There is a huge demand for the development of simple and reliable gas sensors [
1]. In many fields, such as agriculture, medical diagnosis, and industrial waste, especially in environmental monitoring, it is necessary to detect NO
x (especially NO
2), ammonia (NH
3), and volatile organic compounds (VOCs), because of their possible toxicity and related risks to the ecosystem [
2,
3]. In many countries, air pollution is a major environmental problem caused by rapid industrialization. A large amount of NO
2 is emitted into the environment every year due to the industrial combustions and automobile emissions [
4]. Therefore, the detection of NO
2 has aroused widespread concerns, because it is harmful to the plants and respiratory systems of people and animals [
5]. Additionally, NO
2 can cause acid rain and photochemical smog [
6,
7]. Therefore, the United States Environmental Protection Agency (EPA) defines NO
2 as a typical air pollutant, and the exposure limit is only 53 ppb [
8]. Ammonia (NH
3) is also a common dangerous air pollutant, which is produced by the industrial process, agricultural production, and manufacturing process [
9,
10]. Specifically, any overexposure to the high concentrations of NH
3 (>30 ppm, 10 min) can irritate the human eye, skin, and respiratory system [
11,
12,
13]. VOCs are the hydrocarbons that exist as gases or vapor at room temperature, which can be emitted from numerous products and activities, e.g., detergents, paints, solvents, tools, clothes, toys, cleaning, and cooking [
14]. Aldehyde, aromatic, aliphatic, halogenated, and terpenoid compounds are the VOCs commonly detected in commercial buildings [
14,
15]. Toxic VOCs that have been previously detected in air by any type of sensors include formaldehyde, acetaldehyde, benzene, toluene, xylenes, phenol, pyridine, acetone, acetic anhydride, carbon disulfide, dihydroxybenzene, and so on [
14,
15,
16,
17,
18,
19]. For example, phenol is a toxic VOC occurring both naturally but also from industrial processes, which can be rapidly absorbed through the skin and cause skin and eye burns upon contact [
20]. It is considered as a serious pollutant because of the toxicity and persistence in the environment. The short-term exposure limit of phenol is 10 ppm, 60 min [
21]. Because of the serious environmental pollution, phenol monitoring becomes an urgent problem. Therefore, with the monitoring development of air pollution, the demand for gas sensors will increase rapidly in the future.
As a 2D material, graphene has many advantages, such as large conjugated structure, high specific surface area, high conductivity, easy to be synthesized, sensitive to the gas molecules, and so on. It has been proven to be a promising high-performance gas detection material [
22]. Graphene surface can easily absorb some molecules, such as NO
2, NH
3, CO
2, and so on. Moreover, the conductivity of graphene will change after adsorption of target gas molecules. The concentration of target gas in the environment can be detected by monitoring the change of conductivity. There have been many reports on the application of graphene in gas sensors, including pure graphene [
23,
24,
25,
26] and graphene composite materials [
27,
28,
29,
30,
31]. There are many factors affecting graphene-based sensors, including: synthetic method [
32,
33,
34], chemical structure [
35,
36,
37], interlaminar structure [
34,
38], testing environment [
39,
40,
41,
42], and surface properties [
43,
44,
45,
46,
47]. Due to the
π-π accumulation and Van Der Waals force binding between graphene, the 2D graphene nanocomposites tend to agglomerate, resulting in the reduction of specific surface area [
48,
49,
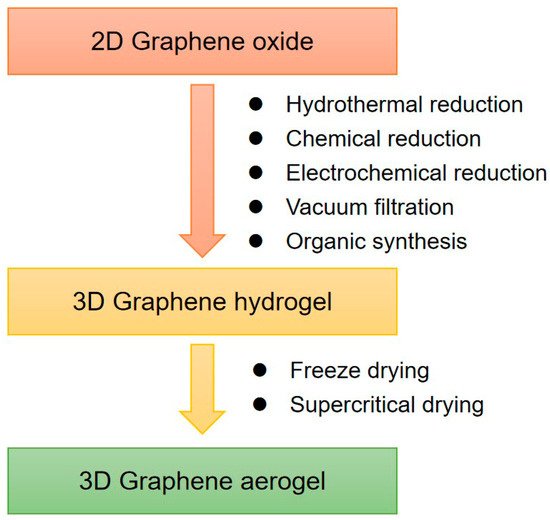
50]. In order to make full use of the characteristics of graphene, 2D graphene is usually assembled into a three-dimensional (3D) framework state by a series of methods. In contrast, due to the combination of 3D porous structure and the inherent characteristics of graphene, 3D graphene provides more space and larger surface area to transport and store electrons. 3D graphene has good conductivity, large specific surface area, and versatile gas adsorption sites. Furthermore, the defects and edge positions on the 3D porous graphene play an important role in promoting gas adsorption [
48]. In recent years, compared with 2D graphene structures, 3D porous graphene structures such as graphene hydrogels, graphene aerogels, and graphene foams have been used as high-performance gas sensors [
49]. Although 3D graphene has broad prospects in the field of gas sensors with the super high sensitivity, the selectivity is not satisfactory. Different gas molecules may adsorb on the same 3D graphene sheets and lead to the total change of the resistance [
50,
51]. It is difficult to quantitatively distinguish one target gas from a gas mixture. To improve the selectivity, defect engineering is generally needed to modulate graphene [
52].
Several reviews have presented the main development of graphene-based gas sensors. For example, in 2015, Meng et al. [
49] reviewed the graphene-based hybrids for chemi-resistive gas sensors. They focused on the sensing principles and synthesis processes of the graphene-based hybrids with noble metals, metal oxides, and conducting polymers. In 2018, Xia et al. [
50] summarized the 3D structure graphene/metal oxide hybrids for gas sensors. They concluded a variety of logical strategies to design the 3D nanohybrids of RGO and MOx. In 2020, Ilnicka et al. [
51] summarized the graphene-based hydrogen gas sensors, a special case of gas sensitivity to H
2. However, the above reviews did not reflect the whole progress of graphene gas sensors, especially for the air pollution monitoring applications. This paper aims to summarize the recent progress of the gas sensors based on 3D graphene frameworks in the detection of air pollutants.