Biochar is most commonly considered for its use as a soil amendment, where it has gained attention for its potential to improve agricultural production and soil health. Twenty years of near exponential growth in investigation has demonstrated that biochar does not consistently deliver these benefits, due to variables in biochar, soil, climate, and cropping systems. While biochar can provide agronomic improvements in marginal soils, it is less likely to do so in temperate climates and fertile soils. Here, biochar and its coproducts may be better utilized for contaminant remediation or the substitution of nonrenewable or mining-intensive materials.
- biochar
- soil
- agriculture
- climate change
- remediation
- bioenergy
- coproducts
- sustainability
- carbon sequestration
- perspective
1. Introduction
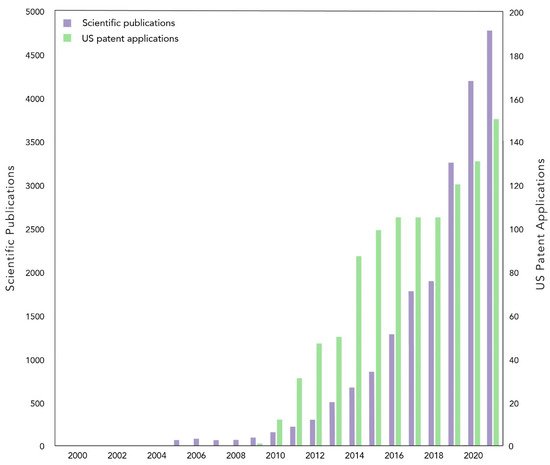
2. What Do We Really Know about Biochar?
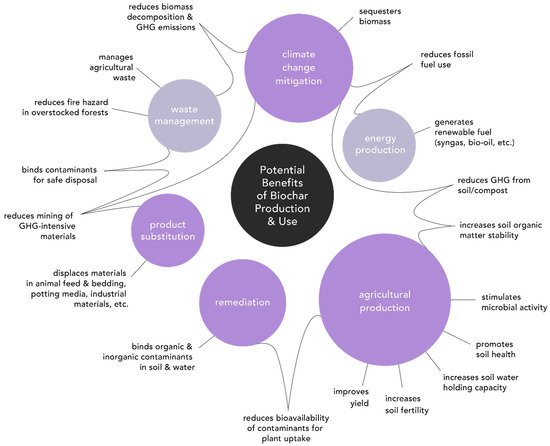
2.1. Agricultural Production
2.2. Climate Change Mitigation
2.3. Environmental Remediation
2.4. Product Substitution
2.5. Potential Consequences to the Widespread Use of Biochar
2.6. Strategies for Mitigating Consequences

3. How Can We Best Move Forward?
This entry is adapted from the peer-reviewed paper 10.3390/su131810079
References
- Web of Science Core Collection Database, “Biochar”. Available online: http://apps.webofknowledge.com (accessed on 1 June 2021).
- US Patent and Trademark Office Patent Application Full Text and Image Database. Available online: http://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=0&f=S&l=50&TERM1=biochar&FIELD1=&co1=AND&TERM2=&FIELD2=&d=PG01 (accessed on 20 May 2021).
- International Biochar Initiative (IBI). IBI July Newsletter. Available online: https://biochar-international.org/wp-content/uploads/2021/07/2021-7-IBI-Newsletter-English.pdf (accessed on 1 June 2021).
- Bradford, M.A.; Carey, C.J.; Atwood, L.; Bossio, D.; Fenichel, E.P.; Gennet, S.; Fargione, J.; Fisher, J.R.B.; Fuller, E.; Kane, D.A.; et al. Soil carbon science for policy and practice. Nat. Sustain. 2019, 2, 1070–1072.
- Baveye, P.C. Soil health at a crossroad. Soil Use Manag. 2021, 37, 215–219.
- De Coninck, H.; Revi, A.; Babiker, M.; Bertoldi, P.; Buckeridge, M.; Cartwright, A.; Dong, W.; Ford, J.; Fuss, S.; Hourcade, J.-C.; et al. Strengthening and implementing the global response. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; IPCC: Geneva, Switzerland, 2018.
- United States Biochar Initiative (USBI) Biochar Introduction. Available online: https://biochar-us.org/biochar-introduction (accessed on 6 August 2021).
- International Biochar Initiative (IBI) About Biochar. Available online: https://biochar-international.org/biochar/ (accessed on 1 June 2021).
- Baveye, P.C. Bypass and hyperbole in soil research: Worrisome practices critically reviewed through examples. Eur. J. Soil Sci. 2021, 72, 31–34.
- MacCarthy, D.S.; Darko, E.; Nartey, E.K.; Adiku, S.G.K.; Tettey, A. Integrating biochar and inorganic fertilizer improves productivity and profitability of irrigated rice in Ghana, West Africa. Agronomy 2020, 10, 904.
- Sadowska, U.; Domagała-Świątkiewicz, I.; Żabiński, A. Biochar and Its Effects on Plant–Soil Macronutrient Cycling during a Three-Year Field Trial on Sandy Soil with Peppermint (Mentha piperita L.). Part I: Yield and Macro Element Content in Soil and Plant Biomass. Agronomy 2020, 10, 1950.
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055.
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711.
- Jeffery, S.; Abalos, D.; Prodana, M.; Bastos, A.C.; van Groenigen, J.W.; Hungate, B.A.; Verheijen, F. Biochar boosts tropical but not temperate crop yields. Environ. Res. Lett. 2017, 12, 053001.
- Zhang, D.; Yan, M.; Niu, Y.; Liu, X.; van Zwieten, L.; Chen, D.; Bian, R.; Cheng, K.; Li, L.; Joseph, S.; et al. Is current biochar research addressing global soil constraints for sustainable agriculture? Agric. Ecosyst. Environ. 2016, 226, 25–32.
- United States Department of Agriculture Natural Resources Conservation Service (NRCS) Soil Carbon Amendment 808-CPS-1 (under Evaluation). Available online: https://www.nrcs.usda.gov/wps/cmis_proxy/https/ecm.nrcs.usda.gov%3A443/fncmis/resources/WEBP/ContentStream/idd_D01F2B77-0000-C211-A3DE-93A6410CE679/0/808+CPS+Soil+Carbon+Amendment+2021+-+DRAFT.pdf (accessed on 1 June 2021).
- Hassan, M.; Liu, Y.; Naidu, R.; Parikh, S.J.; Du, J.; Qi, F.; Willett, I.R. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis. Sci. Total Environ. 2020, 744, 140714.
- Downie, A.; Crosky, A.; Munroe, P. Characteristics of biochar—physical and structural properties. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 89–108.
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187.
- Oladele, S.O. Changes in physicochemical properties and quality index of an Alfisol after three years of rice husk biochar amendment in rainfed rice—Maize cropping sequence. Geoderma 2019, 353, 359–371.
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Zimmerman, A.R.; Pandit, B.H.; Cornelissen, G. Multi-year double cropping biochar field trials in Nepal: Finding the optimal biochar dose through agronomic trials and cost-benefit analysis. Sci. Total Environ. 2018, 637–638, 1333–1341.
- Guo, M. The 3R Principles for Applying Biochar to Improve Soil Health. Soil Syst. 2020, 4, 9.
- Major, J. Guidelines on Practical Aspects of Biochar Application to Field Soil in Various Soil Management Systems. Int. Biochar Initiat. 2010, 1, 1–23.
- Liu, S.; Zhang, Y.; Zong, Y.; Hu, Z.; Wu, S.; Zhou, J.; Jin, Y.; Zou, J. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. GCB Bioenergy 2016, 8, 392–406.
- Zhang, L.; Jing, Y.; Xiang, Y.; Zhang, R.; Lu, H. Responses of soil microbial community structure changes and activities to biochar addition: A meta-analysis. Sci. Total Environ. 2018, 643, 926–935.
- Bonanomi, G.; Ippolito, F.; Scala, F. A “black” future for plant pathology? Biochar as a new soil amendment for controlling plant diseases. J. Plant Pathol. 2015, 97, 223–234.
- Solaiman, Z.M.; Shafi, M.I.; Beamont, E.; Anawar, H.M. Poultry litter biochar increases mycorrhizal colonisation, soil fertility and cucumber yield in a fertigation system on sandy soil. Agriculture 2020, 10, 480.
- Nan, Q.; Wang, C.; Wang, H.; Yi, Q.; Liang, B.; Xu, J.; Wu, W. Biochar drives microbially-mediated rice production by increasing soil carbon. J. Hazard. Mater. 2020, 387, 121680.
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124.
- McDonald, M.R.; Bakker, C.; Motior, M.R. Evaluation of wood biochar and compost soil amendment on cabbage yield and quality. Can. J. Plant. Sci. 2019, 99, 624–638.
- Griffin, D.E.; Wang, D.; Parikh, S.J.; Scow, K.M. Short-lived effects of walnut shell biochar on soils and crop yields in a long-term field experiment. Agric. Ecosyst. Environ. 2017, 236, 21–29.
- Gajić, A.; Koch, H.-J. Sugar Beet (Beta vulgaris L.) Growth Reduction Caused by Hydrochar Is Related to Nitrogen Supply. J. Environ. Qual. 2012, 41, 1067–1075.
- Liu, X.; Zhou, J.; Chi, Z.; Zheng, J.; Li, L.; Zhang, X.; Zheng, J.; Cheng, K.; Bian, R.; Pan, G. Biochar provided limited benefits for rice yield and greenhouse gas mitigation six years following an amendment in a fertile rice paddy. Catena 2019, 179, 20–28.
- Cornelissen, G.; Martinsen, V.; Shitumbanuma, V.; Alling, V.; Breedveld, G.; Rutherford, D.; Sparrevik, M.; Hale, S.; Obia, A.; Mulder, J. Biochar Effect on Maize Yield and Soil Characteristics in Five Conservation Farming Sites in Zambia. Agronomy 2013, 3, 256–274.
- Backer, R.G.M.; Schwinghamer, T.D.; Whalen, J.K.; Seguin, P.; Smith, D.L. Crop yield and SOC responses to biochar application were dependent on soil texture and crop type in southern Quebec, Canada. J. Plant Nutr. Soil Sci. 2016, 179, 399–408.
- Omara, P.; Aula, L.; Oyebiyi, F.B.; Eickhof, E.M.; Carpenter, J.; Raun, W.R. Biochar application in combination with inorganic nitrogen improves maize grain yield, nitrogen uptake, and use efficiency in Temperate Soils. Agronomy 2020, 10, 1241.
- Hestrin, R.; Torres-Rojas, D.; Dynes, J.J.; Hook, J.M.; Regier, T.Z.; Gillespie, A.W.; Smernik, R.J.; Lehmann, J. Fire-derived organic matter retains ammonia through covalent bond formation. Nat. Commun. 2019, 10, 664.
- Sun, Y.; Yang, J.; Yao, R.; Chen, X.; Wang, X. Biochar and fulvic acid amendments mitigate negative effects of coastal saline soil and improve crop yields in a three year field trial. Sci. Rep. 2020, 10, 8946.
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar Mitigates Salinity Stress in Potato. J. Agron. Crop. Sci. 2015, 201, 368–378.
- Mahmoud, E.; El-Beshbeshy, T.; El-Kader, N.A.; El Shal, R.; Khalafallah, N. Impacts of biochar application on soil fertility, plant nutrients uptake and maize (Zea mays L.) yield in saline sodic soil. Arab. J. Geosci. 2019, 12, 719.
- Zhang, M.; Song, G.; Gelardi, D.L.; Huang, L.; Khan, E.; Parikh, S.J.; Ok, Y.S.; Song, G.; Gelardi, D.L.; Huang, L.; et al. Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res. 2020, in press.
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.W.; Conte, P.; Stephen, J. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080.
- Haider, G.; Steffens, D.; Müller, C.; Kammann, C.I. Standard Extraction Methods May Underestimate Nitrate Stocks Captured by Field-Aged Biochar. J. Environ. Qual. 2016, 45, 1196–1204.
- Haider, G.; Joseph, S.; Steffens, D.; Müller, C.; Taherymoosavi, S.; Mitchell, D.; Kammann, C.I. Mineral nitrogen captured in field-aged biochar is plant-available. Sci. Rep. 2020, 10, 13816.
- Ye, L.; Camps-Arbestain, M.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar effects on crop yields with and without fertilizer: A meta-analysis of field studies using separate controls. Soil Use Manag. 2020, 36, 2–18.
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164.
- Hagemann, N.; Kammann, C.I.; Schmidt, H.P.; Kappler, A.; Behrens, S. Nitrate capture and slow release in biochar amended compost and soil. PLoS ONE 2017, 12, e0171214.
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678.
- Kerré, B.; Willaert, B.; Cornelis, Y.; Smolders, E. Long-term presence of charcoal increases maize yield in Belgium due to increased soil water availability. Eur. J. Agron. 2017, 91, 10–15.
- Nawaz, H.; Hussain, N.; Anjum, M.A.; Rehman, H.U.; Jamil, M.; Aown, M.; Raza, S.; Farooq, O. Biochar Application Improves the Wheat Productivity under Different Irrigation Water-Regimes. Int. J. Agric. Biol. 2019, 21, 936–942.
- Madari, B.E.; Silva, M.A.S.; Carvalho, M.T.M.; Maia, A.H.N.; Petter, F.A.; Santos, J.L.S.; Tsai, S.M.; Leal, W.G.O.; Zeviani, W.M. Properties of a sandy clay loam Haplic Ferralsol and soybean grain yield in a five-year field trial as affected by biochar amendment. Geoderma 2017, 305, 100–112.
- Nelissen, V.; Ruysschaert, G.; Mankaabusi, D.; D’Hose, T.; De Beuf, K.; Al-Barri, B.; Cornelis, W.; Boeckx, P. Impact of a woody biochar on properties of a sandy loam soil and spring barley during a two-year field experiment. Eur. J. Agron. 2015, 62, 65–78.
- Glaser, B.; Lehr, V.I. Biochar effects on phosphorus availability in agricultural soils: A meta-analysis. Sci. Rep. 2019, 9, 9338.
- Hale, S.E.; Nurida, N.L.; Jubaedah; Mulder, J.; Sørmo, E.; Silvani, L.; Abiven, S.; Joseph, S.; Taherymoosavi, S.; Cornelissen, G. The effect of biochar, lime and ash on maize yield in a long-term field trial in a Ultisol in the humid tropics. Sci. Total Environ. 2020, 719, 137455.
- Raboin, L.M.; Razafimahafaly, A.H.D.; Rabenjarisoa, M.B.; Rabary, B.; Dusserre, J.; Becquer, T. Improving the fertility of tropical acid soils: Liming versus biochar application? A long term comparison in the highlands of Madagascar. Field Crop. Res. 2016, 199, 99–108.
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287.
- Almaroai, Y.A.; Eissa, M.A. Effect of biochar on yield and quality of tomato grown on a metal-contaminated soil. Sci. Hortic. Amst. 2020, 265, 109210.
- Wang, D.; Fonte, S.J.; Parikh, S.J.; Six, J.; Scow, K.M. Biochar additions can enhance soil structure and the physical stabilization of C in aggregates. Geoderma 2017, 303, 110–117.
- Liu, X.H.; Han, F.P.; Zhang, X.C. Effect of biochar on soil aggregates in the Loess Plateau: Results from incubation experiments. Int. J. Agric. Biol. 2012, 14, 975–979.
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.W.; Kwon, E.E.; Lee, J.; Wang, C.H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253.
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523.
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303.
- Ibarrola, R.; Shackley, S.; Hammond, J. Pyrolysis biochar systems for recovering biodegradable materials: A life cycle carbon assessment. Waste Manag. 2012, 32, 859–868.
- Gaunt, J.L.; Lehmann, J. Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ. Sci. Technol. 2008, 42, 4152–4158.
- Sparrevik, M.; Field, J.L.; Martinsen, V.; Breedveld, G.D.; Cornelissen, G. Life cycle assessment to evaluate the environmental impact of biochar implementation in conservation agriculture in Zambia. Environ. Sci. Technol. 2013, 47, 1206–1215.
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life Cycle Assessment of Biochar Systems: Estimating the Energetic, Economic, and Climate Change Potential. Environ. Sci. Technol. 2010, 44, 827–833.
- Nguyen, T.L.T.; Hermansen, J.E.; Nielsen, R.G. Environmental assessment of gasification technology for biomass conversion to energy in comparison with other alternatives: The case of wheat straw. J. Clean. Prod. 2013, 53, 138–148.
- Dutta, B.; Raghavan, V. A life cycle assessment of environmental and economic balance of biochar systems in Quebec. Int. J. Energy Environ. Eng. 2014, 5, 106.
- Matuštík, J.; Hnátková, T.; Kočí, V. Life cycle assessment of biochar-to-soil systems: A review. J. Clean. Prod. 2020, 259, 120998.
- El-Naggar, A.; Awad, Y.M.; Tang, X.Y.; Liu, C.; Niazi, N.K.; Jien, S.H.; Tsang, D.C.W.; Song, H.; Ok, Y.S.; Lee, S.S. Biochar influences soil carbon pools and facilitates interactions with soil: A field investigation. Land Degrad. Dev. 2018, 29, 2162–2171.
- Hardy, B.; Dufey, J.E. The resistance of centennial soil charcoal to the “Walkley-Black” oxidation. Geoderma 2017, 303, 37–43.
- Lim, B.; Cachier, H. Determination of black carbon by chemical oxidation and thermal treatment in recent marine and lake sediments and Cretaceous-Tertiary clays. Chem. Geol. 1996, 131, 143–154.
- Glaser, B.; Haumaier, L.; Guggenberger, G.; Zech, W. Black carbon in soils: The use of benzenecarboxylic acids as specific markers. Org. Geochem. 1998, 29, 811–819.
- Wiedemeier, D.B.; Hilf, M.D.; Smittenberg, R.H.; Haberle, S.G.; Schmidt, M.W.I. Improved assessment of pyrogenic carbon quantity and quality in environmental samples by high-performance liquid chromatography. J. Chromatogr. A 2013, 1304, 246–250.
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22.
- Bååth, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963.
- Soinne, H.; Hovi, J.; Tammeorg, P.; Turtola, E. Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 2014, 219–220, 162–167.
- Jastrow, D. Soil Aggregate Formation and the Accrual of Particulate and Mineral-Associated Organic Matter. Soil Biol. Biochem. 1996, 28, 665–676.
- Oades, J.M. Soil organic matter and structural stability: Mechanisms and implications for management. Plant. Soil 1984, 76, 319–337.
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial utilisation of biochar-derived carbon. Sci. Total Environ. 2013, 465, 288–297.
- Ameloot, N.; Sleutel, S.; Case, S.D.C.; Alberti, G.; McNamara, N.P.; Zavalloni, C.; Vervisch, B.; delle Vedove, G.; De Neve, S. C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biol. Biochem. 2014, 78, 195–203.
- Hardy, B.; Sleutel, S.; Dufey, J.E.; Cornelis, J.-T. The Long-Term Effect of Biochar on Soil Microbial Abundance, Activity and Community Structure Is Overwritten by Land Management. Front. Environ. Sci. 2019, 7.
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364.
- Sha, Z.; Li, Q.; Lv, T.; Misselbrook, T.; Liu, X. Response of ammonia volatilization to biochar addition: A meta-analysis. Sci. Total Environ. 2019, 655, 1387–1396.
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732.
- Krause, H.-M.; Hüppi, R.; Leifeld, J.; El-Hadidi, M.; Harter, J.; Kappler, A.; Hartmann, M.; Behrens, S.; Mäder, P.; Gattinger, A. Biochar affects community composition of nitrous oxide reducers in a field experiment. Soil Biol. Biochem. 2018, 119, 143–151.
- Akdeniz, N. A systematic review of biochar use in animal waste composting. Waste Manag. 2019, 88, 291–300.
- Sánchez-Monedero, M.A.; Sánchez-García, M.; Alburquerque, J.A.; Cayuela, M.L. Biochar reduces volatile organic compounds generated during chicken manure composting. Bioresour. Technol. 2019, 288, 121584.
- Schmidt, H.P.; Hagemann, N.; Draper, K.; Kammann, C. The use of biochar in animal feeding. PeerJ 2019, 2019, e7373.
- California Food and Agricultural Code Assembly Bill No. 2511. Available online: https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=201520160AB2511 (accessed on 1 June 2021).
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: A review of in situ field trials. Sci. Total Environ. 2018, 619–620, 815–826.
- Wang, Y.; Wang, H.-S.; Tang, C.-S.; Gu, K.; Shi, B. Remediation of heavy-metal-contaminated soils by biochar: A review. Environ. Geotech. 2020, 252.
- Liu, Y.; Lonappan, L.; Brar, S.K.; Yang, S. Impact of biochar amendment in agricultural soils on the sorption, desorption, and degradation of pesticides: A review. Sci. Total Environ. 2018, 645, 60–70.
- Yavari, S.; Malakahmad, A.; Sapari, N.B. Biochar efficiency in pesticides sorption as a function of production variables—A review. Environ. Sci. Pollut. Res. 2015, 22, 13824–13841.
- Zhang, H.; Lin, K.; Wang, H.; Gan, J. Effect of Pinus radiata derived biochars on soil sorption and desorption of phenanthrene. Environ. Pollut. 2010, 158, 2821–2825.
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33.
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X. The role of tailored biochar in increasing plant growth, and reducing bioavailability, phytotoxicity, and uptake of heavy metals in contaminated soil. Environ. Pollut. 2017, 230, 329–338.
- Kumari, I.; Moldrup, P.; Paradelo, M.; de Jonge, L. Phenanthrene sorption on biochar-amended soils: Application rate, aging, and physicochemical properties of soil. Water Air Soil Pollut. 2014, 225, 2105.
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27.
- Ngigi, A.N.; Ok, Y.S.; Thiele-Bruhn, S. Biochar-mediated sorption of antibiotics in pig manure. J. Hazard. Mater. 2019, 364, 663–670.
- Liyanage, A.S.; Canaday, S.; Pittman, C.U.; Mlsna, T. Rapid remediation of pharmaceuticals from wastewater using magnetic Fe3O4/Douglas fir biochar adsorbents. Chemosphere 2020, 258, 127336.
- Bair, D.A.; Mukome, F.N.D.; Popova, I.E.; Ogunyoku, T.A.; Jefferson, A.; Wang, D.; Hafner, S.C.; Young, T.M.; Parikh, S.J. Sorption of Pharmaceuticals, Heavy Metals, and Herbicides to Biochar in the Presence of Biosolids. J. Environ. Qual. 2016, 45, 1998.
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Natasha; Asif Naeem, M.; Niazi, N.K. A critical review of different factors governing the fate of pesticides in soil under biochar application. Sci. Total Environ. 2020, 711, 134645.
- Nguyen, H.N.; Pignatello, J.J. Laboratory tests of biochars as absorbents for use in recovery or containment of marine crude oil spills. Environ. Eng. Sci. 2013, 30, 374–380.
- Xiang, L.; Sheng, H.; Gu, C.; Marc, R.-G.; Wang, Y.; Bian, Y.; Jiang, X.; Wang, F. Biochar combined with compost to reduce the mobility, bioavailability and plant uptake of 2,2’,4,4’-tetrabrominated diphenyl ether in soil. J. Hazard. Mater. 2019, 374, 341–348.
- Bair, D.A.; Anderson, C.G.; Chung, Y.; Scow, K.M.; Franco, R.B.; Parikh, S.J. Impact of biochar on plant growth and uptake of ciprofloxacin, triclocarban and triclosan from biosolids. J. Environ. Sci. Health Part. B Pestic. Food Contam. Agric. Wastes 2020, 55, 990–1001.
- Gomez-Eyles, J.L.; Sizmur, T.; Collins, C.D.; Hodson, M.E. Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ. Pollut. 2011, 159, 616–622.
- Qin, G.; Gong, D.; Fan, M.Y. Bioremediation of petroleum-contaminated soil by biostimulation amended with biochar. Int. Biodeterior. Biodegrad. 2013, 85, 150–155.
- Mukome, F.N.D.; Buelow, M.C.; Shang, J.; Peng, J.; Rodriguez, M.; Mackay, D.M.; Pignatello, J.J.; Sihota, N.; Hoelen, T.P.; Parikh, S.J. Biochar amendment as a remediation strategy for surface soils impacted by crude oil. Environ. Pollut. 2020, 265, 115006.
- Singh, C.; Tiwari, S.; Singh, J.S. Biochar: A Sustainable Tool in Soil Pollutant Bioremediation BT—Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2020; pp. 475–494. ISBN 978-981-13-3426-9.
- Wang, S.; Kwak, J.-H.; Islam, M.S.; Naeth, M.A.; Gamal El-Din, M.; Chang, S.X. Biochar surface complexation and Ni(II), Cu(II), and Cd(II) adsorption in aqueous solutions depend on feedstock type. Sci. Total Environ. 2020, 712, 136538.
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478.
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant. Soil 2011, 348, 439.
- Bandara, T.; Herath, I.; Kumarathilaka, P.; Hseu, Z.-Y.; Ok, Y.S.; Vithanage, M. Efficacy of woody biomass and biochar for alleviating heavy metal bioavailability in serpentine soil. Environ. Geochem. Health 2017, 39, 391–401.
- Visconti, D.; Álvarez-Robles, M.J.; Fiorentino, N.; Fagnano, M.; Clemente, R. Use of Brassica juncea and Dactylis glomerata for the phytostabilization of mine soils amended with compost or biochar. Chemosphere 2020, 260, 127661.
- Prapagdee, S.; Piyatiratitivorakul, S.; Petsom, A.; Tawinteung, N. Application of Biochar for Enhancing Cadmium and Zinc Phytostabilization in Vigna radiata L. Cultivation. Water Air Soil Pollut. 2014, 225, 2233.
- Lebrun, M.; Miard, F.; Nandillon, R.; Léger, J.-C.; Hattab-Hambli, N.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted phytostabilization of a multicontaminated mine technosol using biochar amendment: Early stage evaluation of biochar feedstock and particle size effects on As and Pb accumulation of two Salicaceae species (Salix viminalis and Populus euramericana). Chemosphere 2018, 194, 316–326.
- Sigua, G.C.; Novak, J.M.; Watts, D.W.; Ippolito, J.A.; Ducey, T.F.; Johnson, M.G.; Spokas, K.A. Phytostabilization of Zn and Cd in mine soil using corn in combination with biochars and manure-based compost. Environment 2019, 6, 69.
- Wan, X.; Li, C.; Parikh, S.J. Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ. Pollut. 2020, 261, 114157.
- Wu, J.; Li, Z.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010.
- Lu, H.P.; Li, Z.A.; Gascó, G.; Méndez, A.; Shen, Y.; Paz-Ferreiro, J. Use of magnetic biochars for the immobilization of heavy metals in a multi-contaminated soil. Sci. Total Environ. 2018, 622–623, 892–899.
- Liu, Y.; Huang, J.; Xu, H.; Zhang, Y.; Hu, T.; Chen, W.; Hu, H.; Wu, J.; Li, Y.; Jiang, G. A magnetic macro-porous biochar sphere as vehicle for the activation and removal of heavy metals from contaminated agricultural soil. Chem. Eng. J. 2020, 390, 124638.
- Yang, H.; Ye, S.; Zeng, Z.; Zeng, G.; Tan, X.; Xiao, R.; Wang, J.; Song, B.; Du, L.; Qin, M.; et al. Utilization of biochar for resource recovery from water: A review. Chem. Eng. J. 2020, 397, 125502.
- Kamali, M.; Appels, L.; Kwon, E.E.; Aminabhavi, T.M.; Dewil, R. Biochar in water and wastewater treatment—A sustainability assessment. Chem. Eng. J. 2021, 420, 129946.
- Deng, S.; Chen, J.; Chang, J. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: Performance and ecological benefits. J. Clean. Prod. 2021, 293, 126156.
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309.
- Siddiq, M.O.; Tawabini, B.; Kirmizakis, P.; Kalderis, D.; Ntarlagiannis, D.; Soupios, P. Combining geophysics and material science for environmental remediation: Real-time monitoring of Fe-biochar arsenic wastewater treatment. Chemosphere 2021, 284, 131390.
- Fang, C.; Zhang, T.; Li, P.; Jiang, R.F.; Wang, Y.C. Application of magnesium modified corn biochar for phosphorus removal and recovery from swine wastewater. Int. J. Environ. Res. Public Health 2014, 11, 9217–9237.
- Wang, Z.; Bakshi, S.; Li, C.; Parikh, S.J.; Hsieh, H.-S.; Pignatello, J.J. Modification of pyrogenic carbons for phosphate sorption through binding of a cationic polymer. J. Colloid Interface Sci. 2020, 579, 258–268.
- Fang, Q.; Ye, S.; Yang, H.; Yang, K.; Zhou, J.; Gao, Y.; Lin, Q.; Tan, X.; Yang, Z. Application of layered double hydroxide-biochar composites in wastewater treatment: Recent trends, modification strategies, and outlook. J. Hazard. Mater. 2021, 420, 126569.
- Vu, T.M.; Trinh, V.T.; Doan, D.P.; Van, H.T.; Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H. Removing ammonium from water using modified corncob-biochar. Sci. Total Environ. 2017, 579, 612–619.
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.R.; Nidheesh, P.V.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656.
- Nemati, M.R.; Simard, F.; Fortin, J.-P.; Beaudoin, J. Potential Use of Biochar in Growing Media. Vadose Zone J. 2015, 14, vzj2014.06.0074.
- Headlee, W.L.; Brewer, C.E.; Hall, R.B. Biochar as a Substitute for Vermiculite in Potting Mix for Hybrid Poplar. Bioenergy Res. 2014, 7, 120–131.
- Margenot, A.J.; Gri, D.E.; Alves, B.S.Q.; Rippner, D.A.; Li, C.; Parikh, S.J. Substitution of peat moss with softwood biochar for soil-free marigold growth. Ind. Crop. Prod. 2018, 112, 160–169.
- Yan, J.; Yu, P.; Liu, C.; Li, Q.; Gu, M. Replacing peat moss with mixed hardwood biochar as container substrates to produce five types of mint (Mentha spp.). Ind. Crop. Prod. 2020, 155, 112820.
- Fryda, L.; Visser, R.; Schmidt, J. Biochar replaces peat in horticulture: Environmental impact assessment of combined biochar & bioenergy production. Detritus 2019, 5, 132–149.
- Bridgham, S.D.; Patrick Megonigal, J.; Keller, J.K.; Bliss, N.B.; Trettin, C. The carbon balance of North American wetlands. Wetlands 2006, 26, 889–916.
- Clarkson, B.R.; Ausseil, A.E.; Gerbeaux, P. Wetland Ecosystems Services. In Ecosystem Services in New Zealand: Conditions and Trends; Dymond, J.R., Ed.; Manaaki Whenua Press, Landcare Research: Lincoln, New Zealand, 2013; pp. 192–202.
- Cleary, J.; Roulet, N.T.; Moore, T.R. Greenhouse gas emissions from Canadian peat extraction, 1990–2000: A life-cycle analysis. Ambio 2005, 34, 456–461.
- Głodowska, M.; Husk, B.; Schwinghamer, T.; Smith, D. Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad. Agron. Sustain. Dev. 2016, 36, 21.
- Kalus, K.; Koziel, J.A.; Opaliński, S. A review of biochar properties and their utilization in crop agriculture and livestock production. Appl. Sci. 2019, 9, 3494.
- Vaughn, S.F.; Winkler-Moser, J.K.; Berhow, M.A.; Byars, J.A.; Liu, S.X.; Jackson, M.A.; Peterson, S.C.; Eller, F.J. An odor-reducing, low dust-forming, clumping cat litter produced from Eastern red cedar (Juniperus virginiana L.) wood fibers and biochar1. Ind. Crop. Prod. 2020, 147, 112224.
- Flores, K.R.; Fahrenholz, A.; Grimes, J.L. Effect of pellet quality and biochar litter amendment on male turkey performance. Poult. Sci. 2021, 100, 101002.
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159.
- Schmidt, H.-P. 55 Uses of Biochar. Ithaka J. 2012, 25, 13–25.
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A review of non-soil biochar applications. Materials 2020, 13, 261.
- Guerranti, C.; Martellini, T.; Perra, G.; Scopetani, C.; Cincinelli, A. Microplastics in cosmetics: Environmental issues and needs for global bans. Environ. Toxicol. Pharmacol. 2019, 68, 75–79.
- Genesio, L.; Vaccari, F.P.; Miglietta, F. Black carbon aerosol from biochar threats its negative emission potential. Glob. Chang. Biol. 2016, 22, 2313–2314.
- Li, C.; Bair, D.A.; Parikh, S.J. Estimating potential dust emissions from biochar amended soils under simulated tillage. Sci. Total Environ. 2018, 625, 1093–1101.
- Ravi, S.; Sharratt, B.S.; Li, J.; Olshevski, S.; Meng, Z.; Zhang, J. Particulate matter emissions from biochar-amended soils as a potential tradeoff to the negative emission potential. Sci. Rep. 2016, 6, 35984.
- Chow, J.C.; Watson, J.G.; Lowenthal, D.H.; Solomon, P.A.; Magliano, K.L.; Ziman, S.D.; Willard Richards, L. PM10 source apportionment in California’s San Joaquin valley. Atmos. Environ. Part A Gen. Top. 1992, 26, 3335–3354.
- Madden, N.M.; Southard, R.J.; Mitchell, J.P. Soil Water Content and Soil Disaggregation by Disking Affects PM Emissions. J. Environ. Qual. 2009, 38, 36.
- Madden, N.M.; Southard, R.J.; Mitchell, J.P. Soil water and particle size distribution influence laboratory-generated PM10. Atmos. Environ. 2010, 44, 745–752.
- United States Environmental Protection Agency. Our Nations Air: Status and Trends through 2016. Available online: https://gispub.epa.gov/air/trendsreport/2017/#effects (accessed on 19 February 2019).
- Schenker, M. Exposures and health effects from inorganic agricultural dusts. Environ. Health Perspect. 2000, 108, 661–664.
- Schenker, M.B.; Pinkerton, K.E.; Mitchell, D.; Vallyathan, V.; Elvine-Kreis, B.; Green, F.H.Y. Pneumoconiosis from agricultural dust exposure among young California farmworkers. Environ. Health Perspect. 2009, 117, 988–994.
- Schenker, M.B.; Farrar, J.A.; Mitchell, D.C.; Green, R.S.; Samuels, S.J.; Lawson, R.J.; McCurdy, S.A. Agricultural dust exposure and respiratory symptoms among California farm operators. J. Occup. Environ. Med. 2005, 47, 1157–1166.
- Sigmund, G.; Huber, D.; Bucheli, T.D.; Baumann, M.; Borth, N.; Guebitz, G.M.; Hofmann, T. Cytotoxicity of biochar: A workplace safety concern? Environ. Sci. Technol. Lett. 2017, 4, 362–366.
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. THE DARK SIDE OF BLACK GOLD: Ecotoxicological aspects of biochar and biochar-amended soils. J. Hazard. Mater. 2021, 403, 123833.
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838.
- Liu, G.; Niu, Z.; Van Niekerk, D.; Xue, J.; Zheng, L. Polycyclic aromatic hydrocarbons (PAHs) from coal combustion: Emissions, analysis, and toxicology. Rev. Environ. Contam. Toxicol. 2008, 192, 1–28.
- Buss, W.; Graham, M.C.; MacKinnon, G.; Mašek, O. Strategies for producing biochars with minimum PAH contamination. J. Anal. Appl. Pyrolysis 2016, 119, 24–30.
- Freddo, A.; Cai, C.; Reid, B.J. Environmental contextualisation of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut. 2012, 171, 18–24.
- Shackley, S.; Carter, S.; Knowles, T.; Middelink, E.; Haefele, S.; Sohi, S.; Cross, A.; Haszeldine, S. Sustainable gasification-biochar systems? A case-study of rice-husk gasification in Cambodia, Part I: Context, chemical properties, environmental and health and safety issues. Energy Policy 2012, 42, 49–58.
- Schimmelpfennig, S.; Glaser, B. One Step Forward toward Characterization: Some Important Material Properties to Distinguish Biochars. J. Environ. Qual. 2012, 41, 1001.
- Oleszczuk, P.; Jo, I.; Ku, M. Biochar properties regarding to contaminants content and ecotoxicological assessment. J. Hazard. Mater. 2013, 260, 375–382.
- Hilber, I.; Blum, F.; Leifeld, J.; Schmidt, H.P.; Bucheli, T.D. Quantitative determination of PAHs in biochar: A prerequisite to ensure its quality and safe application. J. Agric. Food Chem. 2012, 60, 3042–3050.
- Keiluweit, M.; Kleber, M.; Sparrow, M.A.; Simoneit, B.R.T.; Prahl, F.G. Solvent-Extractable Polycyclic Aromatic Hydrocarbons in Biochar: Influence of Pyrolysis Temperature and Feedstock. Environ. Sci. Technol. 2012, 46, 9333–9341.
- Anderson, C.G.; Joshi, G.; Bair, D.A.; Oriol, C.; He, G.; Parikh, S.J.; Denison, M.S.; Scow, K.M. Use of nuclear receptor luciferase-based bioassays to detect endocrine active chemicals in a biosolids-biochar amended soil. Chemosphere 2017, 181, 160–167.
- Devi, P.; Saroha, A.K. Risk analysis of pyrolyzed biochar made from paper mill effluent treatment plant sludge for bioavailability and eco-toxicity of heavy metals. Bioresour. Technol. 2014, 162, 308–315.
- Visioli, G.; Conti, F.D.; Menta, C.; Bandiera, M.; Malcevschi, A.; Jones, D.L.; Vamerali, T. Assessing biochar ecotoxicology for soil amendment by root phytotoxicity bioassays. Environ. Monit. Assess. 2016, 188, 166.
- Buss, W.; Mašek, O. Mobile organic compounds in biochar—A potential source of contamination—Phytotoxic effects on cress seed (Lepidiumsativum) germination. J. Environ. Manag. 2014, 137, 111–119.
- Conesa, J.A.; Font, R.; Fullana, A.; Martín-Gullón, I.; Aracil, I.; Gálvez, A.; Moltó, J.; Gómez-Rico, M.F. Comparison between emissions from the pyrolysis and combustion of different wastes. J. Anal. Appl. Pyrolysis 2009, 84, 95–102.
- Gelardi, D.L.; Li, C.; Parikh, S.J. An emerging environmental concern: Biochar-induced dust emissions and their potentially toxic properties. Sci. Total Environ. 2019, 678, 813–820.
- Wang, J.; Xia, K.; Waigi, M.G.; Gao, Y.; Odinga, E.S.; Ling, W.; Liu, J. Application of biochar to soils may result in plant contamination and human cancer risk due to exposure of polycyclic aromatic hydrocarbons. Environ. Int. 2018, 121, 169–177.
- Wang, J.; Odinga, E.S.; Zhang, W.; Zhou, X.; Yang, B.; Waigi, M.G.; Gao, Y. Polyaromatic hydrocarbons in biochars and human health risks of food crops grown in biochar-amended soils: A synthesis study. Environ. Int. 2019, 130, 104899.
- Liu, X.; Ji, R.; Shi, Y.; Wang, F.; Chen, W. Release of polycyclic aromatic hydrocarbons from biochar fine particles in simulated lung fluids: Implications for bioavailability and risks of airborne aromatics. Sci. Total Environ. 2019, 655, 1159–1168.
- Oleszczuk, P. Changes of total and freely dissolved polycyclic aromatic hydrocarbons and toxicity of biochars treated with various aging. Environ. Pollut. 2018, 237, 65–73.
- Sigmund, G.; Bucheli, T.D.; Hilber, I.; Micić, V.; Kah, M.; Hofmann, T. Effect of ageing on the properties and polycyclic aromatic hydrocarbon composition of biochar. Environ. Sci. Process. Impacts 2017, 19, 768–774.
- Ko, J.H.; Wang, J.; Xu, Q. Impact of pyrolysis conditions on polycyclic aromatic hydrocarbons (PAHs) formation in particulate matter (PM) during sewage sludge pyrolysis. Chemosphere 2018, 208, 108–116.
- Madej, J.; Hilber, I.; Bucheli, T.D.; Oleszczuk, P. Biochars with low polycyclic aromatic hydrocarbon concentrations achievable by pyrolysis under high carrier gas flows irrespective of oxygen content or feedstock. J. Anal. Appl. Pyrolysis 2016, 122, 365–369.
- Dunnigan, L.; Morton, B.J.; Hall, P.A.; Kwong, C.W. Production of biochar and bioenergy from rice husk: Influence of feedstock drying on particulate matter and the associated polycyclic aromatic hydrocarbon emissions. Atmos. Environ. 2018, 190, 218–225.
- Kołtowski, M.; Oleszczuk, P. Toxicity of biochars after polycyclic aromatic hydrocarbons removal by thermal treatment. Ecol. Eng. 2015, 75, 79–85.
- Reza, M.T.; Lynam, J.G.; Vasquez, V.R.; Coronella, C.J. Pelletization of biochar from hydrothermally carbonized wood. Environ. Prog. Sustain. Energy 2012, 31, 225–234.
- Hu, Q.; Shao, J.; Yang, H.; Yao, D.; Wang, X.; Chen, H. Effects of binders on the properties of bio-char pellets. Appl. Energy 2015, 157, 508–516.
- European Biochar Foundation (EBC). Guidelines for a Sustainable Production of Biochar. Eur. Biochar Found. 2016, 6.1, 1–22.
- Silva, F.C.; Borrego, C.; Keizer, J.J.; Amorim, J.H.; Verheijen, F.G.A. Effects of moisture content on wind erosion thresholds of biochar. Atmos. Environ. 2015, 123, 121–128.
- Schwab, C.V.; Hanna, H.M. Master Gardeners’ safety precautions for handling, applying, and storing biochar. Agric. Biosyst. Eng. Ext. Outreach Publ. 2012, 5. Available online: https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=1005&context=abe_eng_extensionpubs (accessed on 1 June 2021).
- International Biochar Initiative (IBI). Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. Int. Biochar Initiat. 2015, 2.1, 1–23. Available online: http://www.biochar-international.org/characterizationstandard (accessed on 1 June 2021).
- International Biochar Initiative (IBI). Directory of IBI Certified Biochars. Available online: https://biochar-international.org/ibi-certified-biochars/ (accessed on 9 July 2021).
- European Biochar Foundation (EBC). EBC Producers. Available online: https://www.european-biochar.org/en/ct/9-EBC-Producer (accessed on 7 July 2021).
