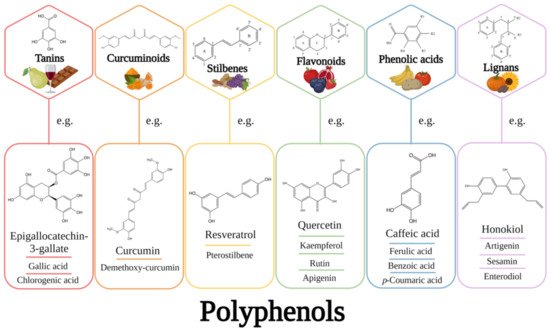
Polyphenols encapsulated in liposomes are known to produce more substantial effects on targeted cells than unencapsulated polyphenols, while having minimal cytotoxicity in healthy cells.
- polyphenols
- liposomes
- cancer therapy
- chemoprevention
- health
1. Introduction
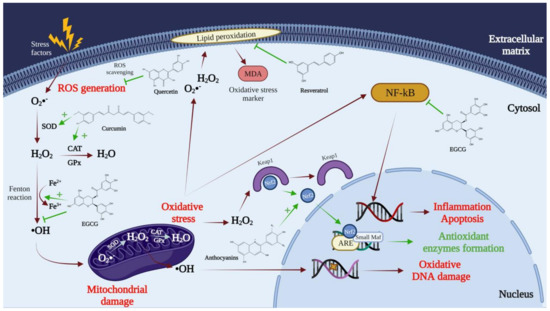
2. The Need to Encapsulate Polyphenols in Liposomes
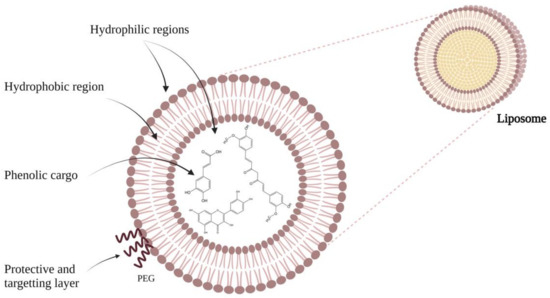
3. What Is a Liposome?
4. Applications of Liposomes
|
Polyphenol |
Production Method |
% (w/w) Polyphenol/ Lipids |
Encapsulation Efficiency |
Biological Effects |
Ref. |
|---|---|---|---|---|---|
|
Curcumin |
Lyophilization (Freeze-drying)
Evaporation method with some modification
Thin-film hydration
Ethanol injection |
10–25
15
N/S
N/S |
45% ± 0.2%
73.7% ± 1.6%
87.8% ± 4.3%
46.6% ± 1.0% |
In vivo: antiangiogenic activity and tumor growth inhibition
Enhanced stability
Slower release and better accumulation
More stable during storage |
[41]
[42]
[43] |
|
Resveratrol |
Lyophilization (Freeze-drying)
Thin-film hydration
Film hydration |
20
10
N/S |
N/S
>90%
78.14% ± 8.04% |
Prostate cancer incidence was minimized, and bioavailability was enhanced
The toxicity of free resveratrol was considerably lowered
Enhanced delivery
|
[44]
[45]
[46] |
|
Quercetin |
Film hydration and lyophilization procedure
Film hydration and sonication
Emulsification/evaporation |
30
N/S
10 |
N/S
87.1% ± 2.7%
69.42–85.72% |
Enhanced solubility, bioavailability, and antitumor activity in vivo
Maintained higher plasma quercetin concentrations
Inhibited growth of glioma cancer cells |
[23]
[47]
[48] |
|
Silymarin |
Film hydration
Reverse evaporation technique
Supercritical fluid technology |
20
10
N/S |
92.56% ± 0.93%
69.22% ± 0.6%
91.4% |
Better oral bioavailability
Higher bioavailability
Enhanced oral bioavailability |
[49]
[50]
[51] |
|
Dehydro- silymarin |
Film hydration and freeze-drying |
25 |
81.59% ± 0.24% |
Better oral bioavailability |
[52] |
|
Epigallocatechin-3-gallate (EGCG) |
Film hydration and sonication/extrusion
Film hydration
Reverse-phase evaporation method |
20
10
N/S |
84.6% ± 3.8%
80% ± 3%
85.79% ± 1.65% |
Protection against deterioration Even at lower doses, there was an increase in carcinoma cell death Enhanced targeted delivery and controlled release
Modulated the proliferation of tumor cells |
[53]
[54]
[55] |
|
Fisetin |
Film hydration and extrusion
Probe sonication |
18
7–15 |
58%
N/S |
Enhanced bioavailability and antitumor activity
Better antiangiogenic and anticancer activities |
[22]
[56] |
|
Honokiol |
Film hydration and sonication
Film hydration |
20
N/S |
95.43% ± 2.76%
90.1% ± 2.3% |
Strong anticancer effect on breast cancer Enhanced cytotoxicity and cellular uptake Enhanced bioavailability and promoted accumulation in tumor |
[57]
[58] |
|
Anthocyanins |
Film hydration
Hydration and ultrasound combined
Improved supercritical carbon dioxide (SC-CO2) |
N/S
4.5–9
20 |
43%
50.6% |
Enhanced antioxidant activity Enhanced chemical stability and bioavailability
Enhanced stability and bioavailability |
[59] [60]
[61] |
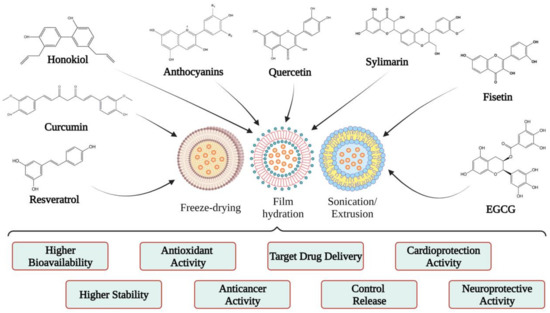
5. Polyphenols Encapsulated into Liposomes and Their Potential Health Benefits
5.1. Quercetin
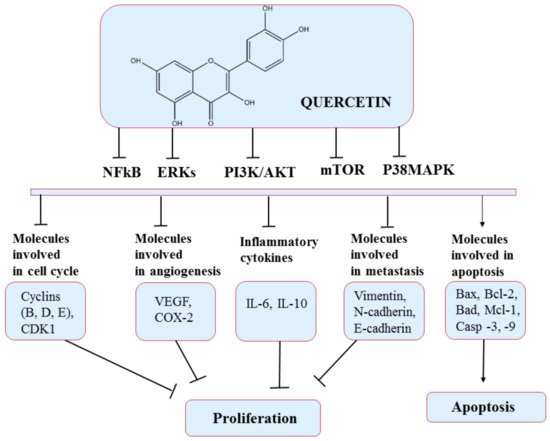
5.2. Curcumin
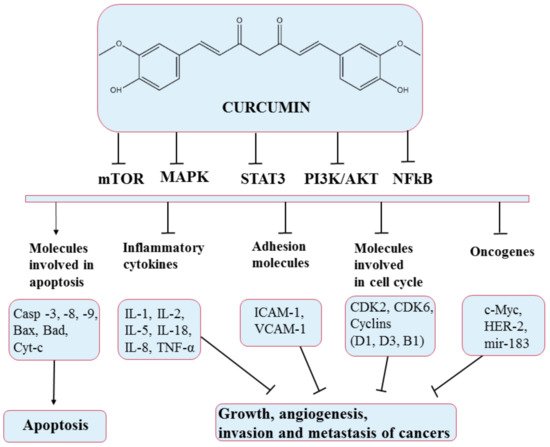
5.3. Honokiol
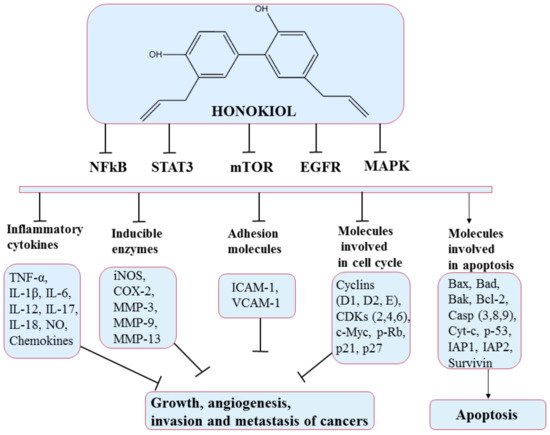
5.4. Resveratrol
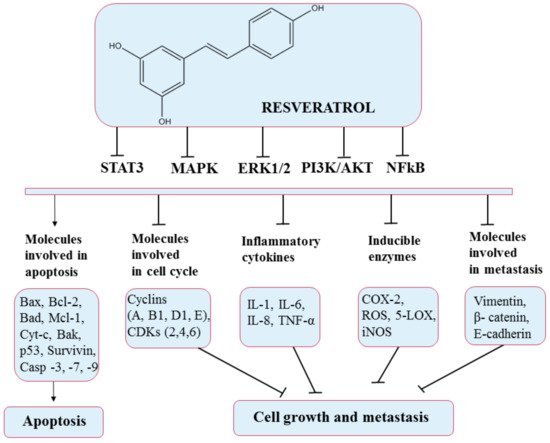
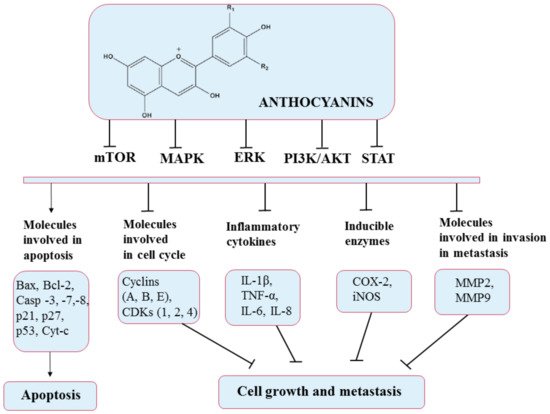
5.5. Anthocyanins
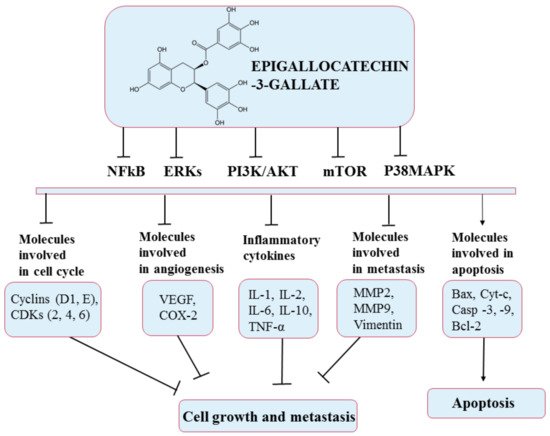
5.6. Epigallocatechin-3-Gallate (EGCG)
This entry is adapted from the peer-reviewed paper 10.3390/ph14100946
References
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047.
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid Nanocarriers for the Loading of Polyphenols—A Comprehensive Review. Adv. Colloid Interface Sci. 2018, 260, 85–94.
- Mocanu, M.-M.; Nagy, P.; Szöllősi, J. Chemoprevention of Breast Cancer by Dietary Polyphenols. Molecules 2015, 20, 22578–22620.
- El Gharras, H. Polyphenols: Food Sources, Properties and Applications—A Review: Nutraceutical Polyphenols. Int. J. Food Sci. Technol. 2009, 44, 2512–2518.
- De Araújo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and Their Applications: An Approach in Food Chemistry and Innovation Potential. Food Chem. 2021, 338, 127535.
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642.
- Parmenter, B.H.; Croft, K.D.; Hodgson, J.M.; Dalgaard, F.; Bondonno, C.P.; Lewis, J.R.; Cassidy, A.; Scalbert, A.; Bondonno, N.P. An Overview and Update on the Epidemiology of Flavonoid Intake and Cardiovascular Disease Risk. Food Funct. 2020, 11, 6777–6806.
- Mignet, N.; Seguin, J.; Chabot, G. Bioavailability of Polyphenol Liposomes: A Challenge Ahead. Pharmaceutics 2013, 5, 457–471.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Perron, N.R.; Brumaghim, J.L. Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100.
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042.
- Zhou, B.; Wu, L.-M.; Yang, L.; Liu, Z.-L. Evidence for α-Tocopherol Regeneration Reaction of Green Tea Polyphenols in SDS Micelles. Free Radic. Biol. Med. 2005, 38, 78–84.
- Du, Y.; Guo, H.; Lou, H. Grape Seed Polyphenols Protect Cardiac Cells from Apoptosis via Induction of Endogenous Antioxidant Enzymes. J. Agric. Food Chem. 2007, 55, 1695–1701.
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829.
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342.
- Watson, R.R.; Preedy, V.R.; Zibadi, S. (Eds.) Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands; Academic Press: Boston, MA, USA, 2014.
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Farina, G.; Iemma, F.; Picci, N. Polyphenols and Their Formulations. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 29–45.
- Pralhad, T.; Rajendrakumar, K. Study of Freeze-Dried Quercetin–Cyclodextrin Binary Systems by DSC, FT-IR, X-Ray Diffraction and SEM Analysis. J. Pharm. Biomed. Anal. 2004, 34, 333–339.
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and Characterization of Polyphenol-Loaded Lipid Nanocapsules. Int. J. Pharm. 2009, 379, 270–277.
- Ragelle, H.; Crauste-Manciet, S.; Seguin, J.; Brossard, D.; Scherman, D.; Arnaud, P.; Chabot, G.G. Nanoemulsion Formulation of Fisetin Improves Bioavailability and Antitumour Activity in Mice. Int. J. Pharm. 2012, 427, 452–459.
- Seguin, J.; Brullé, L.; Boyer, R.; Lu, Y.M.; Ramos Romano, M.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Mignet, N.; Chabot, G.G. Liposomal Encapsulation of the Natural Flavonoid Fisetin Improves Bioavailability and Antitumor Efficacy. Int. J. Pharm. 2013, 444, 146–154.
- Yuan, Z.; Chen, L.; Fan, L.; Tang, M.; Yang, G.; Yang, H.; Du, X.; Wang, G.; Yao, W.; Zhao, Q.; et al. Liposomal Quercetin Efficiently Suppresses Growth of Solid Tumors in Murine Models. Clin. Cancer Res. 2006, 12, 3193–3199.
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative Delivery Systems Loaded with Plant Bioactive Ingredients: Formulation Approaches and Applications. Plants 2021, 10, 1238.
- Ganesan, P.; Choi, D.K. Current Application of Phytocompound-Based Nanocosmeceuticals for Beauty and Skin Therapy. Int. J. Nanomed. 2016, 11, 1987.
- Wu, X.; Guy, R.H. Applications of Nanoparticles in Topical Drug Delivery and in Cosmetics. J. Drug Deliv. Sci. Technol. 2009, 19, 371–384.
- Chen, Z.; Farag, M.A.; Zhong, Z.; Zhang, C.; Yang, Y.; Wang, S.; Wang, Y. Multifaceted Role of Phyto-Derived Polyphenols in Nanodrug Delivery Systems. Adv. Drug Deliv. Rev. 2021, 176, 113870.
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of Univalent Ions across the Lamellae of Swollen Phospholipids. J. Mol. Biol. 1965, 13, 238–252, IN26–IN27.
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of Liposomes in Medicine and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391.
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as Potential Drug Carrier Systems for Drug Delivery; IntechOpen: London, UK, 2014.
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazù, S.; Kiselev, M.A. Soft Interaction in Liposome Nanocarriers for Therapeutic Drug Delivery. Nanomaterials 2016, 6, 125.
- William, B.; Noémie, P.; Brigitte, E.; Géraldine, P. Supercritical Fluid Methods: An Alternative to Conventional Methods to Prepare Liposomes. Chem. Eng. J. 2020, 383, 123106.
- Dwivedi, C.; Verma, S. Review on Preparation and Characterization of Liposomes with Application. Int. J. Sci. Innov. Res. 2013, 2, 23.
- Karami, N.; Moghimipour, E.; Salimi, A. Liposomes as a Novel Drug Delivery System: Fundamental and Pharmaceutical Application. Asian J. Pharm. (AJP) Free. Full Text Artic. Asian J. Pharm. 2018, 12, S31–S41.
- Liu, W.; Ye, A.; Singh, H. Progress in Applications of Liposomes in Food Systems. In Microencapsulation and Microspheres for Food Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 151–170.
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as Carrier Vehicles for Functional Compounds in Food Sector. J. Exp. Nanosci. 2016, 11, 737–759.
- Keller, B.C. Liposomes in Nutrition. Trends Food Sci. Technol. 2001, 12, 25–31.
- Barani, H.; Montazer, M. A Review on Applications of Liposomes in Textile Processing. J. Liposome Res. 2008, 18, 249–262.
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-Encapsulated Curcumin: In Vitro and In Vivo Effects on Proliferation, Apoptosis, Signaling, and Angiogenesis. Cancer 2005, 104, 1322–1331.
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal Curcumin with and without Oxaliplatin: Effects on Cell Growth, Apoptosis, and Angiogenesis in Colorectal Cancer. Mol. Cancer Ther. 2007, 6, 1276–1282.
- Wei, X.-Q.; Zhu, J.-F.; Wang, X.-B.; Ba, K. Improving the Stability of Liposomal Curcumin by Adjusting the Inner Aqueous Chamber PH of Liposomes. ACS Omega 2020, 5, 1120–1126.
- Pamunuwa, G.; Karunaratne, V.; Karunaratne, D.N. Effect of Lipid Composition on In Vitro Release and Skin Deposition of Curcumin Encapsulated Liposomes. J. Nanomater. 2016, 2016, e4535790.
- Cheng, C.; Peng, S.; Li, Z.; Zou, L.; Liu, W.; Liu, C. Improved Bioavailability of Curcumin in Liposomes Prepared Using a PH-Driven, Organic Solvent-Free, Easily Scalable Process. RSC Adv. 2017, 7, 25978–25986.
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome Encapsulation of Curcumin and Resveratrol in Combination Reduces Prostate Cancer Incidence in PTEN Knockout Mice. Int. J. Cancer 2009, 125, 1–8.
- Zhao, Y.N.; Cao, Y.N.; Sun, J.; Liang, Z.; Wu, Q.; Cui, S.H.; Zhi, D.F.; Guo, S.T.; Zhen, Y.H.; Zhang, S.B. Anti-Breast Cancer Activity of Resveratrol Encapsulated in Liposomes. J. Mater. Chem. B 2020, 8, 27–37.
- Jagwani, S.; Jalalpure, S.; Dhamecha, D.; Jadhav, K.; Bohara, R. Pharmacokinetic and Pharmacodynamic Evaluation of Resveratrol Loaded Cationic Liposomes for Targeting Hepatocellular Carcinoma. ACS Biomater. Sci. Eng. 2020, 6, 4969–4984.
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin Liposomes Ameliorate Streptozotocin-Induced Diabetic Nephropathy in Diabetic Rats. Sci. Rep. 2020, 10, 2440.
- Gang, W.; Jie, W.J.; Ping, Z.L.; Ming, D.S.; Ying, L.J.; Lei, W.; Fang, Y. Liposomal Quercetin: Evaluating Drug Delivery In Vitro and Biodistribution In Vivo. Expert Opin. Drug Deliv. 2012, 9, 599–613.
- Yanyu, X.; Yunmei, S.; Zhipeng, C.; Qineng, P. Preparation of Silymarin Proliposome: A New Way to Increase Oral Bioavailability of Silymarin in Beagle Dogs. Int. J. Pharm. 2006, 319, 162–168.
- El-Samaligy, M.S.; Afifi, N.N.; Mahmoud, E.A. Increasing Bioavailability of Silymarin Using a Buccal Liposomal Delivery System: Preparation and Experimental Design Investigation. Int. J. Pharm. 2006, 308, 140–148.
- Yang, G.; Zhao, Y.; Zhang, Y.; Dang, B.; Liu, Y.; Feng, N. Enhanced Oral Bioavailability of Silymarin Using Liposomes Containing a Bile Salt: Preparation by Supercritical Fluid Technology and Evaluation In Vitro and In Vivo. Int. J. Nanomed. 2015, 10, 6633.
- Chu, C.; Tong, S.; Xu, Y.; Wang, L.; Fu, M.; Ge, Y.; Yu, J.; Xu, X. Proliposomes for Oral Delivery of Dehydrosilymarin: Preparation and Evaluation In Vitro and In Vivo. Acta Pharmacol. Sin. 2011, 32, 973–980.
- Fang, J.-Y.; Lee, W.-R.; Shen, S.-C.; Huang, Y.-L. Effect of Liposome Encapsulation of Tea Catechins on Their Accumulation in Basal Cell Carcinomas. J. Dermatol. Sci. 2006, 42, 101–109.
- Marwah, M.; Perrie, Y.; Badhan, R.K.S.; Lowry, D. Intracellular Uptake of EGCG-Loaded Deformable Controlled Release Liposomes for Skin Cancer. J. Liposome Res. 2020, 30, 136–149.
- Luo, X.; Guan, R.; Chen, X.; Tao, M.; Ma, J.; Zhao, J. Optimization on Condition of Epigallocatechin-3-Gallate (EGCG) Nanoliposomes by Response Surface Methodology and Cellular Uptake Studies in Caco-2 Cells. Nanoscale Res. Lett. 2014, 9, 291.
- Mignet, N.; Seguin, J.; Ramos Romano, M.; Brullé, L.; Touil, Y.S.; Scherman, D.; Bessodes, M.; Chabot, G.G. Development of a Liposomal Formulation of the Natural Flavonoid Fisetin. Int. J. Pharm. 2012, 423, 69–76.
- Ju, R.-J.; Cheng, L.; Qiu, X.; Liu, S.; Song, X.-L.; Peng, X.-M.; Wang, T.; Li, C.-Q.; Li, X.-T. Hyaluronic Acid Modified Daunorubicin plus Honokiol Cationic Liposomes for the Treatment of Breast Cancer along with the Elimination Vasculogenic Mimicry Channels. J. Drug Target. 2018, 26, 793–805.
- Zhou, C.; Guo, C.; Li, W.; Zhao, J.; Yang, Q.; Tan, T.; Wan, Z.; Dong, J.; Song, X.; Gong, T. A Novel Honokiol Liposome: Formulation, Pharmacokinetics, and Antitumor Studies. Drug Dev. Ind. Pharm. 2018, 44, 2005–2012.
- Hwang, J.-M.; Kuo, H.-C.; Lin, C.-T.; Kao, E.-S. Inhibitory Effect of Liposome-Encapsulated Anthocyanin on Melanogenesis in Human Melanocytes. Pharm. Biol. 2013, 51, 941–947.
- Homayoonfal, M.; Mousavi, S.M.; Kiani, H.; Askari, G.; Desobry, S.; Arab-Tehrany, E. Encapsulation of Berberis Vulgaris Anthocyanins into Nanoliposome Composed of Rapeseed Lecithin: A Comprehensive Study on Physicochemical Characteristics and Biocompatibility. Foods 2021, 10, 492.
- Zhao, L.; Temelli, F.; Chen, L. Encapsulation of Anthocyanin in Liposomes Using Supercritical Carbon Dioxide: Effects of Anthocyanin and Sterol Concentrations. J. Funct. Foods 2017, 34, 159–167.
- Saraswat, A.L.; Maher, T.J. Development and Optimization of Stealth Liposomal System for Enhanced In Vitro Cytotoxic Effect of Quercetin. J. Drug Deliv. Sci. Technol. 2020, 55, 101477.
- Daneshniya, M.; Maleki, M.H.; Liavali, H.; Hassanjani, M.; Keshavarz Bahadori, N.; Mohammadi, M.; Jalilvand Nezhad, H. Antioxidant Activity of Flavonoids as an Important Phytochemical Compound in Plants. In Proceedings of the 2nd International Congress on Engineering, Technology and Innovation, Darmstadt, Germany, 6 November 2020.
- Chou, C.-C.; Yang, J.-S.; Lu, H.-F.; Ip, S.-W.; Lo, C.; Wu, C.-C.; Lin, J.-P.; Tang, N.-Y.; Chung, J.-G.; Chou, M.-J.; et al. Quercetin-Mediated Cell Cycle Arrest and Apoptosis Involving Activation of a Caspase Cascade through the Mitochondrial Pathway in Human Breast Cancer MCF-7 Cells. Arch. Pharm. Res. 2010, 33, 1181–1191.
- Long, Q.; Xie, Y.; Huang, Y.; Wu, Q.; Zhang, H.; Xiong, S.; Liu, Y.; Chen, L.; Wei, Y.; Zhao, X.; et al. Induction of Apoptosis and Inhibition of Angiogenesis by PEGylated Liposomal Quercetin in Both Cisplatin-Sensitive and Cisplatin-Resistant Ovarian Cancers. J. Biomed. Nanotechnol. 2013, 9, 965–975.
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological Basis and New Insights of Quercetin Action in Respect to Its Anti-Cancer Effects. Biomed. Pharmacother. 2020, 121, 109604.
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and Cancer: New Insights into Its Therapeutic Effects on Ovarian Cancer Cells. Cell Biosci. 2020, 10, 32.
- Kumari, A.; Kumar, V.; Yadav, S.K. Plant Extract Synthesized PLA Nanoparticles for Controlled and Sustained Release of Quercetin: A Green Approach. PLoS ONE 2012, 7, e41230.
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and MiRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214.
- Arora, S.; Singh, S.; Piazza, G.A.; Contreras, C.M.; Panyam, J.; Singh, A.P. Honokiol: A Novel Natural Agent for Cancer Prevention and Therapy. Curr. Mol. Med. 2012, 12, 1244–1252.
- Esumi, T.; Makado, G.; Zhai, H.; Shimizu, Y.; Mitsumoto, Y.; Fukuyama, Y. Efficient Synthesis and Structure–Activity Relationship of Honokiol, a Neurotrophic Biphenyl-Type Neolignan. Bioorg. Med. Chem. Lett. 2004, 14, 2621–2625.
- Ishitsuka, K.; Hideshima, T.; Hamasaki, M.; Raje, N.; Kumar, S.; Hideshima, H.; Shiraishi, N.; Yasui, H.; Roccaro, A.M.; Richardson, P.; et al. Honokiol Overcomes Conventional Drug Resistance in Human Multiple Myeloma by Induction of Caspase-Dependent and -Independent Apoptosis. Blood 2005, 106, 1794–1800.
- Lee, Y.-J.; Lee, Y.M.; Lee, C.-K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic Applications of Compounds in the Magnolia Family. Pharmacol. Ther. 2011, 130, 157–176.
- Ezzat, S.M.; Shouman, S.A.; Elkhoely, A.; Attia, Y.M.; Elsesy, M.S.; El Senousy, A.S.; Choucry, M.A.; El Gayed, S.H.; El Sayed, A.A.; Sattar, E.A.; et al. Anticancer Potentiality of Lignan Rich Fraction of Six Flaxseed Cultivars. Sci. Rep. 2018, 8, 544.
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for Cancer Therapeutics: A Traditional Medicine That Can Modulate Multiple Oncogenic Targets. Pharmacol. Res. 2019, 144, 192–209.
- Qiu, N.; Cai, L.; Xie, D.; Wang, G.; Wu, W.; Zhang, Y.; Song, H.; Yin, H.; Chen, L. Synthesis, Structural and In Vitro Studies of Well-Dispersed Monomethoxy-Poly(Ethylene Glycol)–Honokiol Conjugate Micelles. Biomed. Mater. 2010, 5, 065006.
- Bai, X.; Cerimele, F.; Ushio-Fukai, M.; Waqas, M.; Campbell, P.M.; Govindarajan, B.; Der, C.J.; Battle, T.; Frank, D.A.; Ye, K.; et al. Honokiol, a Small Molecular Weight Natural Product, Inhibits Angiogenesis In Vitro and Tumor Growth In Vivo *. J. Biol. Chem. 2003, 278, 35501–35507.
- Li, Z.; Liu, Y.; Zhao, X.; Pan, X.; Yin, R.; Huang, C.; Chen, L.; Wei, Y. Honokiol, a Natural Therapeutic Candidate, Induces Apoptosis and Inhibits Angiogenesis of Ovarian Tumor Cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 95–102.
- Yang, J.; Pei, H.; Luo, H.; Fu, A.; Yang, H.; Hu, J.; Zhao, C.; Chai, L.; Chen, X.; Shao, X.; et al. Non-Toxic Dose of Liposomal Honokiol Suppresses Metastasis of Hepatocellular Carcinoma through Destabilizing EGFR and Inhibiting the Downstream Pathways. Oncotarget 2016, 8, 915–932.
- Pezzuto, J. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2018, 27, 1–14.
- Harikumar, K.B.; Aggarwal, B.B. Resveratrol: A Multitargeted Agent for Age-Associated Chronic Diseases. Cell Cycle 2008, 7, 1020–1035.
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. 2016, 7, 706–718.
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The In Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506.
- Cardile, V.; Chillemi, R.; Lombardo, L.; Sciuto, S.; Spatafora, C.; Tringali, C. Antiproliferative Activity of Methylated Analogues of E- and Z-Resveratrol. Z. Naturforschung C 2007, 62, 189–195.
- Wang, Y.; Halls, C.; Zhang, J.; Matsuno, M.; Zhang, Y.; Yu, O. Stepwise Increase of Resveratrol Biosynthesis in Yeast Saccharomyces Cerevisiae by Metabolic Engineering. Metab. Eng. 2011, 13, 455–463.
- Tian, B.; Liu, J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020, 100, 1392–1404.
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and Solubility of Trans-Resveratrol Are Strongly Influenced by PH and Temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204.
- Subramanian, L.; Youssef, S.; Bhattacharya, S.; Kenealey, J.; Polans, A.S.; van Ginkel, P.R. Resveratrol: Challenges in Translation to the Clinic—A Critical Discussion. Clin. Cancer Res. 2010, 16, 5942–5948.
- Smoliga, J.M.; Blanchard, O. Enhancing the Delivery of Resveratrol in Humans: If Low Bioavailability Is the Problem, What Is the Solution? Molecules 2014, 19, 17154–17172.
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of Resveratrol: What Formulation Solutions to Bioavailability Limitations? J. Control. Release 2012, 158, 182–193.
- Han, G.; Xia, J.; Gao, J.; Inagaki, Y.; Tang, W.; Kokudo, N. Anti-Tumor Effects and Cellular Mechanisms of Resveratrol. Drug Discov. Ther. 2015, 9, 1–12.
- Shankar, S.; Gyanendra, S.; Rakesh, K.S. Chemoprevention by Resveratrol: Molecular Mechanisms and Therapeutic Potential. Front. Biosci. 2007, 12, 4839.
- Lee, M.-F.; Pan, M.-H.; Chiou, Y.-S.; Cheng, A.-C.; Huang, H. Resveratrol Modulates MED28 (Magicin/EG-1) Expression and Inhibits Epidermal Growth Factor (EGF)-Induced Migration in MDA-MB-231 Human Breast Cancer Cells. J. Agric. Food Chem. 2011, 59, 11853–11861.
- Meng, X.; Zhou, J.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods 2020, 9, 340.
- Van Ginkel, P.R.; Sareen, D.; Subramanian, L.; Walker, Q.; Darjatmoko, S.R.; Lindstrom, M.J.; Kulkarni, A.; Albert, D.M.; Polans, A.S. Resveratrol Inhibits Tumor Growth of Human Neuroblastoma and Mediates Apoptosis by Directly Targeting Mitochondria. Clin. Cancer Res. 2007, 13, 5162–5169.
- Mukherjee, S.; Dudley, J.I.; Das, D.K. Dose-Dependency of Resveratrol in Providing Health Benefits. Dose-Response 2010, 8, 478–500.
- Chi, J.; Ge, J.; Yue, X.; Liang, J.; Sun, Y.; Gao, X.; Yue, P. Preparation of Nanoliposomal Carriers to Improve the Stability of Anthocyanins. LWT 2019, 109, 101–107.
- Diaconeasa, Z.; Frond, A.; Stirbu, I.; Rugină, D.; Socaciu, C. Anthocyanins-Smart Molecules for Cancer Prevention. In Phytochemicals-Source of Antioxidants and Role in Disease Prevention; Asao, T., Asaduzzaman, M., Eds.; IntechOpen: London, UK, 2018.
- Diaconeasa, Z.; Știrbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stǎnilǎ, A.; Nistor, M.; Socaciu, C. Anthocyanins, Vibrant Color Pigments, and Their Role in Skin Cancer Prevention. Biomedicines 2020, 8, 336.
- Fernández, J.; García, L.; Monte, J.; Villar, C.J.; Lombó, F. Functional Anthocyanin-Rich Sausages Diminish Colorectal Cancer in an Animal Model and Reduce Pro-Inflammatory Bacteria in the Intestinal Microbiota. Genes 2018, 9, 133.
- Fakhri, S.; Khodamorady, M.; Naseri, M.; Farzaei, M.H.; Khan, H. The Ameliorating Effects of Anthocyanins on the Cross-Linked Signaling Pathways of Cancer Dysregulated Metabolism. Pharmacol. Res. 2020, 159, 104895.
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic Effects of EGCG: A Patent Review. Expert Opin. Ther. Pat. 2016, 26, 907–916.
- Sanni, O.; Enebi, D. A Multidisciplinary Research Book; Maharani Kasiswari College Kolkata: West Bengal, India, 2021.
- Aggarwal, V.; Tuli, H.S.; Tania, M.; Srivastava, S.; Ritzer, E.E.; Pandey, A.; Aggarwal, D.; Barwal, T.S.; Jain, A.; Kaur, G.; et al. Molecular Mechanisms of Action of Epigallocatechin Gallate in Cancer: Recent Trends and Advancement. Semin. Cancer Biol. 2020, in press.
- Chen, W.; Zou, M.; Ma, X.; Lv, R.; Ding, T.; Liu, D. Co-Encapsulation of EGCG and Quercetin in Liposomes for Optimum Antioxidant Activity. J. Food Sci. 2018, 84, 111–120.
- Gan, R.-Y.; Li, H.-B.; Sui, Z.-Q.; Corke, H. Absorption, Metabolism, Anti-Cancer Effect and Molecular Targets of Epigallocatechin Gallate (EGCG): An Updated Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 924–941.
- Wang, Y.-Q.; Lu, J.-L.; Liang, Y.-R.; Li, Q.-S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334.
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer Preventive and Therapeutic Effects of EGCG, the Major Polyphenol in Green Tea. Egypt. J. Basic Appl. Sci. 2018, 5, 1–23.
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green Tea Extracts Epigallocatechin-3-Gallate for Different Treatments. BioMed Res. Int. 2017, 2017, 5615647.
