Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zengyong Chu | + 1298 word(s) | 1298 | 2021-05-17 08:20:15 | | | |
| 2 | Bruce Ren | -21 word(s) | 1277 | 2021-05-25 04:11:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chu, Z. 3D Graphene-Based Toxic Gas Sensors. Encyclopedia. Available online: https://encyclopedia.pub/entry/10004 (accessed on 08 February 2026).
Chu Z. 3D Graphene-Based Toxic Gas Sensors. Encyclopedia. Available at: https://encyclopedia.pub/entry/10004. Accessed February 08, 2026.
Chu, Zengyong. "3D Graphene-Based Toxic Gas Sensors" Encyclopedia, https://encyclopedia.pub/entry/10004 (accessed February 08, 2026).
Chu, Z. (2021, May 24). 3D Graphene-Based Toxic Gas Sensors. In Encyclopedia. https://encyclopedia.pub/entry/10004
Chu, Zengyong. "3D Graphene-Based Toxic Gas Sensors." Encyclopedia. Web. 24 May, 2021.
Copy Citation
Air pollution is becoming an increasingly important global issue. Toxic gases such as ammonia, nitrogen dioxide, and volatile organic compounds (VOCs) like phenol are very common air pollutants. To date, various sensing methods have been proposed to detect these toxic gases. Researchers are trying their best to build sensors with the lowest detection limit, the highest sensitivity, and the best selectivity. As a 2D material, graphene is very sensitive to many gases and so can be used for gas sensors. Recent studies have shown that graphene with a 3D structure can increase the gas sensitivity of the sensors. The limit of detection (LOD) of the sensors can be upgraded from ppm level to several ppb level.
graphene
graphene hydrogel
graphene aerogel
gas sensor
1. Introduction
There is a huge demand for the development of simple and reliable gas sensors [1]. In many fields, such as agriculture, medical diagnosis, and industrial waste, especially in environmental monitoring, it is necessary to detect NOx (especially NO2), ammonia (NH3), and volatile organic compounds (VOCs), because of their possible toxicity and related risks to the ecosystem [2][3]. In many countries, air pollution is a major environmental problem caused by rapid industrialization. A large amount of NO2 is emitted into the environment every year due to the industrial combustions and automobile emissions [4]. Therefore, the detection of NO2 has aroused widespread concerns, because it is harmful to the plants and respiratory systems of people and animals [5]. Additionally, NO2 can cause acid rain and photochemical smog [6][7]. Therefore, the United States Environmental Protection Agency (EPA) defines NO2 as a typical air pollutant, and the exposure limit is only 53 ppb [8]. Ammonia (NH3) is also a common dangerous air pollutant, which is produced by the industrial process, agricultural production, and manufacturing process [9][10]. Specifically, any overexposure to the high concentrations of NH3 (>30 ppm, 10 min) can irritate the human eye, skin, and respiratory system [11][12][13]. VOCs are the hydrocarbons that exist as gases or vapor at room temperature, which can be emitted from numerous products and activities, e.g., detergents, paints, solvents, tools, clothes, toys, cleaning, and cooking [14]. Aldehyde, aromatic, aliphatic, halogenated, and terpenoid compounds are the VOCs commonly detected in commercial buildings [14][15]. Toxic VOCs that have been previously detected in air by any type of sensors include formaldehyde, acetaldehyde, benzene, toluene, xylenes, phenol, pyridine, acetone, acetic anhydride, carbon disulfide, dihydroxybenzene, and so on [14][15][16][17][18][19]. For example, phenol is a toxic VOC occurring both naturally but also from industrial processes, which can be rapidly absorbed through the skin and cause skin and eye burns upon contact [20]. It is considered as a serious pollutant because of the toxicity and persistence in the environment. The short-term exposure limit of phenol is 10 ppm, 60 min [21]. Because of the serious environmental pollution, phenol monitoring becomes an urgent problem. Therefore, with the monitoring development of air pollution, the demand for gas sensors will increase rapidly in the future.
As a 2D material, graphene has many advantages, such as large conjugated structure, high specific surface area, high conductivity, easy to be synthesized, sensitive to the gas molecules, and so on. It has been proven to be a promising high-performance gas detection material [22]. Graphene surface can easily absorb some molecules, such as NO2, NH3, CO2, and so on. Moreover, the conductivity of graphene will change after adsorption of target gas molecules. The concentration of target gas in the environment can be detected by monitoring the change of conductivity. There have been many reports on the application of graphene in gas sensors, including pure graphene [23][24][25][26] and graphene composite materials [27][28][29][30][31]. There are many factors affecting graphene-based sensors, including: synthetic method [32][33][34], chemical structure [35][36][37], interlaminar structure [34][38], testing environment [39][40][41][42], and surface properties [43][44][45][46][47]. Due to the π-π accumulation and Van Der Waals force binding between graphene, the 2D graphene nanocomposites tend to agglomerate, resulting in the reduction of specific surface area [48][49][50]. In order to make full use of the characteristics of graphene, 2D graphene is usually assembled into a three-dimensional (3D) framework state by a series of methods. In contrast, due to the combination of 3D porous structure and the inherent characteristics of graphene, 3D graphene provides more space and larger surface area to transport and store electrons. 3D graphene has good conductivity, large specific surface area, and versatile gas adsorption sites. Furthermore, the defects and edge positions on the 3D porous graphene play an important role in promoting gas adsorption [48]. In recent years, compared with 2D graphene structures, 3D porous graphene structures such as graphene hydrogels, graphene aerogels, and graphene foams have been used as high-performance gas sensors [49]. Although 3D graphene has broad prospects in the field of gas sensors with the super high sensitivity, the selectivity is not satisfactory. Different gas molecules may adsorb on the same 3D graphene sheets and lead to the total change of the resistance [50][51]. It is difficult to quantitatively distinguish one target gas from a gas mixture. To improve the selectivity, defect engineering is generally needed to modulate graphene [52].
Several reviews have presented the main development of graphene-based gas sensors. For example, in 2015, Meng et al. [49] reviewed the graphene-based hybrids for chemi-resistive gas sensors. They focused on the sensing principles and synthesis processes of the graphene-based hybrids with noble metals, metal oxides, and conducting polymers. In 2018, Xia et al. [50] summarized the 3D structure graphene/metal oxide hybrids for gas sensors. They concluded a variety of logical strategies to design the 3D nanohybrids of RGO and MOx. In 2020, Ilnicka et al. [51] summarized the graphene-based hydrogen gas sensors, a special case of gas sensitivity to H2. However, the above reviews did not reflect the whole progress of graphene gas sensors, especially for the air pollution monitoring applications. This paper aims to summarize the recent progress of the gas sensors based on 3D graphene frameworks in the detection of air pollutants.
2. Synthesis of 3D Graphene Frameworks
Graphene oxide (GO) and reduced graphene oxide (RGO) have a 2D conjugated structure with single-atom thickness and residual oxygen-containing groups, which can be regarded as 2D conjugated macromolecules, structurally. They have rich chemical activities, which are helpful for 3D self-assembly through a series of chemical modification methods to regulate the interaction between the layers [34][38].
Graphene hydrogel is one of the major 3D assemblies. Chemically modified graphene (CMG) hydrogels prepared from GO or RGO can be used for large-scale production. As shown in Figure 1, RGO hydrogels (RGOHs) can be obtained by the following methods:
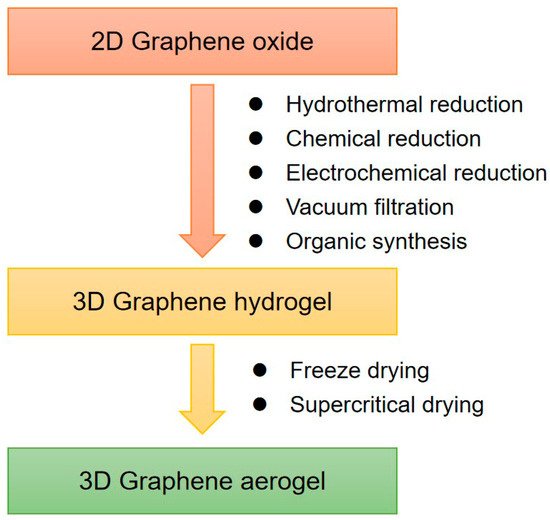
Figure 1. Synthesis methods of 3D graphene frameworks.
- (1)
- (2)
- (3)
- (4)
In addition to the 3D self-assembly of graphene in a water system, the assembly of the graphene in an organic system can also be achieved by thermal solvent reduction [71][72][73].
Graphene aerogel composites are usually prepared by supercritical drying or freeze-drying of hydrogel precursors [74][75]. For example, highly compressible RGO aerogels can be obtained by freeze-drying and microwave treatment. Directional freezing is a well-known processing technology of porous materials. This technology can also be used for the preparation of graphene aerogels [76]. Moreover, the controllable heat treatment technology can also reduce GO to RGO and restore conductivity. The regulation of the chemical structure of GO can adjust the morphology and elasticity of aerogels, for example, the oxygen functional groups in GO have a significant effect on the morphology and elasticity of the gels [77].
References
- Koolen, C.D.; Rothenberg, G. Air pollution in Europe. ChemSusChem 2018, 12, 164–172.
- Tong, S. Air pollution and disease burden. Lancet Planet. Health 2019, 3, 49–50.
- Ranscombe, P. Wearable technology for air pollution. Lancet Respir. Med. 2019, 7, 567–568.
- Liu, J. Mapping high resolution national daily NO2 exposure across mainland China using an ensemble algorithm. Environ. Pollut. 2021, 279, 116932.
- Sinharoy, S.S.; Clasen, T.; Martorell, R. Air pollution and stunting: A missing link? Lancet Glob. Health 2020, 8, 472–475.
- Chen, C.; Li, W.; Dong, L.; Li, X. The effect of meteorological factors, seasonal factors and air pollutions on the formation of particulate matter. IOP Conf. Ser. Earth Environ. Sci. 2020, 450, 0120121.
- Siregar, A.M.; Siregar, C.A.; Yani, M. Engineering of motorcycle exhaust gases to reduce air pollution. IOP Conf. Ser. Mater. Sci. Eng. 2020, 821, 012048.
- U. S. Environmental Protection Agency. Review of the Primary National Ambient Air Quality Standards for Nitrogen Dioxide. 2015. Available online: (accessed on 4 May 2021).
- Zhang, Y.; Xu, W.; Wen, Z.; Wang, D.; Hao, T.; Tang, A.; Liu, X. Atmospheric deposition of inorganic nitrogen in a semi-arid grassland of Inner Mongolia, China. J. Arid Land 2017, 9, 810–822.
- Widiana, D.R.; Wang, Y.F.; You, S.J.; Yang, H.H.; Wang, L.C.; Tsai, J.H.; Chen, H.M. Air pollution profiles and health risk assessment of ambient volatile organic compounds above a municipal wastewater treatment plant, Taiwan. Aerosol. Air Qual. Res. 2019, 19, 375–382.
- Naseem, S.; King, A.J. Ammonia production in poultry houses can affect health of humans, birds, and the environment—techniques for its reduction during poultry production. Environ. Sci. Pollut. Res. 2018, 25, 15269–15293.
- Börü, Ü.T.; Bölük, C.; Taşemir, M.; Gezer, T.; Serim, V.A. Air pollution, a possible risk factor for multiple sclerosis. Acta Neurol. Scand. 2020, 141, 431–437.
- National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 6; The National Academies Press: Washington, DC, USA, 2003. Available online: (accessed on 4 May 2021).
- Jia, C.; Cao, K.; Valaulikar, R.; Fu, X.; Sorin, A.B. Variability of total volatile organic compounds (TVOC) in the indoor air of retail stores. Int. J. Environ. Res. Public Health 2019, 16, 4622.
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520.
- Bocos-Bintintan, V.; Ratiu, I.A. Hunting for toxic industrial chemicals: Real-time detection of carbon disulfide traces by means of ion mobility spectrometry. Toxics 2020, 8, 121.
- Bocos-Bintintan, V.; Ghira, G.B.; Anton, M.; Martiniuc, A.-V.; Ratiu, I.A. Sensing precursors of illegal drugs—Rapid detection of acetic anhydride vapors at trace levels using photoionization detection and ion mobility spectrometry. Molecules 2020, 61, 1852.
- Ghira, G.B.; Ratiu, I.A.; Bocos-Bintintan, V. Fast characterization of pyridine using ion mobility spectrometry and photoionization detection. Environ. Eng. Manag. J. 2013, 12, 251–256.
- Patel, B.R.; Noroozifar, M.; Kerman, K. Review-Nanocomposite-based sensors for voltammetric detection of hazardous phenolic pollutants in water. J. Electrochem. Soc. 2020, 167, 037568.
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288.
- Wolkoff, P.; Wilkins, C.K.; Clausen, P.A.; Nielsen, G.D. Organic compounds in office environments—Sensory irritation, odor, measurements and the role of reactive chemistry. Indoor Air 2006, 16, 7–19.
- Zong, P.A.; Liang, J.; Zhang, P.; Wan, C.; Wang, Y.; Koumoto, K. Graphene-based thermoelectrics. ACS Appl. Energy Mater. 2020, 3, 2224–2239.
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuator B Chem. 2015, 218, 160–183.
- Cui, Y.; Liu, Q.; He, Z.; Gao, X.; Wu, E.; Guo, G.; Zhou, C.; Feng, Z. Epitaxial graphene gas sensors on SiC substrate with high sensitivity. J. Semicond. 2020, 41, 032101.
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.X.; Yang, S.S.; Cheng, M.M.C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuator B Chem. 2011, 157, 310–313.
- Chung, M.G.; Kim, D.H.; Lee, H.M.; Kim, T.; Choi, J.H.; Seo, D.K.; Yoo, J.B.; Hong, S.H.; Kang, T.J.; Kim, Y.H. Highly sensitive NO2 gas sensor based on ozone treated graphene. Sens. Actuator B Chem. 2012, 166–167, 172–176.
- Zhou, L.; Shen, F.; Tian, X.; Wang, D.; Zhang, T.; Chen, W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale 2013, 5, 1564–1569.
- Bian, S.; Shen, C.; Qian, Y.; Liu, J.; Xi, F.; Dong, X. Facile synthesis of sulfur-doped graphene quantum dots as fluorescent sensing probes for Ag+ ions detection. Sens. Actuator B Chem. 2017, 242, 231–237.
- Li, X.; Li, X.; Li, Z.; Wang, J.; Zhang, J. WS2 nanoflakes based selective ammonia sensors at room temperature. Sens. Actuator B Chem. 2017, 240, 273–277.
- Karmakar, N.S.; Fernandes, R.P.; Jain, S.; Patil, U.V.; Shimpi, N.G.; Bhat, N.V.; Kothari, D.C. Room temperature NO2 gas sensing properties of p-toluenesulfonic acid doped silver-polypyrrole nanocomposite. Sens. Actuator B Chem. 2017, 242, 118–126.
- Zou, J.; Liu, Z.; Guo, Y.; Dong, C. Electrochemical sensor for the facile detection of trace amounts of bisphenol A based on cyclodextrin-functionalized graphene/platinum nanoparticles. Anal. Methods 2016, 9, 134–140.
- Wu, W.; Liu, Z.; Jauregui, L.A.; Yu, Q.; Pillai, R.; Cao, H.; Bao, J.; Chen, Y.P.; Pei, S.S. Wafer-scale synthesis of graphene by chemical vapor deposition and its application in hydrogen sensing. Sens. Actuator B Chem. 2010, 150, 296–300.
- Pak, Y.; Kim, S.M.; Jeong, H.; Kang, C.G.; Park, J.S.; Song, H.; Lee, R.; Myoung, N.S.; Lee, B.H.; Seo, S.; et al. Palladium-decorated hydrogen-gas sensors using periodically aligned graphene nanoribbons. ACS Appl. Mater. Interfaces 2014, 6, 13293–13298.
- Ritikos, R.; Whitcher, T.J.; Razib, N.M.; Bien, D.C.S.; Chanlek, N.; Nakajima, H.; Saisopa, T.; Songsiriritthigul, P.; Huang, N.M.; Rahman, S.A. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuator B Chem. 2014, 193, 692–700.
- Yasaei, P.; Kumar, B.; Hantehzadeh, R.; Kayyalha, M.; Baskin, A.; Repnin, N.; Wang, C.H.; Klie, F.R.; Chen, Y.P.; Král, P.; et al. Chemical sensing with switchable transport channels in graphene grain boundaries. Nat. Commun. 2014, 5, 4911.
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuator B Chem. 2013, 178, 485–493.
- Johnson, J.L.; Behnam, A.; Pearton, S.J.; Ural, A. Hydrogen sensing using Pd-functionalized multi-layer graphene nanoribbon networks. Adv. Mater. 2010, 22, 4877–4880.
- Kumar, R.; Avasthi, D.K.; Kaur, A. Fabrication of chemiresistive gas sensors based on multistep reduced graphene oxide for low parts per million monitoring of sulfur dioxide at room temperature. Sens. Actuator B Chem. 2017, 242, 461–468.
- Akhter, F.; Alahi, M.E.E.; Siddiquei, H.R.; Gooneratne, C.P.; Mukhopadhyay, S.C. Graphene oxide (GO) coated impedimetric gas sensor for selective detection of carbon dioxide (CO2) with temperature and humidity compensation. IEEE Sens. J. 2021, 21, 4241–4249.
- Ratinac, K.R.; Yang, W.; Ringer, S.P.; Braet, F. Toward ubiquitous environmental gas sensors—Capitalizing on the promise of graphene. Environ. Sci. Technol. 2010, 44, 1167–1176.
- Ali, S.; Hassan, A.; Hassan, G.; Bae, J.; Lee, C.H. All-printed humidity sensor based on gmethyl-red/methyl-red composite with high sensitivity. Carbon 2016, 105, 23–32.
- Jang, J.; Han, J.I. High performance cylindrical capacitor as a relative humidity sensor for wearable computing device. J. Electrochem. Soc. 2017, 164, B136–B141.
- Chung, M.G.; Kim, D.H.; Seo, D.K.; Kim, T.; Im, H.U.; Lee, H.M.; Yoo, J.B.; Hong, S.H.; Kang, T.J.; Kim, Y.H. Flexible hydrogen sensors using graphene with palladium nanoparticle decoration. Sens. Actuator B Chem. 2012, 169, 387–392.
- Cuong, T.V.; Pham, V.H.; Chung, J.S.; Shin, E.W.; Yoo, D.H.; Hahn, S.H.; Huh, J.S.; Rue, G.H.; Kim, E.J.; Hur, S.H.; et al. Solution-processed ZnO-chemically converted graphene gas sensor. Mater. Lett. 2010, 64, 2479–2482.
- Choi, S.J.; Choi, C.; Kim, S.J.; Cho, H.J.; Hakim, M.; Jeon, S.; Kim, I.D. Highly efficient electronic sensitization of non-oxidized graphene flakes on controlled pore-loaded WO3 nanofibers for selective detection of H2S molecules. Sci. Rep. 2015, 5, 8067.
- Gautam, M.; Jayatissa, A.H. Ammonia gas sensing behavior of graphene surface decorated with gold nanoparticles. Solid State Electron. 2012, 78, 159–165.
- Jiang, L.; Fan, Z. Design of advanced porous graphene materials: From graphene nanomesh to 3D architectures. Nanoscale 2014, 6, 1922–1945.
- Duy, L.T.; Kim, D.J.; Trung, T.Q.; Dang, V.Q.; Kim, B.Y.; Moon, H.K.; Lee, N.E. High performance three-dimensional chemical sensor platform using reduced graphene oxide formed on high aspect-ratio micropillars. Adv. Funct. Mater 2015, 25, 883–890.
- Meng, F.-L.; Guo, Z.; Huang, X.-J. Graphene-based hybrids for chemiresistive gas sensors. Trends Anal. Chem. 2015, 68, 37–47.
- Xia, Y.; Li, R.; Chen, R.; Wang, J.; Xiang, L. 3D Architectured graphene/metal oxide hybrids for gas sensors: A review. Sensors 2018, 18, 1456.
- Ilnicka, A.; Lukaszewicz, J.P. Graphene-based hydrogen gas sensors: A review. Processes 2020, 8, 633.
- Chen, T.; Yan, W.; Xu, J.; Li, J.; Zhang, G.; Ho, D. Highly sensitive and selective NO2 sensor based on 3D MoS2/rGO composites prepared by a low temperature self-assembly method. J. Alloys Compd. 2019, 793, 541–551.
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330.
- Yin, S.; Niu, Z.; Chen, X. Assembly of graphene sheets into 3D macroscopic structures. Small 2012, 8, 2458–2463.
- Li, C.; Shi, G. Functional gels based on chemically modified graphenes. Adv. Mater. 2014, 26, 3992–4012.
- Chen, J.; Sheng, K.; Luo, P.; Li, C.; Shi, G. Graphene hydrogels deposited in nickel foams for high-rate electrochemical capacitors. Adv. Mater. 2012, 24, 4569–4573.
- Li, C.; Shi, G. Three-dimensional graphene architectures. Nanoscale 2012, 4, 5549–5563.
- Sui, Z.; Zhang, X.; Lei, Y.; Luo, Y. Easy and green synthesis of reduced graphite oxide-based hydrogels. Carbon 2011, 49, 4314–4321.
- Zhang, X.; Sui, Z.; Xu, B.; Yue, S.; Luo, Y.; Zhan, W.; Liu, B. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J. Mater. Chem. 2011, 21, 6494–6497.
- Wu, Q.; Sun, Y.; Bai, H.; Shi, G. High-performance supercapacitor electrodes based on graphene hydrogels modified with 2-aminoanthraquinone moieties. Phys. Chem. Chem. Phys 2011, 13, 11193–11198.
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683.
- Pham, H.D.; Pham, V.H.; Cuong, T.V.; Nguyen-Phan, T.D.; Chung, J.S.; Shin, E.W.; Kim, S. Synthesis of the chemically converted graphene xerogel with superior electrical conductivity. Chem. Commun. 2011, 47, 9672–9674.
- Chen, M.; Zhang, C.; Li, X.; Zhang, L.; Ma, Y.; Zhang, L.; Xu, X.; Xia, F.; Wang, W.; Gao, J. A one-step method for reduction and self-assembling of graphene oxide into reduced graphene oxide aerogels. J. Mater. Chem. A 2013, 1, 2869–2877.
- Luan, V.H.; Tien, H.N.; Le, T.H.; Hien, N.T.M.; Hur, S.H. Synthesis of a highly conductive and large surface area graphene oxide hydrogel and its use in a supercapacitor. J. Mater. Chem. A 2012, 1, 208–211.
- Sheng, K.X.; Xu, Y.X.; Li, C.; Shi, Q.G. High-performance self-assembled graphene hydrogels prepared by chemical reduction of graphene oxide. New Carbon Mater. 2011, 26, 9–15.
- Chen, K.; Chen, L.; Chen, Y.; Bai, H.; Li, L. Three-dimensional porous graphene-based composite materials: Electrochemical synthesis and application. J. Mater. Chem. 2012, 22, 20968–20976.
- Shadkam, R.; Naderi, M.; Ghazitabar, A.; Asghari-Alamdari, A.; Shateri, S. Enhanced electrochemical performance of graphene aerogels by using combined reducing agents based on mild chemical reduction method. Ceram. Int. 2020, 46, 22197–22207.
- Li, Y.; Sheng, K.; Yuan, W.; Shi, G. A high-performance flexible fibre-shaped electrochemical capacitor based on electrochemically reduced graphene oxide. Chem. Commun. 2012, 49, 291–293.
- Yang, X.; Qiu, L.; Cheng, C.; Wu, Y.; Ma, Z.F.; Li, D. Ordered gelation of chemically converted graphene for next-generation electroconductive hydrogel films. Angew. Chem. Int. Ed. 2011, 50, 7325–7328.
- Yang, X.; Zhu, J.; Qiu, L.; Li, D. Bioinspired effective prevention of restacking in multilayered graphene films: Towards the next generation of high-performance supercapacitors. Adv. Mater. 2011, 23, 2833–2838.
- Sun, Y.; Wu, Q.; Shi, G. Supercapacitors based on self-assembled graphene organogel. Phys. Chem. Chem. Phys. 2011, 13, 17249–17254.
- Zhou, Q.; Gao, J.; Li, C.; Chen, J.; Shi, G. Composite organogels of graphene and activated carbon for electrochemical capacitors. J. Mater. Chem. A 2013, 1, 9196–9201.
- Gun, J.; Kulkarni, S.A.; Xiu, W.; Batabyal, S.K.; Sladkevich, S.; Prikhodchenko, P.V.; Gutkin, V.; Lev, O. Graphene oxide organogel electrolyte for quasi solid dye sensitized solar cells. Electrochem. Commun. 2012, 19, 108–110.
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; Monte, F.D. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830.
- Hu, H.; Zhao, Z.; Wu, W.; Gogotsi, Y.; Qiu, J. Ultralight and highly compressible graphene aerogels. Adv. Mater. 2012, 25, 2219–2223.
- Li, W.L.; Lu, K.; Walz, J.Y. Freeze casting of porous materials: Review of critical factors in microstructure evolution. Int. Mater. Rev. 2012, 57, 37–60.
- Qiu, L.; Liu, J.Z.; Chang, S.L.Y.; Wu, Y.; Li, D. Biomimetic superelastic graphene-based cellular monoliths. Nat. Commun. 2012, 3, 1241.
More
Information
Subjects:
Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
25 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




