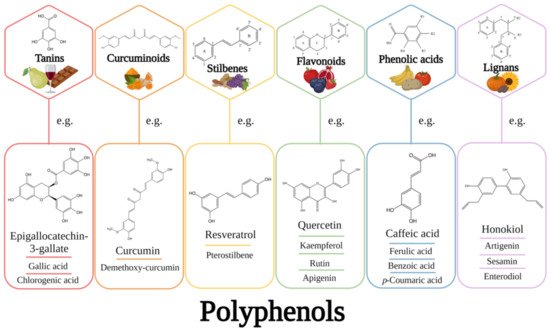
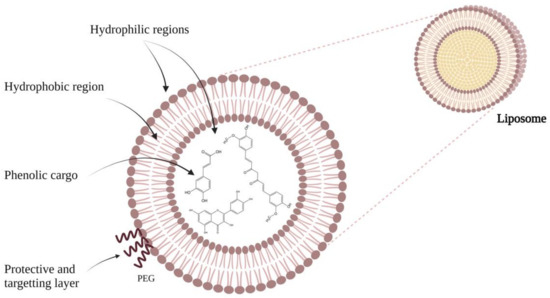
4. Applications of Liposomes
As outlined above, liposomes are delivery systems that have the ability to encapsulate and release controlled different types of substances, including drugs, nutraceuticals, and even genes, making their use extremely wide
[34][35][41,42]. Initially, the applications of liposomes were restricted in the medical field, before being later used in cosmetics. However, over time, their popularity has been expanded to other areas, such as delivering vaccines, hormones, enzymes, and vitamins in the body
[36][43]. Liposomes show great flexibility as they can be injected intravenously, intramuscularly, or subcutaneously (liquid suspensions); furthermore, they can be inhaled (aerosol of liposome suspension or lyophilized powder), applied directly to the skin as a suspension, cream, or gel, or even ingested (any of the physical forms)
[37][44].
Considering the benefits offered by liposomes, involving the possibility of large-scale production, the natural ingredients (such as eggs, milk, and soy) involved in manufacturing, biocompatibility, and the ability to transport a wide range of bioactive compounds, they are currently used in many areas, as overviewed in this section
[36][43].
The first popular topic of discussion is drug targeting, allowing to enhance the specificity of a drug that targets the desired cell/tissue. Liposomes can be encapsulated with opsonin and ligands (containing antibodies, apoproteins, hormones). The ligand will specifically recognize the receptor sites and determine the direction of the liposomes to those target sites, where they will accumulate and achieve the anticipated effect. By doing so, liposomes will not be recognized and eliminated by the reticuloendothelial system (liver, spleen, and bone marrow), while the toxicity produced by drugs in untargeted cells/tissues will be minimized
[38][45].
Due to their basic characteristics, namely, the ability to encapsulate various biological substances that can then be delivered to epidermal cells, liposomes are also used in the pharmaceutical and cosmetic fields, e.g., dermatology. Skin hydration is the most critical aspect in skincare. Accordingly, most applications in the field of cosmetics are concerned with this issue of balancing the moisture of the skin. Liposomes can simply hydrate the skin, thus reducing skin dryness, which is the main factor causing skin aging. In addition to this first applicability of liposomes in cosmetics, they can also encapsulate anti-inflammatory agents, immunostimulants, and enhancers of molecular and cellular detoxification, which can produce some therapeutic effects on several skin problems such as dark circles, wrinkles, and age spots
[34][41].
Likewise, due to the rapid development of the food industry in recent years, adding functional compounds to food products has gained more attention. Thus, functional compounds that help to control the flavor, color, texture, or preservative properties of food products have been more widely employed in liposomes. However, these functional compounds are sensitive to environmental factors, processing, and conditions in the gastrointestinal tract, whereas encapsulation could remedy these inconveniences, making liposomes the suitable candidates in such cases
[36][43].
Although there are drawbacks to the degree of encapsulation of polyphenols in liposomes, several studies have reported that both the bioavailability and the efficacy of polyphenols encapsulated in liposomes are improved compared to the free active substance and other transport systems. Various formulations of liposomes showed improved results in terms of the solubility of many polyphenols, for example, resveratrol, quercetin, curcumin, and puerarin
[8][9], as detailed in
Table 1.
Table 1. Liposomal formulations that have been created for polyphenol-related biological research.
|
| Polyphenol |
|
| Production |
| Method |
|
| % (w/w) |
| Polyphenol/ |
| Lipids |
|
| Encapsulation Efficiency |
|
| Biological Effects |
|
| Ref. |
|
|
| Curcumin |
|
| Lyophilization |
| (Freeze-drying) |
| |
| Evaporation |
| method with some modification |
| |
| Thin-film hydration |
| |
| Ethanol injection |
|
| 10–25 |
| |
| |
| |
| 15 |
| |
| |
| |
| |
| N/S |
| |
| |
| |
| N/S |
|
| 45% ± 0.2% |
| |
| |
| |
| 73.7% ± 1.6% |
| |
| |
| |
| |
| 87.8% ± 4.3% |
| |
| |
| |
| 46.6% ± 1.0% |
|
| In vivo: antiangiogenic activity and tumor growth inhibition |
| |
| Enhanced stability |
| |
| |
| |
| |
| Slower release and better accumulation |
| |
| |
| More stable during storage |
|
[39][40]
|
[46,47]
| |
|
[ 41]
|
[ 48 ]
| |
| |
| |
|
[ 42 ]
|
[ 49 ]
| |
| |
|
[ 43 ]
|
[ 50 ]
|
|
| Resveratrol |
|
| Lyophilization |
| (Freeze-drying) |
| |
| Thin-film hydration |
| |
| Film hydration |
|
| 20 |
| |
| |
| 10 |
| |
| |
| |
| N/S |
|
| N/S |
| |
| |
| >90% |
| |
| |
| |
| 78.14% ± 8.04% |
|
| Prostate cancer incidence was minimized, and bioavailability was enhanced |
| |
| The toxicity of free resveratrol was considerably lowered |
| |
| Enhanced delivery |
| |
|
[44]
|
[51]
| |
|
[ 45]
|
[ 52 ]
| |
|
[ 46 ]
|
[ 53 ]
|
|
| Quercetin |
|
| Film hydration and lyophilization procedure |
| |
| Film hydration and sonication |
| |
| Emulsification/evaporation |
|
| 30 |
| |
| |
| |
| N/S |
| |
| 10 |
|
| N/S |
| |
| |
| |
| |
| 87.1% ± 2.7% |
| |
| |
| 69.42–85.72% |
|
| Enhanced solubility, bioavailability, and antitumor activity in vivo |
| |
| |
| Maintained higher plasma quercetin concentrations |
| |
| Inhibited growth of glioma cancer cells |
|
[23]
|
[24]
| |
| |
| |
|
[ 47 ]
|
[ 54 ]
| |
|
[ 48 ]
|
[ 55 ]
|
|
| Silymarin |
|
| Film hydration |
| |
| Reverse evaporation technique |
| |
| Supercritical fluid technology |
|
| 20 |
| |
| 10 |
| |
| |
| |
| N/S |
|
| 92.56% ± 0.93% |
| |
| 69.22% ± 0.6% |
| |
| |
| 91.4% |
|
| Better oral bioavailability |
| |
| Higher bioavailability |
| |
| |
| |
| Enhanced oral bioavailability |
|
[49]
|
[56]
|
[50]
|
[ 57]
| |
| |
|
[ 51 ]
|
[ 58 ]
|
|
| Dehydro- |
| silymarin |
|
| Film hydration and freeze-drying |
|
| 25 |
|
| 81.59% ± 0.24% |
|
| Better oral bioavailability |
|
[52]
|
[59]
|
|
| Epigallocatechin-3-gallate (EGCG) |
|
| Film hydration and sonication/extrusion |
| |
| Film hydration |
| |
| Reverse-phase evaporation method |
|
| 20 |
| |
| |
| |
| 10 |
| |
| N/S |
|
| 84.6% ± 3.8% |
| |
| |
| |
| 80% ± 3% |
| |
| |
| 85.79% ± 1.65% |
|
| Protection against deterioration |
| Even at lower doses, there was an increase in carcinoma cell death |
| Enhanced targeted delivery and controlled release |
| |
| Modulated the proliferation of tumor cells |
|
[53]
|
[60]
| |
| |
|
[ 54 ]
|
[ 61 ]
|
[ 55 ]
|
[ 62 ]
|
|
| Fisetin |
|
| Film hydration and extrusion |
| |
| Probe sonication |
|
| 18 |
| |
| 7–15 |
|
| 58% |
| |
| N/S |
|
| Enhanced bioavailability and antitumor activity |
| |
| Better antiangiogenic and anticancer activities |
|
[22]
|
[23]
|
[56]
|
[ 63]
|
|
| Honokiol |
|
| Film hydration and sonication |
| |
| |
| Film |
| hydration |
|
| 20 |
| |
| |
| N/S |
|
| 95.43% ± 2.76% |
| |
| |
| |
| 90.1% ± 2.3% |
|
| Strong anticancer effect on breast cancer |
| Enhanced cytotoxicity and cellular uptake |
| Enhanced bioavailability and promoted accumulation in tumor |
|
[57]
|
[64]
| |
|
[ 58]
|
[ 65 ]
|
|
| Anthocyanins |
|
| Film hydration |
| |
| Hydration and ultrasound combined |
| |
| Improved supercritical carbon dioxide (SC-CO 2 ) |
|
| N/S |
| |
| 4.5–9 |
| |
| |
| |
| |
| 20 |
|
| |
| 43% |
| |
| |
| |
| |
| 50.6% |
|
| Enhanced antioxidant activity |
| Enhanced chemical stability and bioavailability |
| |
| |
| |
| Enhanced stability and bioavailability |
|
[59]
|
[66][60]
[67]
| |
| |
| |
| |
|
[ 61 ]
|
[ 68 ]
|
According to
Table 1, the encapsulation ratio varies, even in the case of the same encapsulated polyphenol, and it can be concluded that the encapsulation rate is dependent on the structure of the polyphenol, the lipid composition of the liposome, and its formulation
[8][9].
The liposome–polyphenol complex has another crucial benefit, which is its increased chemical stability, thereby maintaining long-term efficacy
[8][9] or inducing effects that cannot be achieved otherwise by administering free polyphenols
[53][60]. At the same time, the active polyphenols can be injected into nonorganic solvents due to the improved solubility of the substance in the liposomes, which decreases the systemic toxicity and increases the maximum dose tolerated by the body, thus enabling administration of a higher amount of polyphenols in vivo
[8][9].
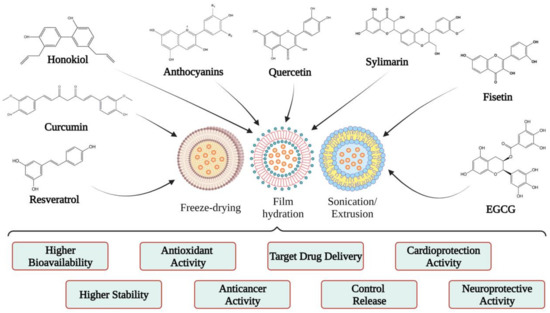
Studies on the use of polyphenols encapsulated in liposomes performed in vitro/in vivo have revealed that they have similar or better efficacy than free polyphenols
[39][56][46,63]. Therefore, starting from this idea, several researchers have encapsulated different polyphenols in liposomes to observe the effects and benefits of each, a topic that is discussed in the next section (
Figure 4).
Figure 4.
Examples of liposomal forms developed for polyphenol biological studies.
5. Polyphenols Encapsulated into Liposomes and Their Potential Health Benefits
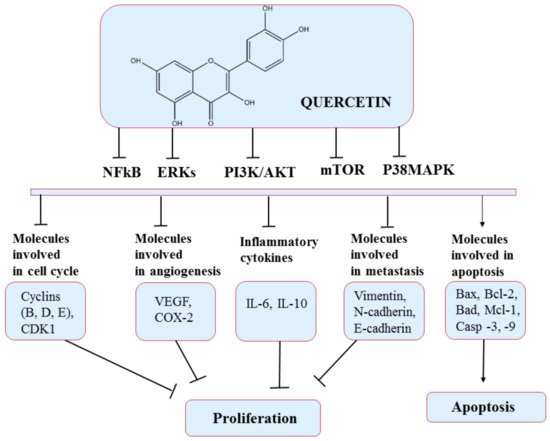
5.1. Quercetin
Quercetin (QC) is a flavonoid plant coming from the word “quercetum” (oak forest), which is part of the Fagaceae family and genus
Quercus. The literature on QC has highlighted several important characteristics such as its antioxidant, anticarcinogenic, antiviral, anti-obesity, anti-inflammatory, and antihypertensive activities
[62][72]. Due to its antioxidant character, quercetin can eliminate free radicals
[63][73]. This property originates from a large number of conjugated hydroxyl and orbital groups through which QC can donate electrons and hydrogen or eliminate H
2O
2 and superoxide anions.
According to existing data, QC has shown anticancer effects on several mechanisms. For example, it has been shown that QC caused cell-cycle arrest in the G2/M phase by activating the p53 tumor suppressor protein, thereby inhibiting the activity of cyclin dependent kinase 2 (CDK2), cyclin A, and cyclin B
[64][74]. In addition, this flavonoid can suppress the synthesis and expression of heat-shock protein and block the signal transduction pathways by inhibiting protein tyrosine kinase and downregulating oncogene expression (c-myc, ki-ras)
[65][75]. Regarding the action of quercetin in angiogenesis, it has been shown that it affects the VEGFR-2 mediated pathway, causing under-expression of the AKT (protein kinase B) regulatory factor, thus inhibiting blood vessel growth and restricting tumor growth in prostate and breast cancer
[66][76]. Quercetin can also determinate apoptosis in tumor cells by stimulating proapoptotic proteins such as BAX and caspases (3, 6, 7, 8, and 9). Furthermore, QC can stop the expression of antiapoptotic proteins such as Bcl-2 and terminate cancer metastasis. In order to form metastases, epithelial-to-mesenchymal transition (EMT), a process that involves downregulation of epithelial-type proteins (e.g., E-cadherin), and stimulation of expression of mesenchymal markers, including N-cadherin and Vimentin, must occur. Thus, in this case, quercetin can decrease the occurrence of EMT by overexpressing E-cadherin and under-expressing N-cadherin and Vimentin
[66][76].
The most recent investigation reported that QC could inhibit the growth of different types of cancer cells, including colorectal, prostate, liver, pancreatic, breast, kidney, lung, and ovarian, via modulation of various cellular processes (
Figure 5). In addition, QC can exhibit selective cytotoxic activity toward cancer cells without producing adverse effects on normal cells
[67][77]. On the other hand, QC has slight aqueous solubility and bioavailability and is rapidly metabolized, and these disadvantages can diminish its effectiveness in treating diseases
[68][78].
Figure 5. Chemical structure of quercetin and mechanism of action of quercetin through different molecular targets resulting in apoptosis or stopping proliferation.
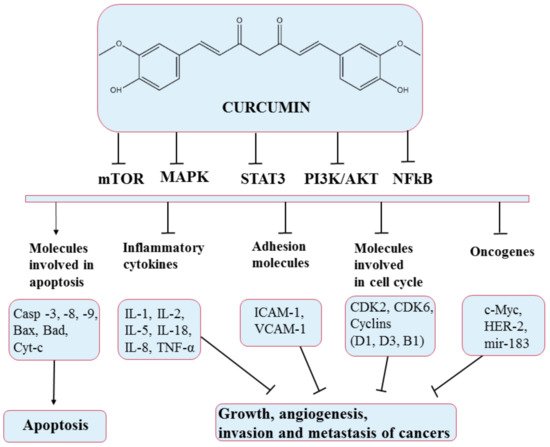
5.2. Curcumin
Curcumin is a natural yellow polyphenolic compound extracted from turmeric roots (
Curcuma longa). It is a multifunctional compound that has been widely used in traditional medicine due to its various therapeutic activities in anti-inflammation, antioxidation, antiproliferation, and anti-angiogenesis
[69][82]. Curcumin acts on several pathways, producing growth suppression and angiogenesis, as illustrated in
Figure 6.
Figure 6. Chemical structure of curcumin and mechanism of action of curcumin through different molecular targets, resulting in apoptosis or growth suppression, angiogenesis, invasion, and metastasis of cancers.
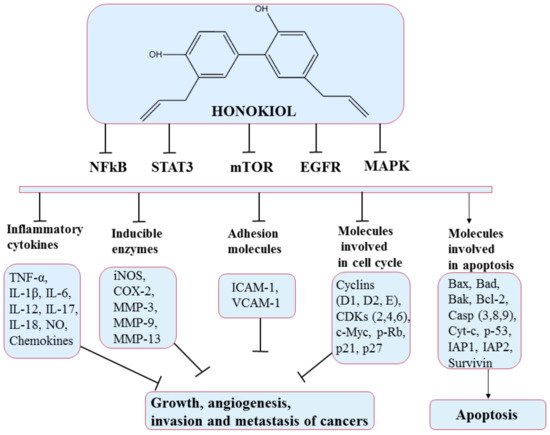
5.3. Honokiol
Honokiol is a lignan found in several species of the genus
Magnolia, which is distributed worldwide
[70][71][91,92]. Honokiol is obtained by purification of the bark and seed cones of the magnolia tree
[72][93]. It is known that extracts from
Magnolia bark are used as traditional herbal medicines in Korea, China and Japan, and other countries
[73][94]. Many lignans with anticancer potential act by shrinking the tumor, decreasing the expression of estrogen, insulin growth factor, vascular endothelial growth factor, and matrix metalloproteinases enzymes, and enhancing caspase 3
[74][95].
This polyphenol produces effects on many molecular targets that modulate the expression of genes controlling the different hallmarks of cancer
[75][96]. Mechanisms of action include retarding the cell cycle (via effects on cyclins D and B)
[76][97], inducing apoptosis (by upregulating the expression of proteins that control apoptosis such as Bcl-xL, Bcl-2), inhibiting angiogenesis (via regulation of hypoxia-inducible factor 1-alpha (HIF1α) and VEGF gene expression)
[77][78][98,99], and interdicting invasion and metastasis
[79][100]. Some of these pathways and molecules implicated are illustrated in
Figure 7. Honokiol has also been shown to produce promising results in the case of chemoresistance. It shows chemosensitization effects when combined with well-known chemotherapeutics
[75][96].
Figure 7. Chemical structure of honokiol and mechanism of action of honokiol through different molecular targets that suppress growth, angiogenesis, and invasion of cancers (adapted from Arora et al., 2012).
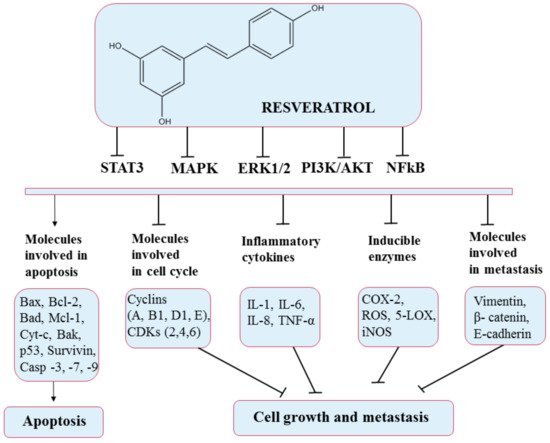
5.4. Resveratrol
Resveratrol is a stilbenoid natural polyphenol, isolated for the first time in 1939 from
Veratrum grandiflorum [80][108]. Since its first certification, resveratrol has been identified in various plants such as plums, pistachios, berries, and peanuts. However, the most abundant source is represented by fresh grape skin, where it occurs in concentrations as high as 50–100 mg/g
[81][82][109,110]. In recent years, due to its beneficial effects on health, resveratrol has received the attention of researchers
[83][111]. It is found in two isomeric forms,
cis and
trans, but the predominant isomer is
trans, which has the most potent therapeutic effects due to its conformation
[82][84][110,112]. In addition, it is also obtained via chemical or biotechnological synthesis from yeast
Saccharomyces cerevisiae for industrial applications
[85][113].
Resveratrol is sensitive to light, pH, and high temperatures due to its unstable hydroxyls and C=C double bond. In this regard, many studies have aimed to increase its stability in an effort to expand its use
[86][114]. The
trans form of resveratrol is stable under acidic conditions at room or body temperature, but resveratrol degrades rapidly when the pH is alkaline. Therefore, by lowering the temperature and pH and by limiting the exposure to light and oxygen, the stability of this isomer can be improved
[87][115].
Similar to the other polyphenols, resveratrol has a low bioavailability due to poor absorption and rapid metabolism of glucuronidated and sulfated compounds, followed by their excretion
[88][116]. Hence, the poor bioavailability of this compound is a major problem in amplifying its effects in humans and, as such, so many approaches have been attempted to increase its bioavailability
[89][117]. The effectiveness of resveratrol depends mainly on a combination of factors such as dosage, method of administration, the origin of the targeted tumor, and other substances present in the diet that may interfere with this polyphenol
[83][111]. Thus, to improve the bioavailability, it is necessary to carry out studies on the delivery routes, the formulations and modulation of resveratrol metabolism, and possible interactions of resveratrol with other food components. On the other hand, another possible approach to its bioavailability is creating novel resveratrol-based derivatives
[90][118].
Despite the extremely low bioavailability of this polyphenol, recent studies found strong evidence that resveratrol can prevent or delay the onset of cancer, heart disease, ischemic and chemically induced damage, diabetes, pathological inflammation, and viral infections
[83][111]. In particular, resveratrol has shown anticancer effects by altering glycolysis and molecules involved in the cell cycle (resveratrol upregulates p53 protein, thereby downregulating the expression of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)
[91][119] and suppressing cancer cell growth (stops cell cycle at G1 and G1/S phases by inducing the expression of CDK inhibitors and proliferation. Resveratrol causes a high production of nitric oxide synthases (NOS), thus inhibiting cell proliferation
[92][120], inducing apoptosis (through the intrinsic pathway via the activation of caspase 3 and caspase 9, the determined release of cytochrome c, upregulation of Bax expression, and downregulation of Bcl-2 expression
[91][119]) promoting antitumor immune responses, and preventing cancer cell adhesion, migration (also reduced by resveratrol through the EGFR/PI3K signaling pathway
[93][121]), and invasion by modulating active molecules and gene expression through various signaling pathways (
Figure 8). In addition, different doses of resveratrol may induce different effects, which can sometimes be opposite
[94][122]. Therefore, it is crucial to identify the most effective dose and administration route. Likewise, it has been documented that resveratrol induces cell death in tumor tissues with relatively no effect on normal tissues in the vicinity of the tumor
[95][123]. Mukherjee et al. (2010) reported that lower doses of resveratrol could result in health benefits, while higher doses affect tumor cells via proapoptotic effects
[96][124]. Thus, future studies based on this polyphenol are needed to fully decipher its effects.
Figure 8. Chemical structure of resveratrol and its mechanism of action through different molecular targets that result in apoptosis or growth suppression and metastasis of cancers.
5.5. Anthocyanins
Considering their great health benefits, this section focuses on anthocyanins. Anthocyanins are pigments that are responsible for the various colors (blue, red, purple) of fruits, flowers, and vegetables. They can be stacked in vegetative tissues, where they fulfill the role of protection against biotic and abiotic factors. In nature, anthocyanins are found in the form of glycosides, which have one or more sugars bound to the aglycone nucleus. The chemical structure is C6–C3–C6, with two benzene rings (A and B) and a heterocyclic C ring. Due to this structure, anthocyanins have been shown to have strong antioxidant, anticancer, anti-inflammatory, and cardioprotective activities, as well as effects related to vision improvement
[97][98][131,132].
The most frequent anthocyanin aglycones found in plants are delphinidin, cyanidin, petunidin, peonidin, pelargonidin, and malvidin. Nevertheless, these compounds are found in plants at different levels. Cyanidin has the largest proportion in plant tissues (50%), followed by pelargonidin, peonidin, and delphinidin, each representing a percentage of 12%; finally, petunidin and malvidin each make up a percentage of 7%. The unique properties of anthocyanins and anthocyanidins may have an impact on their anticancer effectiveness, antioxidant activities, and bioavailability
[99][133].
Like other classes of polyphenols, anthocyanins can cause anti-inflammatory and antitumor effects on several types of cancer cell lines such as breast, liver, colon, prostate, ovarian, and skin cancers. These anticancer effects appear to be linked to cancer cell growth suppression via increased oxidative stress biomarkers and induction of apoptosis via the mitochondrial route
[100][134]. As a consequence, they seem to be attractive therapeutic options.
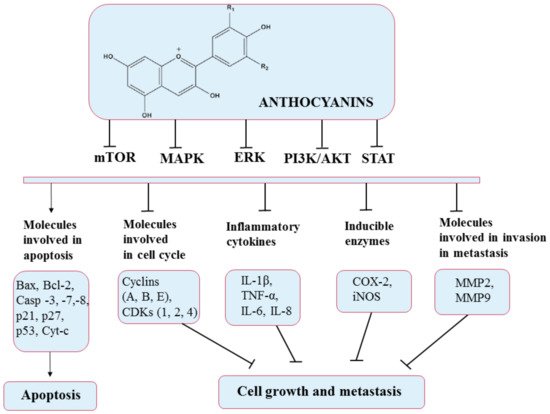
Anthocyanins work as antioxidants by scavenging free radicals and lowering lipid peroxidation and ROS levels. Numerous studies have also reported that anthocyanins can mediate oxidative stress in many signaling pathways such as PI3K/Akt/mTOR and Ras/ERK/MAPK, targeting their component molecules. In addition, anthocyanin-rich formulations have been found to suppress H
2O
2 and TNF-α-induced VEGF expression, as well as promote caspase pathways, thereby exerting anticarcinogenic and antiangiogenic effects. They also exhibit anti-inflammatory activities by significantly reducing cyclooxygenase-2 (COX-2), inflammatory interleukins (ILs), inducible nitric oxide synthase iNOS, and NF-κB. Furthermore, anthocyanins can stimulate the apoptosis of cancer cells by activating cell death receptors such as BAX and Bcl-2 and caspases 3, 7, and 8
[101][135]. Additionally, anthocyanin extracts were reported to mediate cell metastasis by inhibiting matrix metallopeptidase 2 (MMP-2) and matrix metallopeptidase 9 (MMP-9) through the PI3K signaling pathway
[99][133]. All these pathways and the molecules involved are presented in
Figure 9.
Figure 9. Chemical structure of anthocyanins and their mechanism of action through different molecular targets that result in apoptosis or suppressing growth and metastasis of cancers.
5.6. Epigallocatechin-3-Gallate (EGCG)
Tea is one of the most widely consumed beverages worldwide. Green tea (
Camellia sinensis), which is made from unfermented leaves, has been demonstrated to have the highest concentration of effective antioxidants
[102][139]. In this type of tea are found four flavanol derivatives epicatechin (EC), epigallocatechin (EGC), epicatechin gallate (ECG), and epigallocatechin gallate (EGCG)
[103][140]. The main polyphenolic component and the most abundant and physiologically active catechin found in green tea is epigallocatechin-3-gallate (EGCG), which is an ester of epigallocatechin and gallic acid
[104][105][141,142]. EGCG is a flavone-3-ol type polyphenol, which has in its chemical structure eight free hydroxyl groups, an arrangement that makes it bioactive with versatile biological functions. In addition, aside from some tannin compounds, EGCG has the strongest free-radical-scavenging capacity among common phenolic compounds
[106][143].
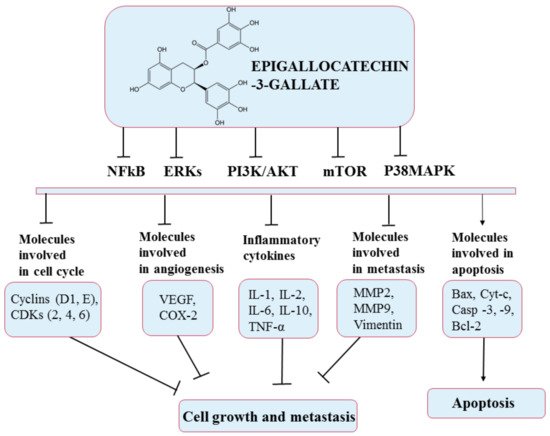
This compound has been intensively studied in recent years; research has shown its beneficial effects through its antioxidant, antibacterial, anticancer, antidiabetic, and antiangiogenic properties
[105][142]. The chemopreventive effect of EGCG has a solid basis in studies conducted both in vivo and in vitro in a number of cancers: breast, duodenum, prostate, colon, skin, lung, cervical, and liver
[103][107][140,144]. Thus, EGCG interferes with various mechanisms such as cell proliferation and differentiation, apoptosis, angiogenesis, and metastasis, with inhibitory effects on several processes in diverse types of cancer such as initiation, promotion, and progression. For example, EGCG has been shown to induce apoptosis in breast cancer by activating apoptosis-related proteins, such as caspase 3 and 9, and in cholangiocarcinoma by inducing apoptotic molecular signals, such as Bax and cytochrome c
[104][141]. Furthermore, EGCG inhibits invasion and epithelial–mesenchymal transition through modulation of MMP-2, MMP-9, and Vimentin
[108][145]. In addition, EGCG has been shown to stop cell division in a variety of cell lines at different stages of the cell cycle
[107][144]. The anticancer effects of EGCG occur via several signal transmission pathways such as MAPK and PI3K/AKT, as presented in
Figure 10 [109][146].
Figure 10. Chemical structure of epigallocatechin-3-gallate and its mechanism of action through different molecular targets that result in apoptosis or growth suppression and metastasis of cancers.