. Chang. Biol. 2016, 22, 2313–2314.
- Li, C.; Bair, D.A.; Parikh, S.J. Estimating potential dust emissions from biochar amended soils under simulated tillage. Sci. Total Environ. 2018, 625, 1093–1101.
- Ravi, S.; Sharratt, B.S.; Li, J.; Olshevski, S.; Meng, Z.; Zhang, J. Particulate matter emissions from biochar-amended soils as a potential tradeoff to the negative emission potential. Sci. Rep. 2016, 6, 35984.
- Chow, J.C.; Watson, J.G.; Lowenthal, D.H.; Solomon, P.A.; Magliano, K.L.; Ziman, S.D.; Willard Richards, L. PM10 source apportionment in California’s San Joaquin valley. Atmos. Environ. Part A Gen. Top. 1992, 26, 3335–3354.
- Madden, N.M.; Southard, R.J.; Mitchell, J.P. Soil Water Content and Soil Disaggregation by Disking Affects PM Emissions. J. Environ. Qual. 2009, 38, 36.
- Madden, N.M.; Southard, R.J.; Mitchell, J.P. Soil water and particle size distribution influence laboratory-generated PM10. Atmos. Environ. 2010, 44, 745–752.
- United States Environmental Protection Agency. Our Nations Air: Status and Trends through 2016. Available online: https://gispub.epa.gov/air/trendsreport/2017/#effects (accessed on 19 February 2019).
- Schenker, M. Exposures and health effects from inorganic agricultural dusts. Environ. Health Perspect. 2000, 108, 661–664.
- Schenker, M.B.; Pinkerton, K.E.; Mitchell, D.; Vallyathan, V.; Elvine-Kreis, B.; Green, F.H.Y. Pneumoconiosis from agricultural dust exposure among young California farmworkers. Environ. Health Perspect. 2009, 117, 988–994.
- Schenker, M.B.; Farrar, J.A.; Mitchell, D.C.; Green, R.S.; Samuels, S.J.; Lawson, R.J.; McCurdy, S.A. Agricultural dust exposure and respiratory symptoms among California farm operators. J. Occup. Environ. Med. 2005, 47, 1157–1166.
- Sigmund, G.; Huber, D.; Bucheli, T.D.; Baumann, M.; Borth, N.; Guebitz, G.M.; Hofmann, T. Cytotoxicity of biochar: A workplace safety concern? Environ. Sci. Technol. Lett. 2017, 4, 362–366.
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. THE DARK SIDE OF BLACK GOLD: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833.
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838.
- Liu, G.; Niu, Z.; Van Niekerk, D.; Xue, J.; Zheng, L. Polycyclic aromatic hydrocarbons (PAHs) from coal combustion: Emissions, analysis, and toxicology. Rev. Environ. Contam. Toxicol. 2008, 192, 1–28.
- Buss, W.; Graham, M.C.; MacKinnon, G.; Mašek, O. Strategies for producing biochars with minimum PAH contamination. J. Anal. Appl. Pyrolysis 2016, 119, 24–30.
- Freddo, A.; Cai, C.; Reid, B.J. Environmental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut. 2012, 171, 18–24.
- Shackley, S.; Carter, S.; Knowles, T.; Middelink, E.; Haefele, S.; Sohi, S.; Cross, A.; Haszeldine, S. Sustainable gasification-biochar systems? A case-study of rice-husk gasification in Cambodia, Part I: Context, chemical properties, environmental and health and safety issues. Energy Policy 2012, 42, 49–58.
- Schimmelpfennig, S.; Glaser, B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001.
- Oleszczuk, P.; Jo, I.; Ku, M. Biochar properties regarding to contaminants content and ecotoxicological assessment. J. Hazard. Mater. 2013, 260, 375–382.
- Hilber, I.; Blum, F.; Leifeld, J.; Schmidt, H.P.; Bucheli, T.D. Quantitative determination of PAHs in biochar: A prerequisite to ensure its quality and safe application. J. Agric. Food Chem. 2012, 60, 3042–3050.
- Keiluweit, M.; Kleber, M.; Sparrow, M.A.; Simoneit, B.R.T.; Prahl, F.G. Solvent-Extractable Polycyclic Aromatic Hydrocarbons in Biochar: Influence of Pyrolysis Temperature and Feedstock. Environ. Sci. Technol. 2012, 46, 9333–9341.
- Anderson, C.G.; Joshi, G.; Bair, D.A.; Oriol, C.; He, G.; Parikh, S.J.; Denison, M.S.; Scow, K.M. Use of nuclear receptor luciferase-based bioassays to detect endocrine active chemicals in a biosolids-biochar amended soil. Chemosphere 2017, 181, 160–167.
- Devi, P.; Saroha, A.K. Risk analysis of pyrolyzed biochar made from paper mill effluent treatment plant sludge for bioavailability and eco-toxicity of heavy metals. Bioresour. Technol. 2014, 162, 308–315.
- Visioli, G.; Conti, F.D.; Menta, C.; Bandiera, M.; Malcevschi, A.; Jones, D.L.; Vamerali, T. Assessing biochar ecotoxicology for soil amendment by root phytotoxicity bioassays. Environ. Monit. Assess. 2016, 188, 166.
- Buss, W.; Mašek, O. Mobile organic compounds in biochar—A potential source of contamination—Phytotoxic effects on cress seed (Lepidiumsativum) germination. J. Environ. Manag. 2014, 137, 111–119.
- Conesa, J.A.; Font, R.; Fullana, A.; Martín-Gullón, I.; Aracil, I.; Gálvez, A.; Moltó, J.; Gómez-Rico, M.F. Comparison between emissions from the pyrolysis and combustion of different wastes. J. Anal. Appl. Pyrolysis 2009, 84, 95–102.
- Gelardi, D.L.; Li, C.; Parikh, S.J. An emerging environmental concern: Biochar-induced dust emissions and their potentially toxic properties. Sci. Total Environ. 2019, 678, 813–820.
- Wang, J.; Xia, K.; Waigi, M.G.; Gao, Y.; Odinga, E.S.; Ling, W.; Liu, J. Application of biochar to soils may result in plant contamination and human cancer risk due to exposure of polycyclic aromatic hydrocarbons. Environ. Int. 2018, 121, 169–177.
- Wang, J.; Odinga, E.S.; Zhang, W.; Zhou, X.; Yang, B.; Waigi, M.G.; Gao, Y. Polyaromatic hydrocarbons in biochars and human health risks of food crops grown in biochar-amended soils: A synthesis study. Environ. Int. 2019, 130, 104899.
- Liu, X.; Ji, R.; Shi, Y.; Wang, F.; Chen, W. Release of polycyclic aromatic hydrocarbons from biochar fine particles in simulated lung fluids: Implications for bioavailability and risks of airborne aromatics. Sci. Total Environ. 2019, 655, 1159–1168.
- Oleszczuk, P. Changes of total and freely dissolved polycyclic aromatic hydrocarbons and toxicity of biochars treated with various aging. Environ. Pollut. 2018, 237, 65–73.
- Sigmund, G.; Bucheli, T.D.; Hilber, I.; Micić, V.; Kah, M.; Hofmann, T. Effect of ageing on the properties and polycyclic aromatic hydrocarbon composition of biochar. Environ. Sci. Process. Impacts 2017, 19, 768–774.
- Ko, J.H.; Wang, J.; Xu, Q. Impact of pyrolysis conditions on polycyclic aromatic hydrocarbons (PAHs) formation in particulate matter (PM) during sewage sludge pyrolysis. Chemosphere 2018, 208, 108–116.
- Madej, J.; Hilber, I.; Bucheli, T.D.; Oleszczuk, P. Biochars with low polycyclic aromatic hydrocarbon concentrations achievable by pyrolysis under high carrier gas flows irrespective of oxygen content or feedstock. J. Anal. Appl. Pyrolysis 2016, 122, 365–369.
- Dunnigan, L.; Morton, B.J.; Hall, P.A.; Kwong, C.W. Production of biochar and bioenergy from rice husk: Influence of feedstock drying on particulate matter and the associated polycyclic aromatic hydrocarbon emissions. Atmos. Environ. 2018, 190, 218–225.
- Kołtowski, M.; Oleszczuk, P. Toxicity of biochars after polycyclic aromatic hydrocarbons removal by thermal treatment. Ecol. Eng. 2015, 75, 79–85.
- Reza, M.T.; Lynam, J.G.; Vasquez, V.R.; Coronella, C.J. Pelletization of biochar from hydrothermally carbonized wood. Environ. Prog. Sustain. Energy 2012, 31, 225–234.
- Hu, Q.; Shao, J.; Yang, H.; Yao, D.; Wang, X.; Chen, H. Effects of binders on the properties of bio-char pellets. Appl. Energy 2015, 157, 508–516.
- European Biochar Foundation (EBC). Guidelines for a Sustainable Production of Biochar. Eur. Biochar Found. 2016, 6.1, 1–22.
- Silva, F.C.; Borrego, C.; Keizer, J.J.; Amorim, J.H.; Verheijen, F.G.A. Effects of moisture content on wind erosion thresholds of biochar. Atmos. Environ. 2015, 123, 121–128.
- Schwab, C.V.; Hanna, H.M. Master Gardeners’ safety precautions for handling, applying, and storing biochar. Agric. Biosyst. Eng. Ext. Outreach Publ. 2012, 5. Available online: https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=1005&context=abe_eng_extensionpubs (accessed on 1 June 2021).
- International Biochar Initiative (IBI). Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. Int. Biochar Initiat. 2015, 2.1, 1–23. Available online: http://www.biochar-international.org/characterizationstandard (accessed on 1 June 2021).
- International Biochar Initiative (IBI). Directory of IBI Certified Biochars. Available online: https://biochar-international.org/ibi-certified-biochars/ (accessed on 9 July 2021).
- European Biochar Foundation (EBC). EBC Producers. Available online: https://www.european-biochar.org/en/ct/9-EBC-Producer (accessed on 7 July 2021).
More
Information
Subjects:
Soil Science; Others; Optics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
18 Oct 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




