
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria Rosaria Plutino | + 4954 word(s) | 4954 | 2021-05-26 09:34:56 | | | |
| 2 | Conner Chen | Meta information modification | 4954 | 2021-05-27 11:46:26 | | |
Video Upload Options
Different nanostructured coatings and surface finishing, characterized by length range between 1 and 100 nm, may be deposited on the external area of a matrix to implement or enhance the materials efficiency for several applications.The use of polymers combined with nanomaterials or functional nanocarriers in drug-delivery systems finds their main application in the medical sector, in particular in cancer diagnostics and therapy and in traditional oral delivery systems. As a matter of fact, in wastewater treatment the simplest and most efficient methodology to modify the surface properties of filtering membranes, thus improving their retention and regeneration properties, involves the use of membrane coatings based on nanomaterials.
1. Background
Different nanostructured coatings and surface finishing, characterized by length range between 1 and 100 nm, may be deposited on the external area of a matrix to implement or enhance the materials efficiency for several applications [1]. Actually, most of the atoms, by aggregating, may contribute to change the size of the grain boundaries thus improving the physical properties of nanostructured materials; this is in accordance with the Hall–Petch reinforcement method, by which the change in grain size can affect the number of dislocations accumulated at the grain boundary and the yield strength. [2]. Conventional materials have followed through the evolution of human civilization by defining the historical ages, as for stone, iron, bronze ages, up to the most recent cement, and silicon ages. Scientific and technological developments allowed the definition of a “new époque” in which materials acquired a more advanced meaning and specific functionalities. During the last century, the synergism between several scientific disciplines such as chemistry, physics, nanotechnologies, computer science, engineering, electronics, medicine led to innovative composite, nano, and smart materials that represent a step forward with respect to conventionally intended ones.
2. Drug-Delivery/Release Nanosystems
2.1. Functional Coating for Drug-Delivery Nanosystems: An Introduction
Active pharmaceuticals are allowed, under specific conditions, to reach the target site and improve its effectiveness, through the use of controlled drug-release nanosystems [3]. Drug-delivery systems contribute to implementing specific properties of ‘free’ drugs by improving biodistribution, solubility in biological media and their in vivo permanence. By employing a nanosystem that incorporates or encapsulates drugs and releases them, under external stimulation such as pH or temperature, controlled release can be achieved [4]. Controlled-release or drug delivery systems are employed to obtain:
-
constant release into the blood of quantities of therapeutic compounds, avoiding drug waste;
-
repeatable and scheduled long-term release rates;
-
reduction of side effects;
-
personalized therapy;
-
drug stabilization [5]
A proper excipient helps keep drugs intact until delivery and facilitates their release at desired sites with maximum efficiency, while an appropriate processing method avoids unwanted degradation and waste of nanosystems [6]. These systems also change disadvantageous pharmacokinetics of some ‘free’ drugs. In addition, extensive loading of pharmaceuticals’ administration on drug-delivery systems can distribute ‘drug reservoirs’, for controlled and continuous release, to provide the drug level within the therapeutic window. Currently, many delivery nanocarriers based on nanometric size compounds such as micelles, dendrimers, nanotube and metallic nanoparticles have been designed. A lot of research has been focused on these delivery systems to support and provide a promising alternative to chemotherapy. Pharmaceutical research is attracted to planning advanced antineoplastic drugs with specific selectivity on cancer cells. In recent years, chemotherapy has principally focused on destroying all rapidly proliferating cells. The disadvantage of this therapy is that the body’s other rapidly dividing cells, such as in the hair follicles and intestinal epithelium are also killed off, leaving the patient to cope with harmful side effects. These new drugs should be able to exceed any resistance of the tumoral cells and they should provoke bland side effects.
2.1.1. Self-Assembled Polymers for Nanocarriers
Chemotherapy drugs, used in current therapies, are often charged into lipophilic self-assembled molecules so, micelles are also excellent carrier systems to make insoluble drugs soluble due to their hydrophobic core and hydrophilic shell [7]. The further functionalization of the micelle’s surface with PEG increases the ability of the nanocarriers to pass through tumoral tissue as a result of passive transport, therefore resulting in higher drug concentration in cancer cells. Numerous polymeric micelles, incorporating anticancer drugs, are under clinical trial [8] and others are already approved for breast cancer patients. Dendrimers are repetitively branched macromolecules with lots of functional groups available for the connection of drugs, which are used as targeting and imaging agents and their absorption, distribution, metabolism and elimination profile is correlated to diversified structural characteristics. Nanoparticle therapeutics based on dendrimers can improve the therapeutic index of cytotoxic drugs by using biocompatible segments, such as PEG, acetyl, glicosane and various amino acids, linked on the surface area [9].
2.1.2. Functional or Coated Nanofillers
There are several other models of functional nanofiller which show promising results in cancer treatment, a system used today contemplates functionalized carbon nanotubes. This carbon nanosystem is an allotropic form of carbon with a cylindrical structure extending on a number of sheets in concentric cylinders (single-walled carbon nanotubes and multiwalled carbon nanotubes) [10]. Water-insoluble drugs can easily be loaded on the hydrophobic hollow interior of carbon nanotubes. The large surface area consents to a specific outer surface functionalization for defined cancer receptors as well as contrast agents [11]. A spherical molecule such as fullerene (C60) and its derivatives are evaluated for the treatment of cancer [12] thanks to its ability to enhance the cytotoxicity of chemotherapeutic agents [13]. A study conducted using doxorubicin uploaded on the complex of fullerene C60 demonstrated that tumor volumes of treated rats were 1.4 times lower compared to the untreated rats [14]. These results are probably due to the direct action of the C60 + doxorubicin complex on tumor cells as well as immunomodulating effect. The development of nanoparticles has contributed to a new route for chemotherapy. With the design of smart nanoparticles, targeted drug delivery at the tumor site or a specific group of cells widely prevent the toxic and unwanted effects on other healthy tissues and organs [15]. Gold nanoparticles take advantage of their unique chemical and physical properties to carry and release drugs, and they are able to deliver the different size of drug molecules from little one to large biomolecules such as peptides, proteins or nucleic acids like DNA or RNA. This nanosystems recognize the surface of anionic protein as a result of interdependent electrostatic interaction and inhibit its activity [16]. Gold nanoparticles could be functionalized with lots of molecules with appropriate functional groups, in the monolayer [16]. Carbazoles, for example, were widely investigated for all their properties, which can be improved by changing functionalized groups or by introducing suitable substituents on carbazole core, with the intent to gain new and unique properties as antioxidant or antimicrobial. These compounds promote antiproliferative activity and considerable apoptotic response approaching cancer cells selectively. An approach to release pharmaceuticals, as carbazoles, involves the use of gold nanoparticles. These functional coated nanocarriers are suitable to deliver different payloads into target cells. In addition to the surface chemistry of gold nanoparticles, a promising perspective, in cancer therapy, is the photothermal damage [17] to cancer cells, which is an additional technique for enhancing the selective damage of unhealthy cells, based on the irradiation of nanoparticles, with 20 ns laser pulses (λ = 532 nm), to produce local heating. Circumstances for effective drug-release therapy can be improved thanks to external stimuli such as light or from the inside, through variations in the pH levels [18]. Tunable size and functionality make them a suitable scaffold for the efficient delivery of biomolecules. It has also been demonstrated that functionalized gold nanoparticles can act as carriers of insulin [19] and chitosan, a green biopolymer stabilized them. Gold chitosan-coated nanoparticles strongly adsorb insulin on their surface and are efficient for transmucosal delivery of insulin. For in vivo applications, the target of nanocarriers is the diseased tissue after the release into the circulatory system. There are two methods to deliver nanocarriers, ‘passive’ targeting and ‘active’ targeting [20]. The first one depends on vectors’ release in unhealthy cell tissues due to extravasation through a cracked blood vessel. Thanks to the nanometric diameter, the nanocarrier systems take advantage of the enhanced permeation and retention (EPR) effect [21]. On the other hand, ‘active’ targeting holds ligands on the carrier surface, for distinct recognition by cell surface receptors. A combination of both types of targeting will distribute an ideal carrier for in vivo delivery. Nanocarriers experience a non-specific uptake and possible degradation in macrophages. Therefore, targeting is essential for maximizing drug efficiency as well as minimizing side effects. Different physicochemical properties, such as size, PEGylation, or the ligand choice, coordinate non-specific versus target-specific uptake [22]. Gold nanoparticles with or without PEGylation of varying sizes (50, 80, 100, or 150 nm) are used for active targeting of cancer cells. Generally, PEGylation increases blood circulation lifetime and a specific ligand should facilitate filtration of nanocarriers into target cells. Two targeting molecules, folic acid (FA) and methotrexate (MTX) are specifically recognized by folate receptors that are overexpressed on the surfaces of many tumor cells [23]. Gold nanoparticles are not only convenient for cell-specific targeting, but also for localization into desired organelles. Recent research [24] proved that PEGylated gold colloids, functionalized with adsorbed protein, better detect a nuclear localization signal, for delivering drugs into nucleus’ cells. Nanoparticles have been developed as a promising scaffold for implementing drug delivery release compared to traditional delivery vehicles. They combine low toxicity, high surface area and tunable stability provides them with unique properties such as bioavailability and nonimmunogenicity.
2.2. Smart Polymers for Drug Nanocarriers
Drug delivery nanosystems are obtained by employing functional biocompatible materials or smart polymers as capping matrices, sensitive to particular external physiochemical stimuli, able to release an active biomolecule in the target site and at an adequate rate in response to specific functions [25]. New smart polymers for the controlled delivery of therapeutic drugs have been developed in the field of polymer engineering. Their physical, chemical, and biological signals can be supported by external sources, employed as triggering stimuli, and they can be promoted by internal environment conditions [26]. Smart polymeric systems may be dissolved in aqueous solutions or may be chemically grafted onto aqueous-solid interfaces, chemically bonded through hydrogen-bond systems, or hydrogel formulations [27]. The desired pharmacological action is not achieved with the rapid release dosage forms and it is, therefore, very important to control the timing of drug release. The release times are established based on the nature of the drug: water-soluble drugs require a slower release and a longer duration of action; those lipophilic require an increase in solubility to have an adequate therapeutic level, those with a short half-life require repeated administration and, finally, those with indefinite action require delivery to the target sites. To receive the drug level required for treatment, the drug-delivery system should deliver exact amounts of a particular drug at a planned rate. Several factors must be considered to design and implement this system, such as the physico-chemical properties of the drug, its route of administration and its pharmacological and biological effects [28]. Some advantages of controlled release systems are (i) the maintenance of drug levels within the desired range, (ii) the reduction of side effects, such as toxicity and frequency of administration, (iii) the improved efficacy [29]. The problems encountered in the use of these systems are due to the possible toxicity of the materials used, the need to operate through surgical procedures to insert or remove the system, possible poor availability of the system and high production costs [28]. New polymeric materials are currently being formulated, which respond to specific environmental changes in biological systems [30]. Stimulus reactive polymers mimic the behavior of biological molecules, thanks to external stimuli or changes in the local environment that can trigger a change in properties, such as solubility, shape, conformation, charge and size. Drug release could be regulated in spatial conformation by targeting and in time as a function of external stimuli [31]. The smart polymers, currently under study, are classified into the following categories: temperature-sensitive, dual stimulus reactivity, phase, light and biomolecule sensitive.
2.2.1. Temperature-Sensitive Smart Polymers
Temperature is an easily controllable condition, so temperature-sensitive smart polymers have practical advantages in vitro and in vivo [32]. Polymers with a low to medium critical solution temperature (T = 30/40 °C) commonly dissolve in aqueous solvents, while polymers with a higher critical solution temperature (T > 50 °C) better dissolve in organic solvents [33]. The drug may be released as a result of changes in body temperature following a fever or local infections. The sensitivity to temperature, in polymers, depends on the equilibrium between hydrophilic and hydrophobic portions, and these intermolecular and intramolecular interactions lead to aggregation of the polymer chain [26]. Poly (N-isopropyl acrylamide) (PNIPAAm), is the most studied intelligent temperature-sensitive polymeric system. The solubility in water decreases drastically when heated to approx. 32 °C in pure water and a few less in physiological saline solution [27]. Pluronic block copolymer is a thermosensitive polymer capable of releasing biomolecules (proteins or lipoproteins) as a function of temperature variations. Pluronic diblock copolymer microspheres have a porous and hydrophilic structure. The release rate of the biomolecules is modulated according to the temperature variations and the surface functionalization of the Pluronic copolymer [34]. Gelatin and carrageenan are natural polymers that show a sol–gel transition and adopt an amorphous spatial conformation in solution at high temperatures, while during cooling, a continuous and homogeneous network is formed. The solubility of a polymer in water depends on factors such as temperature, molecular weight or the addition of an additive or co-solvent. The variation of the balance between the intermolecular forces causes the disassembly of the micelles, thus freeing the encapsulated host. This response can be induced from within, by exploiting the tendency of tumor tissues to have a slightly higher temperature, or by applying heat externally. Poly(N-isopropylacrylamide) (PNIPAAM) undergoes an abrupt phase transition at 32 °C and separates from the aqueous phase. PNIPAAM is non-toxic, and can be chemically adapted by changing the alkyl part or by copolymerizing it with other more hydrophilic monomers but it is not biodegradable. There are natural temperature-sensitive systems and they are suitable carriers. For example, collagen with glycineproline- (hydroxyl) proline (Gly-Pro-Pro (Hyp)) forms a triple helix. The thermo-sensitive polymers can be classified according to the mechanism and the chemistry of the inner groups. These polymers solubilize many hydrophobic drugs such as paclitaxel and have an excellent formulation for poorly water-soluble drugs [26]. Micelles are formed when the concentration of the polymer is increased above the critical micellar concentration (CMC), then below the low critical solution temperature (LCST) the heat-sensitive polymer is hydrated and hydrophilic (Figure 1).
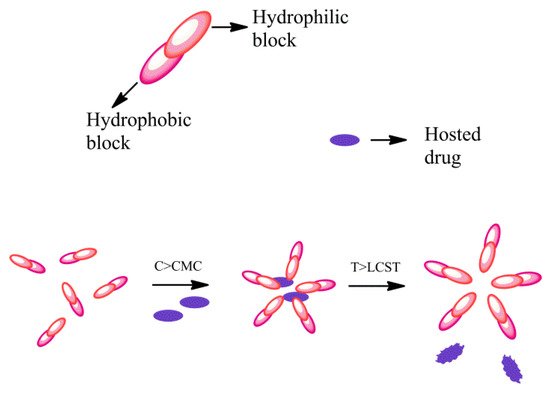
Figure 1. Scheme of behavior of thermo-responsive amphiphilic polymers.
2.2.2. Phase-Sensitive Smart Polymers
Release systems for biomolecules should preserve their biological activity and conformational stability. Intelligent polymer systems were tested that form in situ, injectable, phase-sensitive gels, potentially usable for drug delivery control. These injectable formulations allow single injections of high doses with small volumes and small needles and improve the stability of biomolecules. An instant gel forms in situ after these formulations are injected. The hydrophilic solvent causes the formation of a shell on the outside, while the hydrophobic solvent slows the penetration of water in order to decrease the hydrolysis of the polymer and potentially increases the stability of the biomolecules [35]. In these systems, drug release rates can be controlled by optimizing factors such as drug loading and solvent composition [36]. In drug-delivery systems, the response to light can be introduced through a linker that can be cleaved by irradiation with electromagnetic radiation having a certain wavelength. Encapsulated hosts are activated or released after being irradiated with a radiant source from outside the body, resulting in a spatial and temporal release [37]. These polymers are designed for use in microsystems, medical imaging and tissue engineering. Photo-refractive polymers with near-infrared (NIR) sensitivity have also been reported, such as a matrix of poly (N-vinyl carbazole), N-ethylcarbazole as plasticizer and 2,4,7-trinitro-9-fluorenone (TNF) as a sensitizer. These NIR-sensitive materials could be used to visualize tumors thanks to the different refractive index of tumors compared to that of normal tissues [29].
2.2.3. Light-Sensitive Smart Polymers
Materials sensitive to harmless electromagnetic radiation (mainly UV, visible and near infrared radiation), could be used as drug-delivery systems. Such materials, sensitive to light, trigger an irreversible change that causes the release of the entire drug dose, others are able to undergo reversible structural changes and behave as multi-switchable carriers, releasing the drug in a pulsating manner [25]. These light-sensitive systems have the advantage of delivering drugs to a specific site independent of the conditions of the biological environment [38]. The study of the photo-regulation mechanism of these systems and the development of new biocompatible materials for in vivo applications in drug administration [39]. Electromagnetic radiation, in the wavelength range between 2500–380 nm, applied in vivo can activate and deactivate the release of the biomolecule or drug in a specific organ or tissue, allowing excellent control of the release and reducing damage to adjacent sites [40]. UV light acts as a trigger for topical treatments [41], as the radiation used (λ < 700 nm) undergoes absorption by endogenous biomolecules and is therefore unable to penetrate more than 10 mm deep into the tissue [42]. UV treatment is, therefore, limited to therapies on the superficial layers of the skin or some internal organs. To obtain a slightly deeper tissue penetration, near infrared (NIR) light was used in the wavelength range from 650 to 900 nm, in this area of the spectrum the endogenous molecules have a minimal absorption of light, reducing the interference with the tissue.
NIR imaging techniques are non-invasive in vivo methods that allow to visualize physiological and metabolic processes [43]. Light-induced therapies fall into two categories, photodynamic therapy in which light stimulates apoptosis or necrosis by administering a photosensitizer that reacts with the light and oxygen present in the tissue to produce singlet oxygen. The second approach is photo-polymerization which induces the in situ formation of filling materials. (33) used for the preparation of dental composites or implants that take the shape of the implant and are applied without the use of invasive methods. Some light-sensitive systems that use azobenzene groups and similar aromatic substances are listed among the toxic compounds by the Food and Drug Administration (FDA), which limits their clinical use and pushes researchers to study alternative biocompatible materials [25].
2.2.4. Biomolecule-Sensitive Smart Polymers
Polymers that respond to biomolecules can provide high specificity, higher than those that respond to physical or chemical stimuli, therefore they are being studied in the context of drug-delivery systems. An example is glucose-sensitive polymers, which use phenylboronic acid, glucose oxides or concanavaline A for the treatment of diabetes by administering insulin, which is regulated by a closed-loop feedback system, but the use of proteins such as glucose oxides or concanavaline has limitations. These proteins are difficult to immobilize, so they cause an uncontrolled release of host proteins. Furthermore, several monosaccharides could compete for glucose-binding sites. Glutathione is another polypeptide that regulates cellular redox state [26]. For example, a hybrid hydrogel has been designed, integrating genetically modified calmodulin, which can change swelling based on the response of calcium ions and phenothianzines. These systems have the potential to be used in microfluidics and miniaturized drug-delivery systems [44]. Table 1 summarizes all the smart polymer platforms discussed in this paragraph.
Table 1. Advantages and disadvantages of using the various smart polymer platforms.
| Smart Polymers System | Advantages | Disadvantages | References |
|---|---|---|---|
| Temperature-sensitive | Temperature is an easily controllable parameter. Most of these systems are biodegradable and non-toxic. |
The sensitivity of polymeric systems in response to changes in temperature varies according to different factors, such as molecular weight and solubility. | [27][32][33] |
| Phase-sensitive | The drug release rate can be easily modulated through functional modifications or through the use of different solvents or solvent mixtures. | To have a greater effectiveness of the release it is often necessary to associate to these systems some light sensitive components. Most of these systems are not approved by FDA. |
[35][36][37] |
| Light-sensitive | Targeted drug delivery is independent of the conditions of the biological environment. The therapeutic target can be modulated by modulating the wavelength of the radiation used. |
Most of these systems are not approved by FDA due to their possible toxicity. | [25][38][39] |
| Biomolecule-sensitive | These systems have a high specificity. | Biomolecules are substances that are difficult to immobilize in drug delivery systems, which leads to poor control of the release of host species | [26][44] |
3. Nano/Ultrafiltration Membrane Coatings
Membranes for nanofiltration (NF) and ultrafiltration (UF) processes in the water treatment field are employed to remove the most common organic and inorganic contaminants (e.g., natural organic matter, pharmaceuticals, inorganic salts, organic dyes) with high efficiencies, through different removal mechanisms like electrostatic repulsion, size/steric exclusion, hydrophobic adsorption etc. [45][46][47][48].
The major problem of this technology, mostly in low-pressure processes, is the membrane fouling, that negatively affects membrane performance. In recent years, a proposed solution to reduce the membrane fouling and improve their durability, selectivity, retention and permeate flux, consists in the surface modification of membranes. This can be easily done using coatings that represent the most efficient approach, because of its easy processability, which involves chemical modifications to change surface properties of membranes. In particular, membrane coatings based on nano-sized materials like graphene oxides (GOs), carbon nanotubes (MWCNT), and titanium dioxide (TiO2), thanks to their higher hydrophilicity and the capacity to reduce pore size and increase charge effects of membranes, represents a technology of growing interest in the water treatment field.
GO nanosheet coatings for membranes have good chemical stability, exceptional transport properties, and excellent mechanical stiffness and strength; in particular, the nanochannels of GO sheets 1 nm wide, by a sieving mechanism, can reject larger molecules when the water passes through the membrane filter [49][50][51].
Some studies have reported the removal of several contaminants by a GO membrane in combination with polymeric membranes like polyvinylidene fluoride (PVDF), polysulfone (PS), polyamide (PA), or poly(ether sulfone) (PES), fabricated with various methods in which GO layers are bonded or unbonded together (Figure 2).
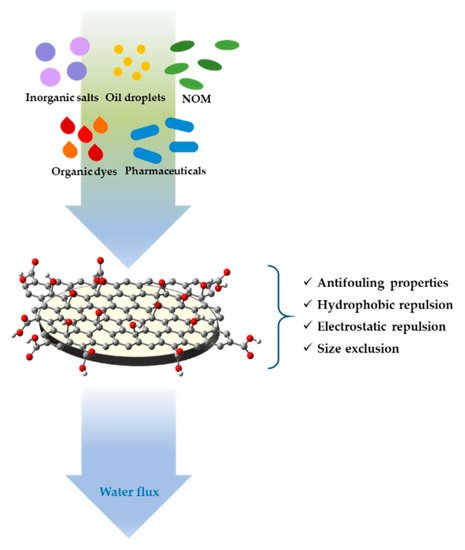
Figure 2. Schematic representation of water contaminants retention of graphene oxide (GO) coated ultrafiltration (UF) membrane.
GO membranes supported on microporous substrates of PVDF exhibit high rates of rejection for organic dyes methylene blue and rhodamin WT, under a transmembrane pressure of 50 psi (0.34 MPa), in a range of 46–66% and 93–95%, respectively and NaCl and Na2SO4 in a range of 6–19% and 26–46% respectively, depending on the specific number of GO layers deposited [52]. Also ceramic membrane coated with GO, at the expense of a low water permeability compared with the pristine membrane, show retention efficiencies of NOM (natural organic matter, humic acid and tannic acid), pharmaceuticals (ibuprofen and sulfamethoxazole), and inorganic salts (NaCl, Na2SO4, CaCl2, and CaSO4), in the order of 93.5%, 51.0%, and 31.4%, respectively, much higher than the pristine membrane [53]. Moreover, the abundant oxygen-containing functional groups feature of GO (carboxyl, carbonyl, hydroxyl and epoxy, distributed at edges and structural defects of GO flakes), which causes a high negative charge on the surface of the membrane, enhancing the hydrophilicity, retention, and antifouling properties. Multiple layers of GO coated on PES ultrafiltration membrane obtained via vacuum filtration of a GO suspension, exhibit a NOM rejection in the order of 31–67%, based on the number of GO layers coated, and a water flux change of the permeate less than ±10% [54]. GO-coated UF membranes, thanks to their underwater superoleophobicity and low oil-adhesion, are very effective in oil-in-water emulsion separation and the removing of oil droplets with sizes in the micrometer range, to obtain water with low oil/grease concentration. Porous polyamide (PA), with 200-nm 3D pores, coated with GO by vacuum filtration for antifouling oil/water separation, show an excellent antifouling performance thanks to the low oil adhesion on the membrane surface, resulting from the optimized micro-/nano-hierarchical roughness of the GO in particular with a 10 nm thickness and exhibit a 100% recovery by surface water flushing [55]. The underwater oleophobicity of GO sheets can be tuned by oxidative etching with ultraviolet (UV) light, to create or enlarge structural defects and introducing oxygen groups around them, to improve potential applications of GO coatings in oil/water separation, oil-repellent materials, microfluidic devices, anti-bioadhesion materials, and robust antifouling materials [56].
The most common fouling agents in water present a negative charge, so they are sensitive to a negatively charged membrane surface due to the electrostatic repulsion, as we have seen in the case of a GO-coated membrane. Several methods to enhance the surface charge of membranes have been developed, and one of them is the fabrication of electrically conducting membranes (ECM) to perform electrofiltration processes by the application of an electrical field. This type of membrane can be produced by coating UF membranes with conductive inorganic materials like carbon nanotubes (CNTs) or multiwalled carbon nanotubes (MWCNTs) that can also be used as inorganic fillers to fabricate nanocomposite membrane, to improve their performance also for the desalination of water [57]. CNTs and MWCNTs, before their use in the fabrication of nanocomposites or coatings for their application in membranes, are treated by acid treatment, that however can lead structural damage of MWCNTs, or by coatings with some molecules like poly(vinyl alcohol), polyaniline or polydopamine, to enhance their hydrophilicity [58]. An example is represented by a thin film made by cross-linked poly(vinyl alcohol) and carboxylated MWCNTs, deposited by pressure on a PS membrane. This system, used in an electrofiltration cell, in which a cell potential of 3–5 V and fields of 9–15 V/cm are applied, demonstrates the inhibition of a negatively charged fouling agent, represented by alginic acid, and the potential of CNT-based coatings on the reduction fouling rates [59]. Also composite membranes based on CNT-conjugated polymers (i.e., PANI, polypyrrole), fabricated through a process of electropolymerization of aniline on a CNT substrate under acidic conditions, the latter obtained by a coating of a PS membrane with a CNT suspension performed by pressure deposition, demonstrate that the application of an anodic potential to the ECM surface, is able to degrade a model organic contaminant (methylene blue) through an electro-oxidation process, also showing an electrochemical in situ membrane cleaning capacity [60]. To reduce membrane fouling by photo-oxidation, there is another solution that can be represented by the use of Titanium dioxide coatings. Photoactivity properties of the semiconductor titanium dioxide, exhibiting under UV irradiation, can be exploited for the photodegradation of smaller organic molecules entrapped in UF membranes. In particular, photoactive anatase membranes on asymmetric ceramic supports, prepared by slip-casting on asymmetric tubular supports in alumina, subsequently immersed in nanocrystalline anatase sols, show a high retention capacity of colloids and macromolecules, ensured by the separative top layer and the photodegradation of smaller organic molecules performed by UV irradiation of the opposite side of the membrane [61].
Manganese oxide and iron oxide coatings for catalytic membranes are also evaluated for the retention and removal of total organic carbon (TOC) in ultrafiltration processes, which is proven to depend on the number of times the membrane was coated with the metal oxide nanoparticles. This type of membrane is produced by coating, using a layer-by-layer self-assembly technique, of commercial UF ceramic membranes, that can also be cleaned from fouling with Distilled DeIonized (DDI) water using an ozonation-filtration. Hybrid ozonation-ceramic membrane filtration performed with Mn oxide-coated membranes, have given the best results in comparison with other metal oxide coatings (titanium oxide and iron oxide), thanks to the excellent catalytic properties of manganese in the oxidation of organic material and then in the reduction of TOC in the permeate [62].
Another application of metal coatings in filtration techniques, is their use as metal mesh coatings for application in oil/water filtration. An example is an eco-friendly iron-based, with a micro/nano-structure, metal mesh coating, produced by immersion of a stainless steel mesh in a solution in which is performed the reduction of FeCl2 with NaBH4, resulting in a membrane with underwater superoleophobicity, with an oil contact angle as high as 152°, that can separate oil/hot corrosive water mixed liquid efficiently, with a separation efficiency >96.2% [63].
A further approach of water filtration in water treatment processes, consists in the use of other types of membranes like chemically-functionalized membranes (CFMs), in which are incorporated selective ligands or ion-exchangers for the extraction specifically chemical species, bulk liquid membranes (BLMs), emulsion liquid membranes (ELMs), supported liquid membranes (SLMs) in which two aqueous phases (feed and stripping) are separated by an organic liquid as interphase and polymer inclusion membranes (PIMs). PIMs, more stable than SLMs, have acquired significant importance in recent years in the field of separations and extraction of organic molecules and metal ions (Au(III), As(V), Cd(II), Co(II)) from water [64]. This type of membrane also has important relevance in the preconcentration of antibiotics (sulfonamides and tetracyclines), which is highly influenced by sample pH, in environmental water samples [65]. PIMs are thin, flexible and stable films made by casting a solution containing an extractant (or carrier), usually an ionic liquid for the selective extraction of the target chemical species, a plasticizer to improve the elasticity of the membrane or modify the solubility of the extracted species and a base polymer such as cellulose triacetate (CTA) or poly(vinyl chloride) (PVC) [66]. The extractant agent in PIMs can also be represented by inorganic species that have a high capability of ion exchange and cation fixation like clays, in particular montmorillonite clay [67]. A PIM membrane based on PVC, whit montmorillonite and ionic liquids (Aliquat 336, Alicy and thiomalic acid) like extractant and plasticising agents, for the absorption and preconcentration in particular of Sn2+ from water samples, can be produced in two different methods: casting the clay modified with organosilanes (by a sol–gel method with dynasylan and APTES) in the solution of membrane (one pot method) or by coating the PIM membrane with the organoclay (layer by layer method).
The research of new technologies for the improvement of retention and regeneration properties of membranes in the waste water treatment, is constantly expanding, but strong evidence, as reported above, shows that the simplest and most efficient methodology for changing the surface properties of membranes, is represented by the use of membrane coatings based on various nanomaterials, which in particular allow easy cleaning, through simple washing or electrochemical methods, from fouling or oil residues for their reuse, in a circular economy perspective.
References
- He, J.; Schoenung, J.M. Nanostructured coatings. Mater. Sci. Eng. A 2002, 336, 274–319.
- Schuh, C.A.; Nieh, T.G. Hardness and Abrasion Resistance of Nanocrystalline Nickel Alloys near the Hall-Petch Breakdown Regime. MRS Proc. 2002, 740, 18.
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717.
- Du, Y.; Chen, W.; Zheng, M.; Meng, F.; Zhong, Z. pH-sensitive degradable chimaeric polymersomes for the intracellular release of doxorubicin hydrochloride. Biomaterials 2012, 33, 7291–7299.
- Wagenaar, B.; Müller, B. Piroxicam release from spray-dried biodegradable microspheres. Biomaterials 1994, 15, 49–54.
- Badruddoza, A.Z.M.; Godfrin, P.D.; Myerson, A.S.; Trout, B.L.; Doyle, P.S. Core-Shell Composite Hydrogels for Controlled Nanocrystal Formation and Release of Hydrophobic Active Pharmaceutical Ingredients. Adv. Healthcare Mater. 2016, 5, 1960–1968.
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2018, 26, 64–70.
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release. Pharm. Res. 2010, 27, 2569–2589.
- Baker, J.R. Dendrimer-based nanoparticles for cancer therapy. Hematology 2009, 1, 708–719.
- Rastogi, V.; Yadav, P.; Bhattacharya, S.S.; Mishra, A.K.; Verma, N.; Verma, A.; Pandit, J.K. Carbon Nanotubes: An Emerging Drug Carrier for Targeting Cancer Cells. J. Drug Deliv. 2014, 2014, 1–23.
- Dinesh, B.; Bianco, A.; Ménard-Moyon, C. Designing multimodal carbon nanotubes by covalent multi-functionalization. Nanoscale 2016, 8, 18596–18611.
- Murugesan, S.; Mousa, S.A.; O’Connor, L.J.; Lincoln, D.W.; Linhardt, R.J. Carbon inhibits vascular endothelial growth factor- and fibroblast growth factor-promoted angiogenesis. FEBS Lett. 2007, 581, 1157–1160.
- Zhang, Q.; Yang, W.; Man, N.; Zheng, F.; Shen, Y.; Sun, K.; Li, Y.; Wen, L.-P. Autophagy-mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy 2009, 5, 1107–1117.
- Prylutska, S.V.; Skivka, L.M.; Didenko, G.V.; Prylutskyy, Y.I.; Evstigneev, M.P.; Potebnya, G.P.; Panchuk, R.R.; Stoika, R.S.; Ritter, U.; Scharff, P. Complex of C60 Fullerene with Doxorubicin as a Promising Agent in Antitumor Therapy. Nanoscale Res. Lett. 2015, 10, 1–7.
- Shen, B.; Ma, Y.; Yu, S.; Ji, C. Smart Multifunctional Magnetic Nanoparticle-Based Drug Delivery System for Cancer Thermo-Chemotherapy and Intracellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 24502–24508.
- Ghosh, P.; Han, G.; De, M.; Kim, C.; Rotello, V.M. Gold nanoparticles in delivery applications☆. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315.
- Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29.
- Saturnino, C.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Caruso, A.; Muià, N.; Longo, P.; Rosace, G.; Galletta, M.; Ielo, I.; et al. N-Thiocarbazole-based gold nanoparticles: Synthesis, characterization and anti-proliferative activity evaluation. IOP Conf. Series Mater. Sci. Eng. 2018, 459, 012023.
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan Reduced Gold Nanoparticles as Novel Carriers for Transmucosal Delivery of Insulin. Pharm. Res. 2007, 24, 1415–1426.
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 206–212.
- Baban, D.F.; Seymour, L.W. Control of tumour vascular permeability. Adv. Drug Deliv. Rev. 1998, 34, 109–119.
- Bergen, J.M.; Von Recum, H.A.; Goodman, T.T.; Massey, A.P.; Pun, S.H. Gold Nanoparticles as a Versatile Platform for Optimizing Physicochemical Parameters for Targeted Drug Delivery. Macromol. Biosci. 2006, 6, 506–516.
- Dixit, V.; Bossche, J.V.D.; Sherman, D.M.; Thompson, D.H.; Andres, R.P. Synthesis and Grafting of Thioctic Acid−PEG−Folate Conjugates onto Au Nanoparticles for Selective Targeting of Folate Receptor-Positive Tumor Cells. Bioconjugate Chem. 2006, 17, 603–609.
- De La Fuente, J.M.; Berry, C.C. Tat Peptide as an Efficient Molecule to Translocate Gold Nanoparticles into the Cell Nucleus. Bioconjugate Chem. 2005, 16, 1176–1180.
- Alvarez-Lorenzo, C.; Bromberg, L.; Concheiro, A. Light-sensitive Intelligent Drug Delivery Systems. Photochem. Photobiol. 2009, 85, 848–860.
- Kim, S.; Kim, J.-H.; Jeon, O.; Kwon, I.C.; Park, K. Engineered polymers for advanced drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 420–430.
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16.
- Rahamathullah, S. Design and Evaluation of Controlled Release Layered Matrix Tablets of Parace-Tamol and Verapamil HCL. 2009; pp. 1–58. Available online: (accessed on 21 May 2021).
- Jeong, B.; Gutowska, A. Lessons from nature: Stimuli-responsive polymers and their biomedical applications. Trends Biotechnol. 2002, 20, 305–311.
- Hoffman, A.S.; Stayton, P.S. Bioconjugates of smart polymers and proteins: Synthesis and applications. Macromol. Symp. 2004, 207, 139–152.
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120.
- He, C.; Kim, S.W.; Lee, D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release 2008, 127, 189–207.
- Hrubý, M.; Filippov, S.; Štěpánek, P. Smart polymers in drug delivery systems on crossroads: Which way deserves following? Eur. Polym. J. 2015, 65, 82–97.
- Adhikari, U.; Goliaei, A.; Tsereteli, L.; Berkowitz, M.L. Properties of Poloxamer Molecules and Poloxamer Micelles Dissolved in Water and Next to Lipid Bilayers: Results from Computer Simulations. J. Phys. Chem. B 2016, 120, 5823–5830.
- Singh, S.A.; Shukla, S.R. Adsorptive removal of cobalt ions on raw and alkali-treated lemon peels. Int. J. Environ. Sci. Technol. 2015, 13, 165–178.
- Chen, S.; Singh, J. Controlled delivery of testosterone from smart polymer solution based systems: In vitro evaluation. Int. J. Pharm. 2005, 295, 183–190.
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884.
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483.
- Sumaru, K.; Ohi, K.; Takagi, T.; Kanamori, T.; Shinbo, T. Photoresponsive Properties of Poly(N-isopropylacrylamide) Hydrogel Partly Modified with Spirobenzopyran. Langmuir 2006, 22, 4353–4356.
- Jiang, J.; Tong, X.; Morris, D.; Zhao, Y. Toward Photocontrolled Release Using Light-Dissociable Block Copolymer Micelles. Macromolecules 2006, 39, 4633–4640.
- McCoy, C.P.; Rooney, C.; Edwards, C.R.; Jones, A.D.S.; Gorman, S.P. Light-Triggered Molecule-Scale Drug Dosing Devices. J. Am. Chem. Soc. 2007, 129, 9572–9573.
- Klohs, J.; Wunder, A.; Licha, K. Near-infrared fluorescent probes for imaging vascular pathophysiology. Basic Res. Cardiol. 2008, 103, 144–151.
- You, J.; Shao, R.; Wei, X.; Gupta, S.; Li, C. Near-Infrared Light Triggers Release of Paclitaxel from Biodegradable Microspheres: Photothermal Effect and Enhanced Antitumor Activity. Small 2010, 6, 1022–1031.
- Mano, J.F. Stimuli-Responsive Polymeric Systems for Biomedical Applications. Adv. Eng. Mater. 2008, 10, 515–527.
- Lin, J.; Tang, C.Y.; Ye, W.; Sun, S.-P.; Hamdan, S.H.; Volodin, A.; Van Haesendonck, C.; Sotto, A.; Luis, P.; Van der Bruggen, B. Unraveling flux behavior of superhydrophilic loose nanofiltration membranes during textile wastewater treatment. J. Membr. Sci. 2015, 493, 690–702.
- Cho, J.; Amya, G.; Pellegrinob, J. Membrane filtration of natural organic matter: Initial comparison of rejection and flux decline characteristics with ultrafiltration and nanofiltration membranes. Water Res. 1999, 33, 2517–2526.
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23.
- Mohammad, A.; Teow, Y.; Ang, W.; Chung, Y.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254.
- Chu, K.H.; Huang, Y.; Yu, M.; Heo, J.; Flora, J.R.; Jang, A.; Jang, M.; Jung, C.; Park, C.M.; Kim, D.-H.; et al. Evaluation of graphene oxide-coated ultrafiltration membranes for humic acid removal at different pH and conductivity conditions. Sep. Purif. Technol. 2017, 181, 139–147.
- Mi, B. Graphene Oxide Membranes for Ionic and Molecular Sieving. Science 2014, 343, 740–742.
- Zinadini, S.; Zinatizadeh, A.A.; Rahimi, M.; Vatanpour, V.; Zangeneh, H. Preparation of a novel antifouling mixed matrix PES membrane by embedding graphene oxide nanoplates. J. Membr. Sci. 2014, 453, 292–301.
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723.
- Chu, K.H.; Fathizadeh, M.; Yu, M.; Flora, J.R.V.; Jang, A.; Jang, M.; Park, C.M.; Yoo, S.S.; Her, N.; Yoon, Y. Evaluation of Removal Mechanisms in a Graphene Oxide-Coated Ceramic Ultrafiltration Membrane for Retention of Natural Organic Matter, Pharmaceuticals, and Inorganic Salts. ACS Appl. Mater. Interfaces 2017, 9, 40369–40377.
- Song, J.J.; Huang, Y.; Nam, S.-W.; Yu, M.; Heo, J.; Her, N.; Flora, J.R.; Yoon, Y. Ultrathin graphene oxide membranes for the removal of humic acid. Sep. Purif. Technol. 2015, 144, 162–167.
- Huang, Y.; Li, H.; Wang, L.; Qiao, Y.; Tang, C.; Jung, C.; Yoon, Y.; Li, S.; Yu, M. Ultrafiltration Membranes with Structure-Optimized Graphene-Oxide Coatings for Antifouling Oil/Water Separation. Adv. Mater. Interfaces 2015, 2, 1–7.
- Li, H.; Huang, Y.; Mao, Y.; Xu, W.L.; Ploehn, H.J.; Yu, M. Tuning the underwater oleophobicity of graphene oxide coatings via UV irradiation. Chem. Commun. 2014, 50, 9849–9851.
- Ahn, C.H.; Baek, Y.; Lee, C.; Kim, S.O.; Kim, S.; Lee, S.; Kim, S.-H.; Bae, S.S.; Park, J.; Yoon, J. Carbon nanotube-based membranes: Fabrication and application to desalination. J. Ind. Eng. Chem. 2012, 18, 1551–1559.
- Sianipar, M.; Kim, S.H.; Min, C.; Tijing, L.D.; Shon, H.K. Potential and performance of a polydopamine-coated multiwalled carbon nanotube/polysulfone nanocomposite membrane for ultrafiltration application. J. Ind. Eng. Chem. 2016, 34, 364–373.
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic fouling inhibition on electrically conducting carbon nanotube–polyvinyl alcohol composite ultrafiltration membranes. J. Membr. Sci. 2014, 468, 1–10.
- Duan, W.; Ronen, A.; Walker, S.; Jassby, D. Polyaniline-Coated Carbon Nanotube Ultrafiltration Membranes: Enhanced Anodic Stability for In Situ Cleaning and Electro-Oxidation Processes. ACS Appl. Mater. Interfaces 2016, 8, 22574–22584.
- Bosc, F.; Ayral, A.; Guizard, C. Mesoporous anatase coatings for coupling membrane separation and photocatalyzed reactions. J. Membr. Sci. 2005, 265, 13–19.
- Byun, S.; Davies, S.; Alpatova, A.; Corneal, L.; Baumann, M.; Tarabara, V.; Masten, S. Mn oxide coated catalytic membranes for a hybrid ozonation–membrane filtration: Comparison of Ti, Fe and Mn oxide coated membranes for water quality. Water Res. 2011, 45, 163–170.
- Wang, J.; Wang, S. A simple and eco-friendly route for fabricating iron-based coating on metal mesh for efficient oil/water separation. Sep. Purif. Technol. 2019, 226, 31–38.
- Nghiem, L.; Mornane, P.; Potter, I.; Perera, J.; Cattrall, R.; Kolev, S. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41.
- Garcia-Rodríguez, A.; Matamoros, V.; Kolev, S.; Fontàs, C. Development of a polymer inclusion membrane (PIM) for the preconcentration of antibiotics in environmental water samples. J. Membr. Sci. 2015, 492, 32–39.
- Vázquez, M.; Romero, V.; Fontas, C.; Antico, E.; Benavente, J. Polymer inclusion membranes (PIMs) with the ionic liquid (IL) Aliquat 336 as extractant: Effect of base polymer and IL concentration on their physical–chemical and elastic characteristics. J. Membr. Sci. 2014, 455, 312–319.
- Laudelout, H.; Van Bladel, R.; Bolt, G.H.; Page, A.L. Thermodynamics of heterovalent cation exchange reactions in a montmorillonite clay. Trans. Faraday Soc. 1968, 64, 1477–1488.




