1000/1000
Hot
Most Recent

The exact connection between Alzheimer’s disease (AD) and type 2 diabetes is still in debate. However, poorly controlled blood sugar may increase the risk of developing Alzheimer’s. This relationship is so strong that some have called Alzheimer’s “diabetes of the brain” or “type 3 diabetes (T3D)”. Given more recent studies continue to indicate evidence linking T3D with AD, this state-of-the-art aimed to demonstrate the relationship between T3D and AD based on the fact that both the processing of amyloid-β (Aβ) precursor protein toxicity and the clearance of Aβ are attributed to impaired insulin signaling, and that insulin resistance mediates the dysregulation of bioenergetics and progress to AD.
The key to understanding the relationship between diabetes and these other areas begins with the role of energy homeostasis in diabetes. Energy homeostasis is a well-regulated process that depends on the coordination between feeding behavior and energy expenditure. The control of energy homeostasis in humans has received much attention in recent years due to alterations caused by onset of conditions such as obesity and diabetes. There are two distinct features of adult neurons that make them vulnerable to either neuronal cell death or a diseased state such as neurodegeneration or neuronal loss. The first feature is that fully differentiated (adult) neurons are permanently postmitotic cells, which lack regenerative ability [1]. Therefore, when adult neurons are exposed to any cellular stresses such as lack of adenosine triphosphate (ATP) moieties or energy crisis or oxidative stress, they either die or experience apoptosis, or degenerate or cause neuronal degeneration and loss, and thus predispose neurodegenerative diseases [1]. The second important feature is that brain neurons or tissues are highly demanding excitable cells, in which more than 40% of the present ATP is used to keep neurons viable or alive [2]. There are two sources of brain glucose that involve cortical glucose metabolism stimulation through basal insulin levels [3] and astrocytic glycogen conversion to glucose that is stimulated by the activation of glial β-adrenoceptors. The increase in glucose uptake is transported by insulin-sensitive glial glucose transporter type 1 (GLUT1) to the plasma membrane for neuronal use. Therefore, the balanced cellular glucose transportation depends on astrocytes and glucose transporters that are expressed in the brain [4].
Moreover, a glucose homeostasis defect might be important in the pathogenesis of T3DM due to impaired glucose uptake as a result of impaired glucose metabolism in the brain. The mechanisms that are involved in glucose transportation abnormalities include brain insulin resistance and intracellular glucose metabolic disturbance. These two abnormalities may contribute to cerebral glucose hypometabolism in T3DM or the brain insulin resistance disease state. A decreased glucose transporters correlated to abnormal hyperphosphorylation of tau in neurodegenerative diseases was reported [5]. Therefore, impairment of insulin signaling not only affects systemic glucose blood levels but also causes various degenerative processes or neuronal cell death or loss [6]. In addition, insulin resistance in T2DM has been defined as “reduced sensitivity in body tissues to the action of insulin” [7]. Similarly, brain insulin resistance can be defined as the failure of brain cells to respond to insulin and its corresponding IRs [8]. Consequently, this leads to insulin deficiency and impaired glucose transport inside the neurons due decreased number of expressed GLUTs in the cell membrane. Furthermore, insulin resistance in the CNS correlates with insulin resistance in the periphery. Therefore, loss of responsiveness to insulin could make neurons more susceptible to neurotoxic insults due to their being devoid of protective effect of insulin [9]. Furthermore, insulin-resistant patients have many increased pathologic features such as apoptosis, neurodegeneration, and the resultant decline in cognition.
The desensitization of the neuronal insulin receptor in brain insulin resistance, similar to the process in T2DM, may play a key role in causing T3DM and its future complications [10]. Besides, T2DM is a metabolic syndrome characterized by insulin resistance, which is also a pathological feature of neurodegeneration or neuroendocrine disorder or T3DM [3]. Thus, glucose homeostasis plays a role in T3DM pathogenesis. Brain glucose uptake or metabolism is impaired in T3DM. Therefore, the combination of T2DM and neurodegenerative brain diseases may be considered as this new classification of diabetes, called T3DM or a neuroendocrine disorder.
Amyloidosis is a pathological condition which consists of the accumulation of fibrillary proteins, characterizing by extracellular amyloid deposits with a clinical variability depending on the affected tissue. Recently, there has been newly emerged evidence regarding the relationship between the pathogenesis of AD and insulin resistance. It is important to consider T2DM as a risk factor essential for the formation of deposits of amyloid-β in patients’ brains with dementia. There was a toxic cycle between continuous insulin exposure and Aβ accumulation inside the neurons [11]. According to Farris et al., insulin degrading enzyme (IDE) regulates the levels of insulin, Aβ protein, and amyloid precursor protein (APP) intracellular domain in vivo [11]. This study showed that a rat model of T2DM of mutant IDE was associated with hyperinsulinemia and glucose intolerance, as hallmarks of T2DM and T3DM or brain insulin resistance. This implies that IDE hypofunction may underlie or contribute to some forms of T3DM and T2DM and provide a mechanism for the recently recognized association among hyperinsulinemia, diabetes, and neurodegeneration or neuronal loss [11]. Therefore, in normal subjects, IDE reduces Aβ, regulates insulin and also degrades APP intracellular domain (AICD). Thus, there was a regulatory relationship among insulin, IDE and Aβ. In the case of brain insulin resistance, insulin possibly failed to stimulate the clearance of Aβ, which permits its buildup inside the neurons causing neurodegeneration or neuronal loss, as hallmarks of T3DM or brain insulin resistance [11]. There is a debate about T3DM and brain insulin resistance as to whether it is a consequence or a cause of abnormal Aβ expression and protein processing [12]. In terms of the concept of T3DM being a consequence, Aβ toxicity may cause insulin resistance in the brain. The Aβ disturbs insulin signaling by competing with insulin on its receptors [13], reducing the surface expression of IRs, and reducing the insulin affinity to its relative receptors, and interfering directly with phosphatidylinositol-4, 5- bisphosphate 3-kinase (PI3K)/Akt activation, causing a blockade of its signaling and leading to impaired survival signaling, increased activation of GSK-3β activity, and increased hyperphosphorylation of tau [14].
On the other hand, in terms of the concept of T3DM being the cause, the brain insulin resistance with oxidative stress and neuroinflammation may cause Aβ accumulation, as shown in Figure 1. The studies that incorporate this concept claim that insulin stimulation may increase or accelerate trafficking of Aβ from the Golgi network to the plasma membrane. Therefore, insulin may activate Aβ extracellular excretion and, at the same time, inhibit its intracellular accumulation by activating its degradation by the insulin-degrading enzyme (IDE) [15]. Thus, impaired insulin signaling can disturb both APP processing and Aβ clearance [16]. This leads to increased neurotoxic effects of Aβ on neurons, resulting in possible neurodegeneration and neuronal cell death. T2DM and AD patients have similar amyloid beta deposits both in pancreas and in the brain. Several researchers have suggested this new pathology should be addressed as T3D [17][18][19][20]. Some of the target receptors of T2DM such as the insulin-like growth factor 1 (IGF-1) and peroxisome proliferator-activated receptor gamma (PPARG) are also involved in the regulation of the expression and phosphorylation of tau protein [20].
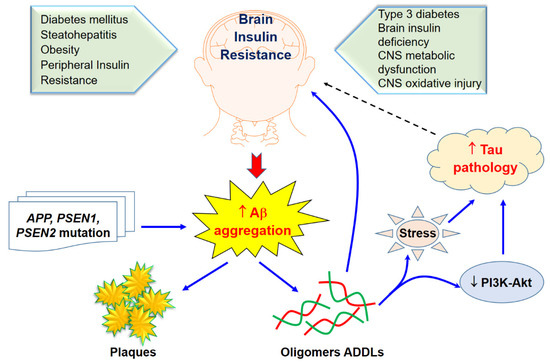
Figure 1. Brain insulin resistance and Aβ aggregation and its toxicity. Solid arrows indicate the interactions of Aβ aggregation on brain insulin resistance through sone potential pathways while tau pathology would likely effect of brain insulin as revealed in a dasher arrow.
Insulin resistance in AD and diabetes can lead to hyperinsulinemia, thereby, saturating insulin-degrading enzymes (IDE) for insulin and Aβ degradation. Recently, many studies indicated that the incidence of AD is higher in T2D patients and obese individuals, implying common mechanisms driving these disorders [21][22][23]. Insulin resistance could be a main feature which is shared among diabetes, obesity, and AD [24]. The neuronal glucose uptake may not depend on insulin totally, thus the concept of insulin resistance in the brain is more related to impaired insulin signaling pathways. The malfunction of insulin signaling pathways and resultant state of hypometabolism observed are considering among factors in altered bioenergetics that connects AD and T2D [25]. The insulin resistant state could lead to compromised neuron functions and cognitive skills, accompanied by an extreme rise in insulin and relatively declined insulin activity in the periphery as important predictors of T2D [26][27]. Consequently, this leads to the development of neuritic plaques, hippocampal atrophy, cognitive performance and lower cerebrocortical glucose metabolism which may closely correlate with memory impairments [19]. A previous study revealed that increased p-Ser312IRS1 manifested in prodromal AD patients that sustained these alterations a decade ago as AD patients [28], suggesting that insulin resistance in AD develops years before clinical manifestations and that neural-derived exosomes carry potential for early AD diagnosis. Due to lack of insulin response, down regulation of insulin receptors, reduced binding of insulin receptors or faulty activation of the insulin signaling cascade cause the defective brain insulin signaling in AD and T2D. The major consequence of this altered cascade is the decreased neuronal glucose uptake that is manifested as impaired neuroplasticity, neurotransmitter deficits, collapse of bioenergetics mechanism and initiation of fateful inflammatory cascade. Overall, the consequences of impaired insulin signaling are attributed to impaired metabolism in the brain that may lead to brain malfunction, providing possible explanations for the connection between diabetes, obesity, and AD [29], as shown in Table 1.
Table 1. Causal model for the potential associated with between T3D and AD.
| Upstream Risk Factors | Metabolic Precursors | Pathways | Subclinical Pathology | Disease Outcome | |
|---|---|---|---|---|---|
| Social factors: stress, low socioeconomic status, certain ethnic and racial groups | Obesity Visceral Adiposity |
Vascular Processes Blood pressure and hypertension Hyperlipidemia Apolipoprotein E |
Cerebral blood flow Atherosclerosis |
Amyloid precursor proteins | Alzheimer’s disease |
| Poor diet: high in calories, fat and sugar, low in fiver | Inflammatory/Oxidative processes Inflammation Oxidative stress Endothelial function |
Neurofibrillary Tangles Amyloid B deposits |
|||
| Physical inactivity Genetics and family history |
Hyperglycemia Hyperinsulinemia |
Metabolic processes Insulin resistance Insulin-degrading enzyme Peroxisome proliferative-activated receptors |
|||
| Early childhood exposures in utero and birth weight | Brain and hippocampal atrophy White matter hyperintensities |
||||
Insulin resistance or dysfunction of insulin signaling is a universal feature of T2D, due to altered glucose metabolism and its interdependence on cell death pathways form the basis of linking T3D with AD, as shown in Figure 2. T3D occurs when neurons in the brain become unable to respond to insulin, which is essential for basic tasks, including memory and learning. Some researchers believe insulin deficiency is central to the cognitive decline of AD. Dysfunctional insulin pathways and resistance of insulin is a status of receptor dysfunction, altered receptor expression, deviations in receptor binding and malfunctioned events in the phosphorylation chain or the altered activities related to kinases involved in phosphorylation. At the molecular level, a cell senses insulin through insulin receptors, with the signal propagating through a signaling cascade collectively known as the PI3K/Akt/mTOR signaling pathway. Recent studies suggested that the pathway operates as a bistable switch under physiologic conditions for certain types of cells, and insulin response may well be a threshold phenomenon [30][31][32]. The pathway’s sensitivity to insulin may be blunted by many factors such as free fatty acids, causing insulin resistance. It also is based on the finding that insulin resistance may be reversed rapidly by exposing cells to mitochondrial uncouplers, electron transport chain inhibitors, or mitochondrial superoxide dismutase mimetics [33][34].
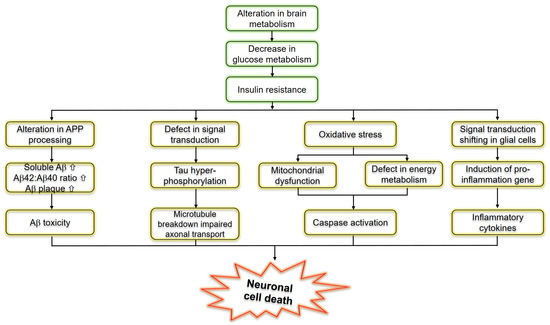
Figure 2. Schematic representation of molecular pathways linking insulin resistance and Alzheimer’s disease.
Interestingly, impaired insulin signaling is present in several transgenic and nontransgenic mouse models of AD. Some previous clinical studies have reported that AD patients could have glucose intolerance, suggesting a bidirectional relationship between the two conditions [35][36]. Reduced levels of IRS-1 associated to the membrane of hippocampal extracts [37] and a decreased activation of IRS-1 and PI3K in the hippocampus and cortex were observed in ten-month-old mice [38]. Markers of insulin resistance were also reported in the hypothalamus of APP/PS1 mice [39] since the IRS-1 phosphorylated in serine 616 in the hippocampus at nine months of age was higher than that of the control group [40], and increased levels of IRS-1 phosphorylated in serine 636 and 312 in the frontal cortex at 13 months [41] were also demonstrated. In combination with peripheral insulin resistance, there was also a report of an increased inhibitory phosphorylation of IRS-1 in serine 612 in the hippocampus of five-month-old tg2576 mice [38]. Remarkably, the central infusion of AβOs lead to peripheral insulin resistance, which was further observed in the APP/PS1 and in the 3xTgAD mouse models of AD [42]. To confirm these concepts, further evidence is still required to investigate the mechanisms whereby AD affects the diabetic phenotype. T3D regarding AD and its approaches for treatment and prevention using naturally synthetic compounds, as shown in Figure 1.
In addition, in the wake of the worldwide increase in T2DM, a major focus of research aims to understand the signaling pathways impacting this disease. Insulin signaling regulates glucose, lipid, and energy homeostasis, predominantly via action on liver, skeletal muscle, and adipose tissue. Cell signaling pathways can be described by a list of biomolecular reactions which occur between the pathway components. T2DM associated with impaired insulin and insulin-like growth factor-1 (IGF1) signaling (IIS) is a risk factor for cognitive impairment and dementia including AD [43]. Importantly, systemic heterozygous inactivation of IGF1R (IGF1R+/−) or neuronal deletion of IGF1R (nIGF1R−/−) could improve the survival in the Tg2576 mouse model of AD while reducing behavioral impairment and Aβ accumulation [44]. Reduced IRS2 signaling throughout the body or in the brain prolongs life span [45] may lead to systemic reduction of IRS2 (IRS2−/−), improves cognitive function, and reduces Aβ deposition and premature mortality in Tg2576 mice with normal blood glucose levels [44][46]. Hence, more recent animal studies have revealed that a reduction in intracellular signaling mediated by IGF1R-IRS2 signaling but not the IR cascade in the CNS exerts neuroprotective effects in AD animal models [43].
Insulin resistance is well known as an essential feature of T3D, therefore treatment strategies for T3D, particularly those aimed at improving insulin sensitivity, may also benefit those patients at risk for AD at the early stages. Due to the overlapping yet distinct pathological features among diabetes, insulin resistance and cognitive decline, multitargeted drug therapies along with lifestyle interventions are also explored [47] from the perspective of research in the pharmaceutical industry, including nutraceuticals, antioxidant activity [48], polyphenols, omega-3 fatty acids as well as the brain–gut connections [49].
Among nutraceuticals produce, a brain-permeable compound, curcumin is able to target abnormal protein aggregates [50]. Curcumin may also thwart “proapoptotic signaling pathways in primary hippocampal neuron cultures”. Previous research has also shown the benefit of metformin in mice when coupled with curcumin and piperine supplementation, particularly regarding enhanced insulin sensitivity, signaling, and better systemic glucose tolerance [50], promising natural substances for AD patients. However, the anti-inflammatory benefits of fruits and vegetables have been widely publicized for decades, particularly regarding antioxidant action in reducing inflammatory damage [51]. Rodent research has linked various vegetables and fruits as protective “against cognitive and brain neuropathology from dietary oxidative stress” due to innumerable bioactive constituents such as carotenoids, antioxidant vitamins, polyphenols and flavonoids [52]. A various families of flavonoids have been suggested to be the potential therapeutic implications via in vivo models [53]. This has significant potential to advance our understanding of proactive approaches toward preventing AD and inhibiting progression. The essential role of omega-3 fatty acids in brain development and maintenance has been well recognized, particularly in the past ten years, yet only recently “have their effects on brain aging been explored” [54]. Diets rich in omega-3 fatty acids and naturally low in omega-6fatty acids may hold the key for nutritional therapy for AD patients [55]. The ketogenic diet may even diminish and clear beta amyloid plaques within the brain, while convalescing damaged mitochondria and reducing universal inflammation [56]. New research has shown that glycated APOE4 protein and faulty insulin signaling leads not only to impaired energy transport for brain tissues, but also impaired lipid transportation, mainly cholesterol [56][57]. APOE4 accounted for approximately 20% of the general population and >50% among Alzheimer’s cases, is responsible for interrupting how the brain processes insulin [58]. The gene and the peripheral insulin resistance caused by the high-fat diet together induced insulin resistance in the brain [59]. The APOE4 protein produced by the gene can bind more aggressively to insulin receptors on the surfaces of neurons than its normal counterpart, APOE3. APOE4 goes on to do lasting damage to brain cells. After blocking the receptor, the sticky APOE4 protein begins to clump and becomes toxic [59]. Furthermore, once the protein enters the interior of the neuron, the clumps get trapped within the cell’s machinery, impeding the receptors from returning to the neuron surface to do their work. The insulin signal processing gets increasingly more impaired, starving brain cells. There is no pharmaceutical intervention that has ever existed that has been more potent in improving overall vasculature throughout the body, than exercise [60]. This also has extensive implications for AD patients and type 2 diabetics due to increases in quality of life, neurochemical messaging within the brain, restorative power over insulin resistance, and the ability to clear Aβ plaques in certain individuals [60]. The concept of the gut–brain axis, the bidirectional communication between gut and brain, contributing significantly to the pathogenesis of AD that has been supported by many experimental and clinical studies [49]. Representatives of some compounds and drugs for the treatment or prevention of T3D regarding AD progression are presented in Table 2.
Table 2. Summary of representative of preclinical and clinical studies on the efficacy of antidiabetic, insulin-sensitizing drugs on multiple aspects of AD pathology.
| Compound | Potential Pathway | Study Design | Reference |
|---|---|---|---|
| DA5-CH | Reduces tau phosphorylation and normalizes theta rhythm | Injected intracerebroventricula (ICV), streptozotocin on rat | [61] |
| DA-JC1 | Antagonizing circadian rhythm disorders induced by Aβ31–35 | ICV, amyloid(31–35) AD model | [62] |
| DA5-CH | Improved of hippocampal synaptic plasticity and activation of the PI3K/AKT signaling pathway | APP/PS1 mouse model of AD | [63] |
| DA-CH3 | Reduced ER stress and apoptotic signaling, reduced amyloid plaque load in the brain | APP/PS1 mouse model of AD | [64] |
| Insulin | Prevention of Aβ oligomer induced synapse loss and insulin receptor reduction, amelioration of PKR-mediated ER stress | Rat hippocampal neuronal cultures | [65][66] |
| Insulin | AD patients that are not ε4 carriers have reduced sensitivity to insulin, effecting cognitive performance | AD patients homozygous or not for the ApoE-ε4 allele and normal subjects intravenously injected | [67] |
| Insulin | Improved verbal memory in MCI AD ε4-subjects after acute insulin administration, but not in ε4 carriers | AD patients homozygous or not for the ApoE-ε4 allele, MCI patients and most subjects intranasally administrated | [68][69] |
| Insulin | Chromic intranasal insulin doses enhanced selective attention, retention of new information and functional status of MCI and early AD subjects | AD patients, MCI patients and normal subjects intranasally administrated | [70] |
| Insulin | Only women presented improved working memory after treatment | Healthy men and woman intranasally administrated | [71] |
| Liraglutide | Reduction of tau phosphorylation; protection of insulin reception and synapse loss in a c-AMP dependent manner | Cynomolgus monkeys ICV with Aβ oligomer | [72] |
| Liraglutide | Improvement of memory deficits in novel object recognition test and fear conditioning | Swiss mice injected ICV with Aβ oligomer | [72] |
| Liraglutide | Restored memory deficits in object recognition test and Morris water maze; enhanced LTP; reduced microglial activation; diminished Aβ plaque load | APP/PSEN1 mice | [73][74] |
| Exendin-4 | Decrease in the inhibitory phosphorylation of Ser312IRS1, Ser66IRS1 of INK, while restoring activating Tyr465 IRS1 phosphorylation | Rat hippocampal neural cultures | [41] |
| Exendin-4 | Improvement of spatial memory in the Morris water maze; reduced amyloid plaque LOAD | APP/PS1 mice | [41] |
| Exedin4- Liraglutide | eIF2α phosphorylation reduction | Rat hippocampal neural cultures, APP/PS1 mice, cynomolgus monkeys injected ICV with Aβ oligomer | [66] |
| GLP-1 Exendin-4 | Reduction of neural excitotoxicity | Rat hippocampal neural cultures, rats injected on the basal nucleus with ibotenic acid | [75] |
| Rosiglitazone | Reversal of memory deficits in objects recognition test and the Morris water maze; Aβ levels reduction | AD transgenic mice J20 line | [76] |