
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Diaconeasa Zorita | + 4683 word(s) | 4683 | 2021-09-27 11:07:14 | | | |
| 2 | Peter Tang | Meta information modification | 4683 | 2021-09-28 04:21:56 | | |
Video Upload Options
Polyphenols encapsulated in liposomes are known to produce more substantial effects on targeted cells than unencapsulated polyphenols, while having minimal cytotoxicity in healthy cells.
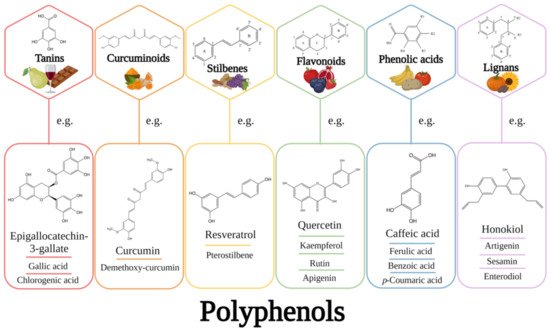
1. Introduction
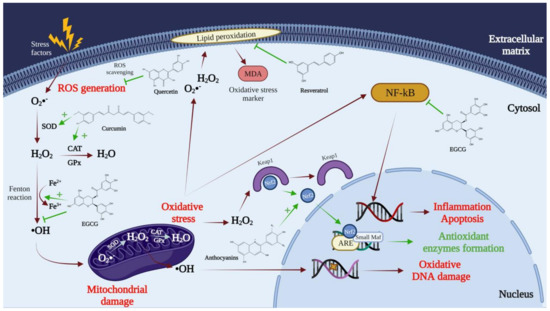
2. The Need to Encapsulate Polyphenols in Liposomes
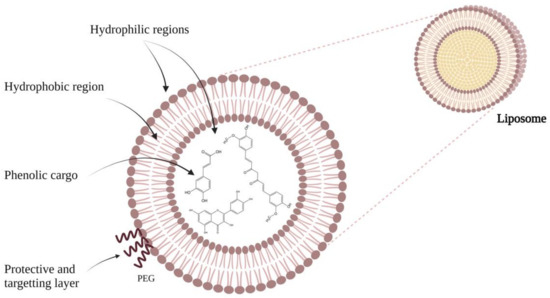
3. What Is a Liposome?
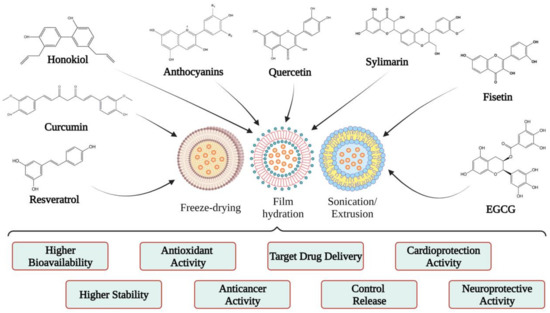
4. Applications of Liposomes
|
Polyphenol |
Production Method |
% (w/w) Polyphenol/ Lipids |
Encapsulation Efficiency |
Biological Effects |
Ref. |
|---|---|---|---|---|---|
|
Curcumin |
Lyophilization (Freeze-drying)
Evaporation method with some modification
Thin-film hydration
Ethanol injection |
10–25
15
N/S
N/S |
45% ± 0.2%
73.7% ± 1.6%
87.8% ± 4.3%
46.6% ± 1.0% |
In vivo: antiangiogenic activity and tumor growth inhibition
Enhanced stability
Slower release and better accumulation
More stable during storage |
[41]
[42]
[43] |
|
Resveratrol |
Lyophilization (Freeze-drying)
Thin-film hydration
Film hydration |
20
10
N/S |
N/S
>90%
78.14% ± 8.04% |
Prostate cancer incidence was minimized, and bioavailability was enhanced
The toxicity of free resveratrol was considerably lowered
Enhanced delivery
|
[44]
[45]
[46] |
|
Quercetin |
Film hydration and lyophilization procedure
Film hydration and sonication
Emulsification/evaporation |
30
N/S
10 |
N/S
87.1% ± 2.7%
69.42–85.72% |
Enhanced solubility, bioavailability, and antitumor activity in vivo
Maintained higher plasma quercetin concentrations
Inhibited growth of glioma cancer cells |
[23]
[47]
[48] |
|
Silymarin |
Film hydration
Reverse evaporation technique
Supercritical fluid technology |
20
10
N/S |
92.56% ± 0.93%
69.22% ± 0.6%
91.4% |
Better oral bioavailability
Higher bioavailability
Enhanced oral bioavailability |
[49]
[50]
[51] |
|
Dehydro- silymarin |
Film hydration and freeze-drying |
25 |
81.59% ± 0.24% |
Better oral bioavailability |
[52] |
|
Epigallocatechin-3-gallate (EGCG) |
Film hydration and sonication/extrusion
Film hydration
Reverse-phase evaporation method |
20
10
N/S |
84.6% ± 3.8%
80% ± 3%
85.79% ± 1.65% |
Protection against deterioration Even at lower doses, there was an increase in carcinoma cell death Enhanced targeted delivery and controlled release
Modulated the proliferation of tumor cells |
[53]
[54]
[55] |
|
Fisetin |
Film hydration and extrusion
Probe sonication |
18
7–15 |
58%
N/S |
Enhanced bioavailability and antitumor activity
Better antiangiogenic and anticancer activities |
[22]
[56] |
|
Honokiol |
Film hydration and sonication
Film hydration |
20
N/S |
95.43% ± 2.76%
90.1% ± 2.3% |
Strong anticancer effect on breast cancer Enhanced cytotoxicity and cellular uptake Enhanced bioavailability and promoted accumulation in tumor |
[57]
[58] |
|
Anthocyanins |
Film hydration
Hydration and ultrasound combined
Improved supercritical carbon dioxide (SC-CO2) |
N/S
4.5–9
20 |
43%
50.6% |
Enhanced antioxidant activity Enhanced chemical stability and bioavailability
Enhanced stability and bioavailability |
[59] [60]
[61] |
5. Polyphenols Encapsulated into Liposomes and Their Potential Health Benefits
5.1. Quercetin
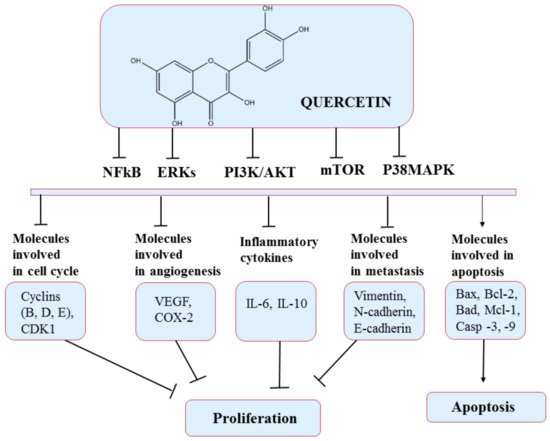
5.2. Curcumin
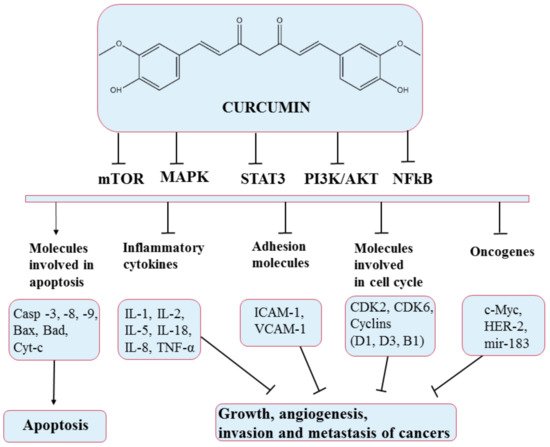
5.3. Honokiol
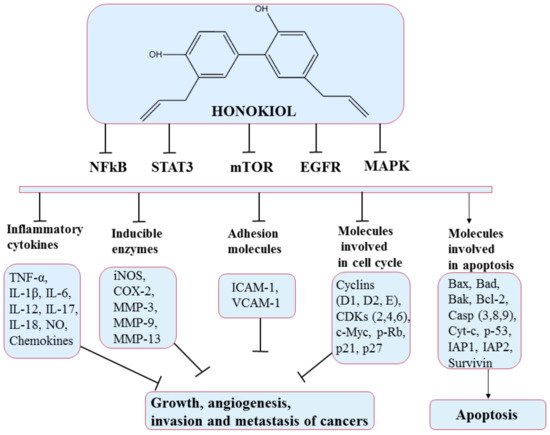
5.4. Resveratrol
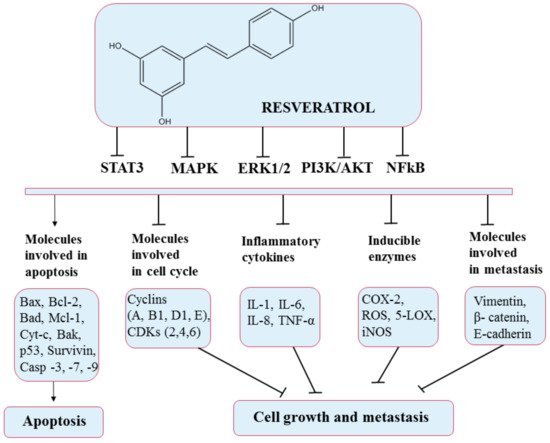
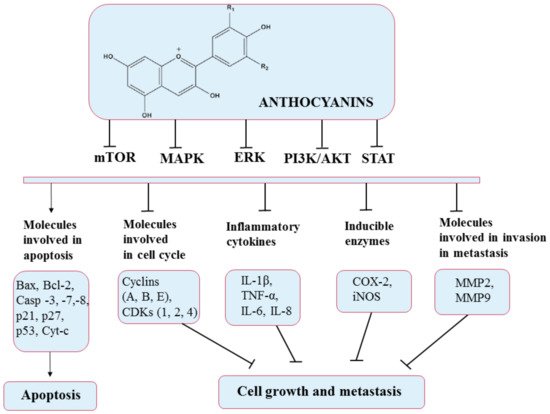
5.5. Anthocyanins
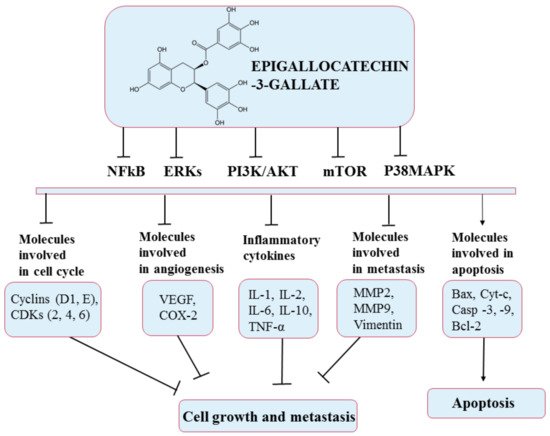
5.6. Epigallocatechin-3-Gallate (EGCG)
References
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047.
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid Nanocarriers for the Loading of Polyphenols—A Comprehensive Review. Adv. Colloid Interface Sci. 2018, 260, 85–94.
- Mocanu, M.-M.; Nagy, P.; Szöllősi, J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578–22620.
- El Gharras, H. Polyphenols: Food Sources, Properties and Applications—A Review: Nutraceutical Polyphenols. Int. J. Food Sci. Technol. 2009, 44, 2512–2518.
- De Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535.
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642.
- Parmenter, B.H.; Croft, K.D.; Hodgson, J.M.; Dalgaard, F.; Bondonno, C.P.; Lewis, J.R.; Cassidy, A.; Scalbert, A.; Bondonno, N.P. An Overview and Update on the Epidemiology of Flavonoid Intake and Cardiovascular Disease Risk. Food Funct. 2020, 11, 6777–6806.
- Mignet, N.; Seguin, J.; Chabot, G. Bioavailability of Polyphenol Liposomes: A Challenge Ahead. Pharmaceutics 2013, 5, 457–471.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Perron, N.R.; Brumaghim, J.L. Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100.
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042.
- Zhou, B.; Wu, L.-M.; Yang, L.; Liu, Z.-L. Evidence for α-Tocopherol Regeneration Reaction of Green Tea Polyphenols in SDS Micelles. Free Radic. Biol. Med. 2005, 38, 78–84.
- Du, Y.; Guo, H.; Lou, H. Grape Seed Polyphenols Protect Cardiac Cells from Apoptosis via Induction of Endogenous Antioxidant Enzymes. J. Agric. Food Chem. 2007, 55, 1695–1701.
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829.
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342.
- Watson, R.R.; Preedy, V.R.; Zibadi, S. (Eds.) Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2014.
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Farina, G.; Iemma, F.; Picci, N. Polyphenols and Their Formulations. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 29–45.
- Pralhad, T.; Rajendrakumar, K. Study of Freeze-Dried Quercetin–Cyclodextrin Binary Systems by DSC, FT-IR, X-Ray Diffraction and SEM Analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339.
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and Characterization of Polyphenol-Loaded Lipid Nanocapsules. Int. J. Pharm. 2009, 379, 270–277.
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion Formulation of Fisetin Improves Bioavailability and Antitumour Activity in Mice. Int. J. Pharm. 2012, 427, 452–459.
- Seguin, J.; Brullé, L.; Boyer, R.; Lu, Y.M.; Ramos Romano, M.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Mignet, N.; Chabot, G.G. Liposomal Encapsulation of the Natural Flavonoid Fisetin Improves Bioavailability and Antitumor Efficacy. Int. J. Pharm. 2013, 444, 146–154.
- Yuan, Z.; Chen, L.; Fan, L.; Tang, M.; Yang, G.; Yang, H.; Du, X.; Wang, G.; Yao, W.; Zhao, Q.; et al. Liposomal Quercetin Efficiently Suppresses Growth of Solid Tumors in Murine Models. Clin. Cancer Res. 2006, 12, 3193–3199.
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative Delivery Systems Loaded with Plant Bioactive Ingredients: Formulation Approaches and Applications. Plants 2021, 10, 1238.
- Ganesan, P.; Choi, D.K. Current Application of Phytocompound-Based Nanocosmeceuticals for Beauty and Skin Therapy. Int. J. Nanomed. 2016, 11, 1987.
- Wu, X.; Guy, R.H. Applications of Nanoparticles in Topical Drug Delivery and in Cosmetics. J. Drug Deliv. Sci. Technol. 2009, 19, 371–384.
- Chen, Z.; Farag, M.A.; Zhong, Z.; Zhang, C.; Yang, Y.; Wang, S.; Wang, Y. Multifaceted Role of Phyto-Derived Polyphenols in Nanodrug Delivery Systems. Adv. Drug Deliv. Rev. 2021, 176, 113870.
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27.
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of Liposomes in Medicine and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391.
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as Potential Drug Carrier Systems for Drug Delivery; IntechOpen: London, UK, 2014.
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazù, S.; Kiselev, M.A. Soft Interaction in Liposome Nanocarriers for Therapeutic Drug Delivery. Nanomaterials 2016, 6, 125.
- William, B.; Noémie, P.; Brigitte, E.; Géraldine, P. Supercritical Fluid Methods: An Alternative to Conventional Methods to Prepare Liposomes. Chem. Eng. J. 2020, 383, 123106.
- Dwivedi, C.; Verma, S. Review on Preparation and Characterization of Liposomes with Application. Int. J. Sci. Innov. Res. 2013, 2, 23.
- Karami, N.; Moghimipour, E.; Salimi, A. Liposomes as a Novel Drug Delivery System: Fundamental and Pharmaceutical Application. Asian J. Pharm. (AJP) Free. Full Text Artic. Asian J. Pharm. 2018, 12, S31–S41.
- Liu, W.; Ye, A.; Singh, H. Progress in Applications of Liposomes in Food Systems. In Microencapsulation and Microspheres for Food Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 151–170.
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as Carrier Vehicles for Functional Compounds in Food Sector. J. Exp. Nanosci. 2016, 11, 737–759.
- Keller, B.C. Liposomes in Nutrition. Trends Food Sci. Technol. 2001, 12, 25–31.
- Barani, H.; Montazer, M. A Review on Applications of Liposomes in Textile Processing. J. Liposome Res. 2008, 18, 249–262.
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-Encapsulated Curcumin: In Vitro and In Vivo Effects on Proliferation, Apoptosis, Signaling, and Angiogenesis. Cancer 2005, 104, 1322–1331.
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal Curcumin with and without Oxaliplatin: Effects on Cell Growth, Apoptosis, and Angiogenesis in Colorectal Cancer. Mol. Cancer Ther. 2007, 6, 1276–1282.
- Wei, X.-Q.; Zhu, J.-F.; Wang, X.-B.; Ba, K. Improving the Stability of Liposomal Curcumin by Adjusting the Inner Aqueous Chamber PH of Liposomes. ACS Omega 2020, 5, 1120–1126.
- Pamunuwa, G.; Karunaratne, V.; Karunaratne, D.N. Effect of Lipid Composition on In Vitro Release and Skin Deposition of Curcumin Encapsulated Liposomes. J. Nanomater. 2016, 2016, e4535790.
- Cheng, C.; Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C. Improved Bioavailability of Curcumin in Liposomes Prepared Using a PH-Driven, Organic Solvent-Free, Easily Scalable Process. RSC Adv. 2017, 7, 25978–25986.
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome Encapsulation of Curcumin and Resveratrol in Combination Reduces Prostate Cancer Incidence in PTEN Knockout Mice. Int. J. Cancer 2009, 125, 1–8.
- Zhao, Y.N.; Cao, Y.N.; Sun, J.; Liang, Z.; Wu, Q.; Cui, S.H.; Zhi, D.F.; Guo, S.T.; Zhen, Y.H.; Zhang, S.B. Anti-Breast Cancer Activity of Resveratrol Encapsulated in Liposomes. J. Mater. Chem. B 2020, 8, 27–37.
- Jagwani, S.; Jalalpure, S.; Dhamecha, D.; Jadhav, K.; Bohara, R. Pharmacokinetic and Pharmacodynamic Evaluation of Resveratrol Loaded Cationic Liposomes for Targeting Hepatocellular Carcinoma. ACS Biomater. Sci. Eng. 2020, 6, 4969–4984.
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin Liposomes Ameliorate Streptozotocin-Induced Diabetic Nephropathy in Diabetic Rats. Sci. Rep. 2020, 10, 2440.
- Gang, W.; Jie, W.J.; Ping, Z.L.; Ming, D.S.; Ying, L.J.; Lei, W.; Fang, Y. Liposomal Quercetin: Evaluating Drug Delivery In Vitro and Biodistribution In Vivo. Expert Opin. Drug Deliv. 2012, 9, 599–613.
- Yanyu, X.; Yunmei, S.; Zhipeng, C.; Qineng, P. Preparation of Silymarin Proliposome: A New Way to Increase Oral Bioavailability of Silymarin in Beagle Dogs. Int. J. Pharm. 2006, 319, 162–168.
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Increasing Bioavailability of Silymarin Using a Buccal Liposomal Delivery System: Preparation and Experimental Design Investigation. Int. J. Pharm. 2006, 308, 140–148.
- Yang, G.; Zhao, Y.; Zhang, Y.; Dang, B.; Liu, Y.; Feng, N. Enhanced Oral Bioavailability of Silymarin Using Liposomes Containing a Bile Salt: Preparation by Supercritical Fluid Technology and Evaluation In Vitro and In Vivo. Int. J. Nanomed. 2015, 10, 6633.
- Chu, C.; Tong, S.; Xu, Y.; Wang, L.; Fu, M.; Ge, Y.; Yu, J.; Xu, X. Proliposomes for Oral Delivery of Dehydrosilymarin: Preparation and Evaluation In Vitro and In Vivo. Acta Pharmacol. Sin. 2011, 32, 973–980.
- Fang, J.-Y.; Lee, W.-R.; Shen, S.-C.; Huang, Y.-L. Effect of Liposome Encapsulation of Tea Catechins on Their Accumulation in Basal Cell Carcinomas. J. Dermatol. Sci. 2006, 42, 101–109.
- Marwah, M.; Perrie, Y.; Badhan, R.K.S.; Lowry, D. Intracellular Uptake of EGCG-Loaded Deformable Controlled Release Liposomes for Skin Cancer. J. Liposome Res. 2020, 30, 136–149.
- Luo, X.; Guan, R.; Chen, X.; Tao, M.; Ma, J.; Zhao, J. Optimization on Condition of Epigallocatechin-3-Gallate (EGCG) Nanoliposomes by Response Surface Methodology and Cellular Uptake Studies in Caco-2 Cells. Nanoscale Res. Lett. 2014, 9, 291.
- Mignet, N.; Seguin, J.; Ramos Romano, M.; Brullé, L.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a Liposomal Formulation of the Natural Flavonoid Fisetin. Int. J. Pharm. 2012, 423, 69–76.
- Ju, R.-J.; Cheng, L.; Qiu, X.; Liu, S.; Song, X.-L.; Peng, X.-M.; Wang, T.; Li, C.-Q.; Li, X.-T. Hyaluronic Acid Modified Daunorubicin plus Honokiol Cationic Liposomes for the Treatment of Breast Cancer along with the Elimination Vasculogenic Mimicry Channels. J. Drug Target. 2018, 26, 793–805.
- Zhou, C.; Guo, C.; Li, W.; Zhao, J.; Yang, Q.; Tan, T.; Wan, Z.; Dong, J.; Song, X.; Gong, T. A Novel Honokiol Liposome: Formulation, Pharmacokinetics, and Antitumor Studies. Drug Dev. Ind. Pharm. 2018, 44, 2005–2012.
- Hwang, J.-M.; Kuo, H.-C.; Lin, C.-T.; Kao, E.-S. Inhibitory Effect of Liposome-Encapsulated Anthocyanin on Melanogenesis in Human Melanocytes. Pharm. Biol. 2013, 51, 941–947.
- Homayoonfal, M.; Mousavi, S.M.; Kiani, H.; Askari, G.; Desobry, S.; Arab-Tehrany, E. Encapsulation of Berberis Vulgaris Anthocyanins into Nanoliposome Composed of Rapeseed Lecithin: A Comprehensive Study on Physicochemical Characteristics and Biocompatibility. Foods 2021, 10, 492.
- Zhao, L.; Temelli, F.; Chen, L. Encapsulation of Anthocyanin in Liposomes Using Supercritical Carbon Dioxide: Effects of Anthocyanin and Sterol Concentrations. J. Funct. Foods 2017, 34, 159–167.
- Saraswat, A.L.; Maher, T.J. Development and Optimization of Stealth Liposomal System for Enhanced In Vitro Cytotoxic Effect of Quercetin. J. Drug Deliv. Sci. Technol. 2020, 55, 101477.
- Daneshniya, M.; Maleki, M.H.; Liavali, H.; Hassanjani, M.; Keshavarz Bahadori, N.; Mohammadi, M.; Jalilvand Nezhad, H. Antioxidant Activity of Flavonoids as an Important Phytochemical Compound in Plants. In Proceedings of the 2nd International Congress on Engineering, Technology and Innovation, Darmstadt, Germany, 6 November 2020.
- Chou, C.-C.; Yang, J.-S.; Lu, H.-F.; Ip, S.-W.; Lo, C.; Wu, C.-C.; Lin, J.-P.; Tang, N.-Y.; Chung, J.-G.; Chou, M.-J.; et al. Quercetin-Mediated Cell Cycle Arrest and Apoptosis Involving Activation of a Caspase Cascade through the Mitochondrial Pathway in Human Breast Cancer MCF-7 Cells. Arch. Pharm. Res. 2010, 33, 1181–1191.
- Long, Q.; Xie, Y.; Huang, Y.; Wu, Q.; Zhang, H.; Xiong, S.; Liu, Y.; Chen, L.; Wei, Y.; Zhao, X.; et al. Induction of Apoptosis and Inhibition of Angiogenesis by PEGylated Liposomal Quercetin in Both Cisplatin-Sensitive and Cisplatin-Resistant Ovarian Cancers. J. Biomed. Nanotechnol. 2013, 9, 965–975.
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological Basis and New Insights of Quercetin Action in Respect to Its Anti-Cancer Effects. Biomed. Pharmacother. 2020, 121, 109604.
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 32.
- Kumari, A.; Kumar, V.; Yadav, S.K. Plant Extract Synthesized PLA Nanoparticles for Controlled and Sustained Release of Quercetin: A Green Approach. PLoS ONE 2012, 7, e41230.
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and MiRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214.
- Arora, S.; Singh, S.; Piazza, G.A.; Contreras, C.M.; Panyam, J.; Singh, A.P. Honokiol: A Novel Natural Agent for Cancer Prevention and Therapy. Curr. Mol. Med. 2012, 12, 1244–1252.
- Esumi, T.; Makado, G.; Zhai, H.; Shimizu, Y.; Mitsumoto, Y.; Fukuyama, Y. Efficient Synthesis and Structure–Activity Relationship of Honokiol, a Neurotrophic Biphenyl-Type Neolignan. Bioorg. Med. Chem. Lett. 2004, 14, 2621–2625.
- Ishitsuka, K.; Hideshima, T.; Hamasaki, M.; Raje, N.; Kumar, S.; Hideshima, H.; Shiraishi, N.; Yasui, H.; Roccaro, A.M.; Richardson, P.; et al. Honokiol Overcomes Conventional Drug Resistance in Human Multiple Myeloma by Induction of Caspase-Dependent and -Independent Apoptosis. Blood 2005, 106, 1794–1800.
- Lee, Y.-J.; Lee, Y.M.; Lee, C.-K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic Applications of Compounds in the Magnolia Family. Pharmacol. Ther. 2011, 130, 157–176.
- Ezzat, S.M.; Shouman, S.A.; Elkhoely, A.; Attia, Y.M.; Elsesy, M.S.; El Senousy, A.S.; Choucry, M.A.; El Gayed, S.H.; El Sayed, A.A.; Sattar, E.A.; et al. Anticancer Potentiality of Lignan Rich Fraction of Six Flaxseed Cultivars. Sci. Rep. 2018, 8, 544.
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for Cancer Therapeutics: A Traditional Medicine That Can Modulate Multiple Oncogenic Targets. Pharmacol. Res. 2019, 144, 192–209.
- Qiu, N.; Cai, L.; Xie, D.; Wang, G.; Wu, W.; Zhang, Y.; Song, H.; Yin, H.; Chen, L. Synthesis, Structural and In Vitro Studies of Well-Dispersed Monomethoxy-Poly(Ethylene Glycol)–Honokiol Conjugate Micelles. Biomed. Mater. 2010, 5, 065006.
- Bai, X.; Cerimele, F.; Ushio-Fukai, M.; Waqas, M.; Campbell, P.M.; Govindarajan, B.; Der, C.J.; Battle, T.; Frank, D.A.; Ye, K.; et al. Honokiol, a Small Molecular Weight Natural Product, Inhibits Angiogenesis In Vitro and Tumor Growth In Vivo *. J. Biol. Chem. 2003, 278, 35501–35507.
- Li, Z.; Liu, Y.; Zhao, X.; Pan, X.; Yin, R.; Huang, C.; Chen, L.; Wei, Y. Honokiol, a Natural Therapeutic Candidate, Induces Apoptosis and Inhibits Angiogenesis of Ovarian Tumor Cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 95–102.
- Yang, J.; Pei, H.; Luo, H.; Fu, A.; Yang, H.; Hu, J.; Zhao, C.; Chai, L.; Chen, X.; Shao, X.; et al. Non-Toxic Dose of Liposomal Honokiol Suppresses Metastasis of Hepatocellular Carcinoma through Destabilizing EGFR and Inhibiting the Downstream Pathways. Oncotarget 2016, 8, 915–932.
- Pezzuto, J. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2018, 27, 1–14.
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A Multitargeted Agent for Age-Associated Chronic Diseases. Cell Cycle 2008, 7, 1020–1035.
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718.
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The In Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506.
- Cardile, V.; Chillemi, R.; Lombardo, L.; Sciuto, S.; Spatafora, C.; Tringali, C. Antiproliferative Activity of Methylated Analogues of E- and Z-Resveratrol. Z. Naturforschung C 2007, 62, 189–195.
- Wang, Y.; Halls, C.; Zhang, J.; Matsuno, M.; Zhang, Y.; Yu, O. Stepwise Increase of Resveratrol Biosynthesis in Yeast Saccharomyces Cerevisiae by Metabolic Engineering. Metab. Eng. 2011, 13, 455–463.
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404.
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and Solubility of Trans-Resveratrol Are Strongly Influenced by PH and Temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204.
- Subramanian, L.; Youssef, S.; Bhattacharya, S.; Kenealey, J.; Polans, A.S.; van Ginkel, P.R. Resveratrol: Challenges in Translation to the Clinic—A Critical Discussion. Clin. Cancer Res. 2010, 16, 5942–5948.
- Smoliga, J.M.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability Is the Problem, What Is the Solution? Molecules 2014, 19, 17154–17172.
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of Resveratrol: What Formulation Solutions to Bioavailability Limitations? J. Control. Release 2012, 158, 182–193.
- Han, G.; Xia, J.; Gao, J.; Inagaki, Y.; Tang, W.; Kokudo, N. Anti-Tumor Effects and Cellular Mechanisms of Resveratrol. Drug Discov. Ther. 2015, 9, 1–12.
- Shankar, S.; Gyanendra, S.; Rakesh, K.S. Chemoprevention by Resveratrol: Molecular Mechanisms and Therapeutic Potential. Front. Biosci. 2007, 12, 4839.
- Lee, M.-F.; Pan, M.-H.; Chiou, Y.-S.; Cheng, A.-C.; Huang, H. Resveratrol Modulates MED28 (Magicin/EG-1) Expression and Inhibits Epidermal Growth Factor (EGF)-Induced Migration in MDA-MB-231 Human Breast Cancer Cells. J. Agric. Food Chem. 2011, 59, 11853–11861.
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340.
- Van Ginkel, P.R.; Sareen, D.; Subramanian, L.; Walker, Q.; Darjatmoko, S.R.; Lindstrom, M.J.; Kulkarni, A.; Albert, D.M.; Polans, A.S. Resveratrol Inhibits Tumor Growth of Human Neuroblastoma and Mediates Apoptosis by Directly Targeting Mitochondria. Clin. Cancer Res. 2007, 13, 5162–5169.
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-Dependency of Resveratrol in Providing Health Benefits. Dose-Response 2010, 8, 478–500.
- Chi, J.; Ge, J.; Yue, X.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Preparation of Nanoliposomal Carriers to Improve the Stability of Anthocyanins. LWT 2019, 109, 101–107.
- Diaconeasa, Z.; Frond, A.; Stirbu, I.; Rugină, D.; Socaciu, C. Anthocyanins-Smart Molecules for Cancer Prevention. In Phytochemicals-Source of Antioxidants and Role in Disease Prevention; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: London, UK, 2018.
- Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines 2020, 8, 336.
- Fernández, J.; García, L.; Monte, J.; Villar, C.J.; Lombó, F. Functional Anthocyanin-Rich Sausages Diminish Colorectal Cancer in an Animal Model and Reduce Pro-Inflammatory Bacteria in the Intestinal Microbiota. Genes 2018, 9, 133.
- Fakhri, S.; Khodamorady, M.; Naseri, M.; Farzaei, M.H.; Khan, H. The Ameliorating Effects of Anthocyanins on the Cross-Linked Signaling Pathways of Cancer Dysregulated Metabolism. Pharmacol. Res. 2020, 159, 104895.
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic Effects of EGCG: A Patent Review. Expert Opin. Ther. Pat. 2016, 26, 907–916.
- Sanni, O.; Enebi, D. A Multidisciplinary Research Book; Maharani Kasiswari College Kolkata: West Bengal, India, 2021.
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular Mechanisms of Action of Epigallocatechin Gallate in Cancer: Recent Trends and Advancement. Semin. Cancer Biol. 2020, in press.
- Chen, W.; Zou, M.; Ma, X.; Lv, R.; Ding, T.; Liu, D. Co-Encapsulation of EGCG and Quercetin in Liposomes for Optimum Antioxidant Activity. J. Food Sci. 2018, 84, 111–120.
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, Metabolism, Anti-Cancer Effect and Molecular Targets of Epigallocatechin Gallate (EGCG): An Updated Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941.
- Wang, Y.-Q.; Lu, J.-L.; Liang, Y.-R.; Li, Q.-S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334.
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer Preventive and Therapeutic Effects of EGCG, the Major Polyphenol in Green Tea. Egypt. J. Basic Appl. Sci. 2018, 5, 1–23.
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-Gallate for Different Treatments. BioMed Res. Int. 2017, 2017, 5615647.





