| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Farid Menaa | + 3419 word(s) | 3419 | 2020-11-03 15:25:54 | | | |
| 2 | Peter Tang | -827 word(s) | 2592 | 2020-11-09 08:59:55 | | |
Video Upload Options
The unique properties of graphene make graphene (and its derivatives) a valuable nanomaterial for 2D or 3D stem cells culture maintenance and differentiation. To the best of our knowledge, this is the first review worldwide that besides provide clues and insights on the way we can much better manage and study stem cells especially for tissue engineering, regenerative medicine, transplantation, orthopedic surgery.
1. Introduction
Nanomaterials encompass a variety of materials with nanoscale structural features, including nanoparticles, nanofibres, nanosurfaces and nanocomposites. As nanomaterials become increasingly more sophisticated in their range of physical properties, e.g. two-dimensional (2D) surfaces, three-dimensional (3D) structures, variable porosity, stiffness, biocompatibility and biodegradability, their diversity of use for medical applications continues to expand. Both physical and chemical properties of biomaterials are now more readily altered, providing opportunities to improve efficacy and safety [1].
Since Langer and Vacanti [2] proposed the combined use of stem cells (SCs), nanomaterial-based scaffolds and inductive factors as the basis for tissue engineering (TE), researchers have been able to fabricate increasingly complex tissue/organ constructs and some are used clinically today as standard treatment for a variety of conditions. Scaffolds are processed in order to produce 3D structures, with appropriate shape, size, architecture and physical properties tailored to fulfil specific functions. In other words, TE products are designed to mimic tissue architecture and responses. Therefore, key scaffold requirements are biocompatibility, controlled porosity and permeability, suitable mechanical and degradation kinetics properties comparable to those of the targeted tissue and, additionally, support for cell attachment and proliferation by the addition of nanotopography to the biomaterial surface [3][4].
TE and regenerative medicine (RM) represent areas of increasing interest, due to the major progress in cell and organ transplantation, as well as advances in materials science and engineering. Isolated from a variety of embryonic, fetal and adult tissues, SC populations consequently differ in their ease of in vitro culture, proliferation rates and capacity to form specialized cell types. Moreover, their unique, pluripotent characteristics related to the differentiation into derivatives of all germ layers in vitro, ex vivo and in vivo, regeneration (i.e. high self-renewal capacity), development, remodelling, and replenishment of aged and diseased tissues, make them leading candidates in TE research and RM (e.g. the treatment of currently incurable diseases).
Conceptually, SCs can be divided into two major types:
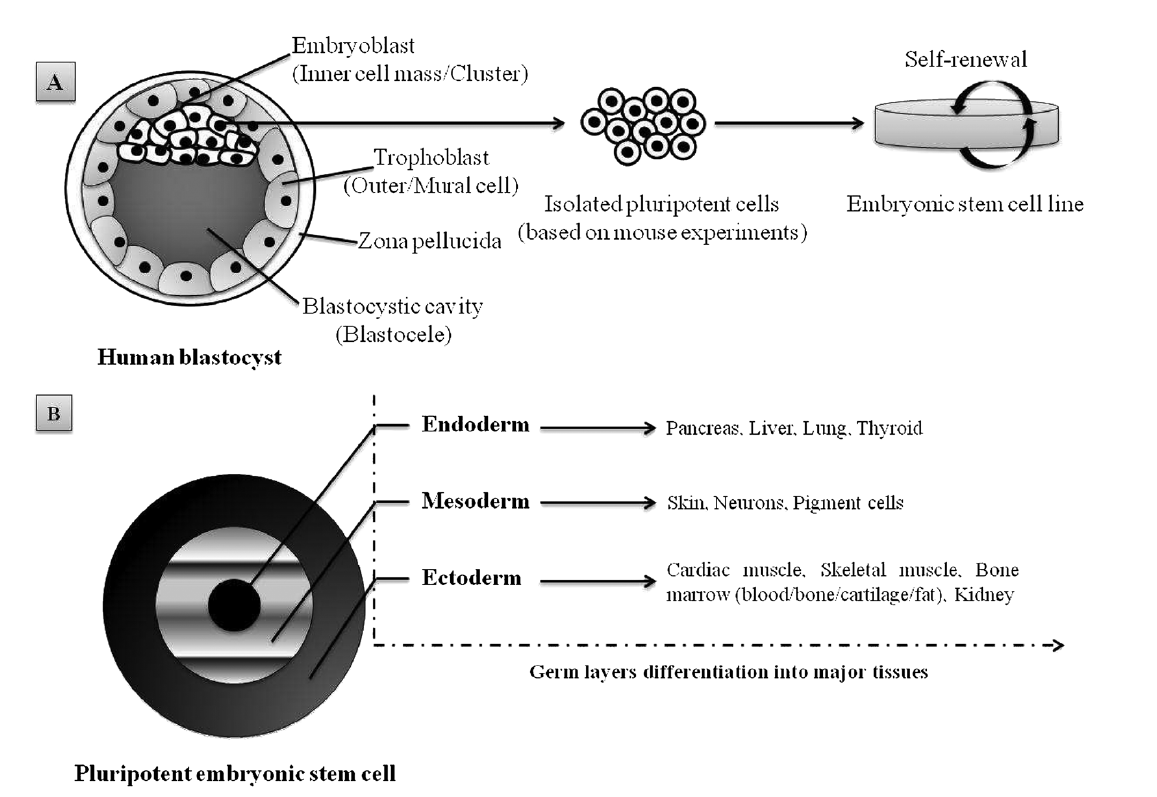
1. Embryonic stem cells (ESCs), derived from a very early embryo and adult stem cells, can be found in postnatal tissues (e.g. bone marrow, adipose tissue, umbilical cord) [5]. In 1981, the first ESCs derived from mice were isolated and grown in culture [6] and almost two decades later the isolation of human ESCs was reported by Thomson et al. [7]. ESCs are considered pluripotent notably due to their ability to self-renew (Figure 1A) and differentiate in various cells lineages (Figure 1B). The pluripotency of ESCs can be demonstrated by either: (a) injection of ESCs into immune-deficientmice to produce teratomas containing cells expressing markers of each of the three primary germ layers, endoderm, ectoderm and mesoderm [7][8]; (b) injection of ESCs into a mouse blastocyst to form a chimeric mouse and subsequent assessment of offspring to confirm incorporation of these cells into the germline [9]. Evidence suggests that human ESC-derived cell populations display low immunogenicity and could, potentially, be transplanted with minimal immune suppression [10][11][12]. However, the conditions required for maintaining pluripotency and self-renewal of mESCs and hESCs in vitro are quite different, and thus studies in one animal ESC line are not always transferable to another. In addition to ethical and political concerns, their clinical application is severely limited by their lack of accessibility and the difficulties that impede purification and manipulation techniques, as well as concerns related to the risk of teratoma formation [13].
Figure 1. Schematic representation of pluripotent human ESC lines isolated from the inner cell mass of a blastocyst-stage embryo: (A) appropriate culture maintenance of ESCs allows them to undergo self-renewal, proliferation with retention of the SC state; (B) alternatively, upon stimulus (e.g. growth factors, electrostatic interactions/electrical pulses), ESCs can differentiate into any cell type of the three germ layers (i.e. endoderm, mesoderm or ectoderm). Adapted from Kingham and Oreffo [14], with permission.
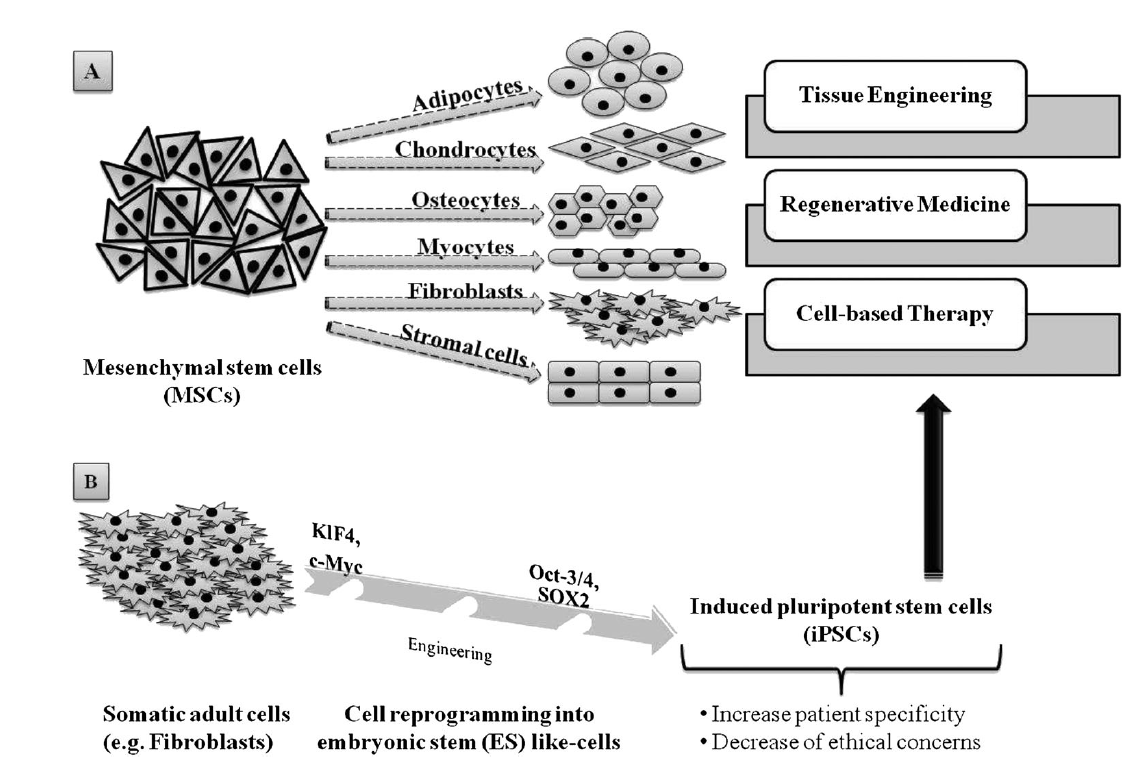
2. Adult SCs, which are ethically preferable to ESCs but, unlike the latter, their sources are somewhat lineagerestricted in humans. Also, their isolation can prove complex and can be painful for the patient, besides their capacity to self-renew that makes their expansion in vitro a significant challenge. However, mesenchymal stem cells (MSCs), one of the many types of adult SCs, display interesting features that make them suitable for tissue regeneration and cell therapy, such as versatility in changing their phenotype during differentiation, and ease of isolatation and culture [15]. Because MSCs of multiple adult vertebrate species originate from extraembryonic mesoderm, their capacity to differentiate into adipogenic, chondrogenic, osteogenic, myogenic and fibroblastic lineages (Figure 2A) has been extensively studied [13][15][16]. Similarly to human ESCs, MSCs and human ESC-derived MSCs are also able to provide immune-suppressive properties [17][18][19] which are important to consider when it comes toTE, RM and global cell-based therapy. Interestingly, adult somatic cell-derived ESC-like induced pluripotent stem cells (iPSCs; Figure 2B) are increasingly being investigated as a patient-specific alternative to human ESCs, with less controversy. Importantly, Yamanaka and colleagues demonstrated that mouse fibroblasts could be reprogrammed to mouse ESC-like cells by the expression of four mouse ESC-specific transcription factor genes (i.e. Klf4, c-Myc, Oct-3/4 and Sox2) [20][21]. Similarly, adult human fibroblasts have been genetically manipulated to form human iPSCs [22][23]. Subsequently, further reports have described iPSCs formed from non-pluripotent, somatic adult cells, and additional strategies have been developed to limit genetic manipulation or to incorporate reprogramming factor-free methods [24]. The high degree of similarity between iPSCs and ESCs, while meanwhile enhancing patient specificity and lowering ethical concerns about iPSCS, undeniably constitutes a new hope for stem cell-based therapy and, therefore, for TE and regenerative therapies [25][26][27].
Figure 2. Adult stem cells capabilities and applications: (A) mesenchymal stem cells (MSCs) are able to differentiate into various cell lineages, making them highly valuable in tissue engineering, regenerative medicine and cell-based therapy; (B) somatic adult cells, such as fibroblasts, can be genetically reprogrammed using four transcription factors, Klf4, c-Myc, Oct-3/4 and SOX2, in order to produce induced pluripotent stem cells (iPSCs), which constitute a consequent alternative source for TE, RM and and cell-based therapy.
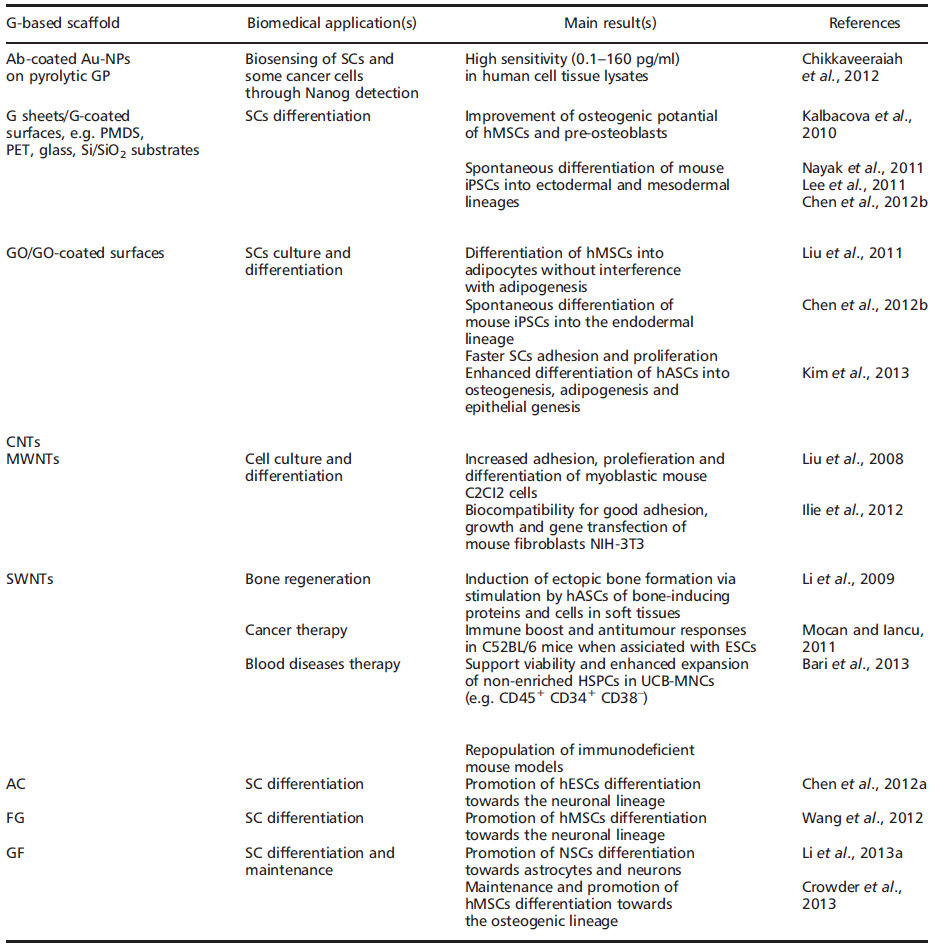
In fact, regardless of stemcell type, current focus remains on SC expansion, maintenance of the SC state, differentiation and, ultimately, transplantation and clinical applications. In some ways, nanomaterials such as graphene (G) and derivatives may hold the key for future advances in TE and RM (Table 1).
G (also known as free-standing 2D crystals or single atom-thick crystallites) is an allotrope of pure carbon with atoms arranged in a regular hexagonal pattern, similar to graphite (GP) but in a single planar sheet (i.e. a flat monolayer) (Figure 3A) of sp2-bonded carbon atoms that are densely/tightly packed in a 2D honeycomb crystal lattice [28][29][30]. It can be wrapped up into 0D fullerenes, rolled into 1D nanotubes or stacked into 3D-GP [30].
Figure 3. SEM images of graphene foams at low magnification: (A) 2D graphene foam; paper models and SEM images showing stacks of (a) flat graphene sheets, (b) heavily wrinkled sheets and (c) crumpled graphene balls; (B) 3D graphene foam. Reproduced with permission from Luo et al. [31] and Li et al. [32], respectively
The name ‘graphene' was coined to describe single-layer carbon foils [33][34][35] and is a derivative of 3D-GP, for which the electronic properties were first studied by Wallace [36][37]. In 1987, the term ‘graphene' was used to describe single sheets/layers of GP (i.e. monolayer GP, also known as atomic plane structure of GP [38], fullerenes including carbon nanotubes (CNTs) [39], epitaxial G [40] and polycyclic aromatic hydrocarbons [41]. Almost a decade ago, Geim and Novoselov [30] demonstrated the possibility of isolating G by a ‘Scotch tape' technique from bulk GP; the latter was then defined as a stack ofmulti-G sheets [42]. The ‘Scotch tape' method (also called micro-mechanical exfoliation) is defined as the cleavage/alleviation of GP in the presence of silicon dioxide (SiO2), which could be used as a ‘back gate' electrode to vary the charge density in the extracted, nearly-neutral G (also known as a zero-gap semiconductor) [42]. The process consists of using adhesive tape to repeatedly split GP crystals into increasingly thinner pieces (i.e. 0.01 thousandths of an inch) in a silicon wafer [42][43]. From 2004, electronic properties have been increasingly studied, and scaling up of innovative procedures to produce G sheets, e.g. by exfoliation by the dry deposition or drawing method [28][29][30], by layer-by-layer (LbL) self-assembly [44][45], by epitaxial growth in GP or metals as substrates [46][47][48][49], notably via chemical vapour deposition (CVD) [50], by a carbon dioxide reduction method [44] or from GP sonication [51][52], has allowed companies to sell good quality G inexpensively [53][50]. In 2010, the Nobel Prize in Physics was awarded to Geim and Novoselov for groundbreaking experiments regarding G (http://www.nobelprize.org). Since then, the isolation of free-standing G sheets [54][55] has caused widespread attention and immense excitement amongst scientists, because of its large potential in industry (e.g. for innovative biosensors, functionalized carbon nanoconstructs) [44][45][56] and theranostic broad applications (e.g. oncology, regenerative medicine) [57][58][59][60][61][62][63][64][65][66][67][68][69][70]. Indeed, G displays extraordinary physicochemical properties, e.g. electronic [30][71][72][73][74][75], optical [76][77][78][79][80][81][82][83], mechanical [84] and thermal [85][86][87][88][89], in addition to being small (i.e. carbon–carbon bond length about 0.142 nm; interplanar spacing of G sheets about 0.335 nm), light (i.e. about 0.77 mg/m2), strong, flexible, cost-effective and ecological [90][91]. However, it is worth noting that ab initio calculations showed that a G sheet is thermodynamically unstable if its size is ca. < 20 nm, certainly because of G´ s lower-energy state [92][93]. Eventually, G´s modifiable chemistry, large surface area, atomic thickness and molecular gate-tunable structure make antibody-functionalized G sheets excellent candidates for cells (e.g. mammalian, microbial) and molecular (e.g. blood biomarkers) detection, as well as for the development of innovative theranostic tools [57][58][59][60][61][62][63][64][65][66][67][68][94](Table 1). Interestingly, recent findings have shown that G-based devices and methods can be also used to detect SCs as well as facilitate growth, maintenance and differentiation [69][95][96][97][98][99][100]. G and derivatives, e.g. graphene oxide (GO) and CNTs, might then be of high importance for SC-based therapies, such as bone regeneration, and oncology, such as the detection and isolation of ‘cancer SCs' (Table 1). They also represent valuable alternatives to other nanobiomaterials, e.g. silica and/or polysaccharide-based scaffolds [14][101][102][103][104][105][106][107][108][109][110][111][112]. Nevertheless, whether G and derivatives provide better properties in terms of efficacy and safety than other nanomaterials for biomedical and pharmaceutical applications, such as TE and RM, SCs culture and maintenance, drug delivery and bio-imaging, is not yet known, therefore careful, comparative and comparable studies are needed. Indeed, if G and its derivatives offer exceptional physicochemical properties and are relatively safe in vitro, one should also be aware of their potential cell and systemic nanotoxicity in vivo unless data clearly prove acceptable human safety [113][114][115][116][117]. Overall, the cell/tissue biocompatibility and biodegradability of G and derivatives may depend on: (a) their concentration and time of incubation under in vitro, ex vivo or in vivo conditions [116][117]; (b) their surface-area design, e.g. chemical surface functionalization, such as covalent attachment of amines to increase the dispersability and/or non-linear optical performance of chemically converted/ modified G [113][114]; (c) their exposure environment, which may or may not lead to G sheets aggregation [118]; and (d) the exposure route/mode of interaction with cells, i.e. suspension vs adherent cell types [118].
2. Graphene and derivatives: speeding up stem cell research?
Current TE approaches combine different scaffold materials with living cells to provide biological substitutes that can repair and eventually improve tissue functions [102][104][105][107][108][109][110][111][112]. Indeed, several available natural and synthetic nanomaterials are useful for transplantation of SCs and their specific differentiation into muscle, bone and cartilage [102][104][105][107][108][109][110][111][112]. One of the key objectives for bone regeneration therapy to be successful is to direct the proliferation of SCs and accelerate their differentiation in a controlled manner through the use of growth factors and osteogenic inducers [14][104].
The culture of bone marrow-derivedMSCs, as well as the control of their differentiation towards a different tissue lineage, represents a very important part of TE, where cells are combined with artificial scaffolds to regenerate tissues [95][96][107][109][110][111].
Further, neural stemcells (NSCs)-based therapy provides a promising approach for neural regeneration. Indeed, NSCs represent a self-renewing and multipotent cell population in the central nervous system (CNS) which exhibits promising prospects in developing cell therapies for neural regeneration [119][120]. For the success of clinical application of NSCs, a scaffold is required to provide 3D cell-growth microenvironments and appropriate synergistic cell-guidance cues, and so, in this context, 3D graphene foams (3D-GFs) (Figure 3B) would be a better option than 2D-GFs (Figure 3A) or 2D-G derivatives, such as GO (Figure 4) [32]. Besides, transplantation of biomaterial scaffolds encasing either human ESCs [121][122][123][124] or adult SCs [124][125] has been proposed as a clinical therapy for various neurological lesions and disorders, such as spinal cord injury, cerebral ischaemia and stroke. Eventually, iPSCs hold great promise as a cell source for RM; however their culture, maintenance of pluripotency and induction of differentiation remain challenging [99]. In light of recent developments, artificially synthesized carbon-based biomaterials/carbon allotropes, such as G, GO and CNTs, have demonstrated feasibility in supporting stem cell attachment and differentiation [32][95][96][97][98][99][100][123] and so have already found a wide variety of applications in biomedicine. Nevertheless, despite the recent progress in human SC research, only a few attempts to use carbon nanotechnology in the TE and RM fields have been reported. Also, the applicability of carbon nanotechnology is significantly hampered by evidence of nanotoxic effects on multiple cell types, which tends to be minimized with appropriate surface designs, i.e. surface physical or chemical functionalizations [114]. Nonetheless, an emergent drive for an innovative carbonaceous biomaterial calls for a safer platform with comparable advantageous characteristics.
Figure 4. A proposed schematic (Lerf–Klinowski model) of graphene oxide structure. The variations of the model indicate ambiguity regarding (A) the presence or (B) the absence of carboxylic acids at the periphery of the basal plane of the graphitic platelets of GO. Reproduced with permission from Dreyer et al [126].
3. Conclusions and perspectives
The mechanical properties of G, such as high elasticity, flexibility and adaptability to flat or irregular surfaces, are suitable for the structural reinforcement of biocompatible films, hydrogels such as polyvinyl alcohol (PVA) and poly(methyl methacrylate) (PMMA), and other scaffold materials, e.g. polysaccharides such as chitosan and alginate, frequently used for TE and RM. Although mostly in its initial stage, the research on biomedical applications of G has seen exciting and encouraging advances. Nevertheless, some challenges must be overcome, such as: (a) a better understanding of cellular interactions with G and derivatives, especially the cellular uptake mechanism. This might facilitate the development of more efficient G or Gderivatives-based nanoplatforms for biosensing, drug delivery, cell culture and maintenance, among other applications; (b) the relative nanotoxicity of G and derivatives. Preliminary results indicate that the physicochemical properties, e.g. flat shape, surface charges, of G and derivatives are closely related to their cytotoxicity, e.g. in vitro induction of cellular oxidative stress and DNA damage, and affect the in vivo biodistribution and fate. Thus, before clinical applications, a systematic comparative study, e.g. a deep meta-analysis, is highly desired to address the relative safety concerns (subtracting false-negative and -positive effects) of G and derivatives. Eventually, the research on G and derivatives-based scaffold materials for cell culture is a relatively new direction that deserves special attention. Indeed, studies in this field so far have demonstrated that G and derivatives are able to accelerate the growth, proliferation and differentiation of SCs, therefore holding great promise in TE, RM and other biomedical fields.We strongly believe that the trend of this emerging field will continue and even speed up in the future.
References
- Nathaniel Huebsch; David J. Mooney; Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426-432, 10.1038/nature08601.
- Langer R., Vacanti J.P.; Tissue engineering. Science 1993, 260, 920-926.
- Sangwon Chung; Martin W. King; Design concepts and strategies for tissue engineering scaffolds. Biotechnology and Applied Biochemistry 2011, 58, 423-438, 10.1002/bab.60.
- Carletti E., Motta A., Migliaresi C.; Scaffolds for tissue engineering and 3D cell culture. Methods Mol. Biol. 2011, 695, 17-39.
- Timothy J. Nelson; Zhi-Dong Ge; Jordan Van Orman; Matthew Barron; Diane Rudy-Reil; Timothy A. Hacker; Ravi Misra; Stephen A. Duncan; John A. Auchampach; John Lough; et al. Improved cardiac function in infarcted mice after treatment with pluripotent embryonic stem cells. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology 2006, 288, 1216-1224, 10.1002/ar.a.20388.
- M. J. Evans; M. H. Kaufman; Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154-156, 10.1038/292154a0.
- James A. Thomson; Joseph Itskovitz-Eldor; Sander S. Shapiro; Michelle A. Waknitz; Jennifer J. Swiergiel; Vivienne S. Marshall; Jeffrey M. Jones; Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145-1147, 10.1126/science.282.5391.1145.
- G. R. Martin; Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells.. Proceedings of the National Academy of Sciences 1981, 78, 7634-7638, 10.1073/pnas.78.12.7634.
- Allan Bradley; Martin Evans; Matthew H. Kaufman; Elizabeth Robertson; Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 1984, 309, 255-256, 10.1038/309255a0.
- Micha Drukker; Gil Katz; Achia Urbach; Maya Schuldiner; Gal Markel; Joseph Itskovitz-Eldor; Benjamin Reubinoff; Ofer Mandelboim; Nissim Benvenisty; Characterization of the expression of MHC proteins in human embryonic stem cells. Proceedings of the National Academy of Sciences 2002, 99, 9864-9869, 10.1073/pnas.142298299.
- Fred Fändrich; Xiongbin Lin; Gui X. Chai; Maren Schulze; Detlev Ganten; Michael Bader; Julia Holle; Dong-Sheng Huang; Reza Parwaresch; Nicholaus Zavazava; et al.Bert Binas Preimplantation-stage stem cells induce long-term allogeneic graft acceptance without supplementary host conditioning. Nature Medicine 2002, 8, 171-178, 10.1038/nm0202-171.
- L. Li; Miren L. Baroja; Anish Majumdar; Kristin Chadwick; Anne Rouleau; Lisa Gallacher; Iris Ferber; Jane Lebkowski; Tanya Martin; Joaquin Madrenas; et al.Mickie Bhatia Human Embryonic Stem Cells Possess Immune-Privileged Properties. STEM CELLS 2004, 22, 448-456, 10.1634/stemcells.22-4-448.
- Cun-Gang Fan; Qing-Jun Zhang; Jing-Ru Zhou; Therapeutic Potentials of Mesenchymal Stem Cells Derived from Human Umbilical Cord. Stem Cell Reviews and Reports 2010, 7, 195-207, 10.1007/s12015-010-9168-8.
- Emmajayne Kingham; R.O.C. Oreffo; Embryonic and Induced Pluripotent Stem Cells: Understanding, Creating, and Exploiting the Nano-Niche for Regenerative Medicine. ACS Nano 2013, 7, 1867-1881, 10.1021/nn3037094.
- Mark F. Pittenger; Alastair M. Mackay; Stephen C. Beck; Rama K. Jaiswal; Robin Douglas; Joseph D. Mosca; Mark A. Moorman; Donald W. Simonetti; Stewart Craig; Daniel R. Marshak; et al. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143-147, 10.1126/science.284.5411.143.
- Iancu C., Ilie I., Mocan L., et al.. Human cord blood-derived stem cells in transplantation and regenerative medicine. In Stem Cells in Clinic and Research, Gholamrezanezhad A (ed.); InTech: Rijeka, Croatia, 2011; pp. 21-28.
- Yen B., Chang C., Liu K.J., et al.; Brief report – human embryonic stem cellderived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells 2009, 27, 451–456.
- Soufiane Ghannam; Carine Bouffi; Farida Djouad; Christian Jorgensen; Danièle Noël; Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Research & Therapy 2010, 1, 1-7, 10.1186/scrt2.
- Somayeh Shahrokhi; Saeed Daneshmandi; Farid Menaa; Tumor Necrosis Factor-α/CD40 Ligand-Engineered Mesenchymal Stem Cells Greatly Enhanced the Antitumor Immune Response and Lifespan in Mice. Human Gene Therapy 2014, 25, 240-253, 10.1089/hum.2013.193.
- Kazutoshi Takahashi; Shinya Yamanaka; Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663-676, 10.1016/j.cell.2006.07.024.
- Keisuke Okita; Tomoko Ichisaka; Shinya Yamanaka; Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313-317, 10.1038/nature05934.
- Kazutoshi Takahashi; Koji Tanabe; Mari Ohnuki; Megumi Narita; Tomoko Ichisaka; Kiichiro Tomoda; Shinya Yamanaka; Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861-872, 10.1016/j.cell.2007.11.019.
- Masato Nakagawa; Michiyo Koyanagi; Koji Tanabe; Kazutoshi Takahashi; Tomoko Ichisaka; Takashi Aoi; Keisuke Okita; Yuji Mochiduki; Nanako Takizawa; Shinya Yamanaka; et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nature Biotechnology 2007, 26, 101-106, 10.1038/nbt1374.
- James O’Malley; Knut Woltjen; Keisuke Kaji; New strategies to generate induced pluripotent stem cells. Current Opinion in Biotechnology 2009, 20, 516-521, 10.1016/j.copbio.2009.09.005.
- Maureen L. Condic; Mahendra Rao; Regulatory Issues for Personalized Pluripotent Cells. STEM CELLS 2008, 26, 2753-2758, 10.1634/stemcells.2008-0421.
- Giovanni Amabile; Alexander Meissner; Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends in Molecular Medicine 2009, 15, 59-68, 10.1016/j.molmed.2008.12.003.
- Han Lee; Jung Park; Bernard G Forget; Peter Gaines; Induced pluripotent stem cells in regenerative medicine: an argument for continued research on human embryonic stem cells. Regenerative Medicine 2009, 4, 759-769, 10.2217/rme.09.46.
- A. K. Geim; Graphene: Status and Prospects. Science 2009, 324, 1530-1534, 10.1126/science.1158877.
- Andrey K. Bodenmann; Allan H. Macdonald; Graphene: Exploring carbon flatland. Physics Today 2007, 60, 35-41, 10.1063/1.2774096.
- A. K. Geim; K. S. Novoselov; The rise of graphene. Nature Materials 2007, 6, 183-191, 10.1038/nmat1849.
- Jiayan Luo; Hee Dong Jang; Jiaxing Huang; Effect of Sheet Morphology on the Scalability of Graphene-Based Ultracapacitors. ACS Nano 2013, 7, 1464-1471, 10.1021/nn3052378.
- Ning Li; Qi Zhang; Song Gao; Qin Song; Rong Huang; Long Wang; Liwei Liu; Jianwu Dai; Mingliang Tang; Guosheng Cheng; et al. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Scientific Reports 2013, 3, 1604, 10.1038/srep01604.
- H.P. Boehm; A. Clauss; Georg Fischer; U. Hofmann; SURFACE PROPERTIES OF EXTREMELY THIN GRAPHITE LAMELLAE. Proceedings of the Fifth Conference on Carbon 1962, 1, 73-80, 10.1016/b978-0-08-009707-7.50013-3.
- Boehm H.P., Clauss A., Fischer G.O., et al.; Das Adsorptionsverhalten sehr dünner Kohlenstoffolien. Zeitschr Anorgan Allgem Chemie 1962, 316, 119–127.
- H. P. Boehm; R. Setton; E. Stumpp; Nomenclature and terminology of graphite intercalation compounds (IUPAC Recommendations 1994). Pure and Applied Chemistry 1994, 66, 1893-1901, 10.1351/pac199466091893.
- P. R. Wallace; Erratum: The Band Theory of Graphite [Phys. Rev. 71, 622 (1947)]. Physical Review 1947, 72, 258-258, 10.1103/physrev.72.258.
- Avouris P., Chen Z., Perebeinos V.; Carbon-based electronics. Nat. Nanotechnol. 2007, 2, 605-615.
- A. Hamwi; S. Mouras; D. Djurado; J.C. Cousseins; New synthesis of first stage graphite intercalation compounds with fluorides. Journal of Fluorine Chemistry 1987, 35, 151, 10.1016/0022-1139(87)95120-7.
- Riichiro Saito; Mitsutaka Fujita; G. Dresselhaus; M. S. Dresselhaus; Electronic structure of graphene tubules based onC60. Physical Review B 1992, 46, 1804-1811, 10.1103/physrevb.46.1804.
- I. Forbeaux; J.-M. Themlin; J.-M. Debever; Heteroepitaxial graphite on6H−SiC(0001): Interface formation through conduction-band electronic structure. Physical Review B 1998, 58, 16396-16406, 10.1103/physrevb.58.16396.
- S. Wang; S. Yata; J. Nagano; Y. Okano; H. Kinoshita; H. Kikuta; T. Yamabe; A New Carbonaceous Material with Large Capacity and High Efficiency for Rechargeable Li-Ion Batteries. Journal of The Electrochemical Society 2000, 147, 2498-2502, 10.1149/1.1393559.
- K. S. Novoselov; A. K. Geim; S. V. Morozov; D. Jiang; Y. Zhang; S. V. Dubonos; I. V. Grigorieva; A. A. Firsov; Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666-669, 10.1126/science.1102896.
- Rutherford RB, Dudman RL. 2002; Ultra-thin flexible expanded graphite heating element. US Patent No. 6 667 100 B2.
- Hui-Lin Guo; Xian-Fei Wang; Qing-Yun Qian; Feng-Bin Wang; Xing-Hua Xia; A Green Approach to the Synthesis of Graphene Nanosheets. ACS Nano 2009, 3, 2653-2659, 10.1021/nn900227d.
- Je Seob Park; Sung Min Cho; Woo-Jae Kim; Juhyun Park; Pil J. Yoo; Fabrication of Graphene Thin Films Based on Layer-by-Layer Self-Assembly of Functionalized Graphene Nanosheets. ACS Applied Materials & Interfaces 2011, 3, 360-368, 10.1021/am100977p.
- C. Riedl; C. Coletti; T. Iwasaki; A. A. Zakharov; Ulrich Starke; Quasi-Free-Standing Epitaxial Graphene on SiC Obtained by Hydrogen Intercalation. Physical Review Letters 2009, 103, 246804, 10.1103/physrevlett.103.246804.
- Sutter P.; Epitaxial graphene: how silicon leaves the scene. Nat. Mat. 2009, 8, 171-172.
- I. Pletikosić; M. Kralj; P. Pervan; R. Brako; J. Coraux; A. T. N’Diaye; C. Busse; T. Michely; Dirac Cones and Minigaps for Graphene on Ir(111). Physical Review Letters 2009, 102, 056808, 10.1103/physrevlett.102.056808.
- Bo Song; Di Li; Wenpeng Qi; Marcus Elstner; Chunhai Fan; Haiping Fang; Graphene on Au(111): A Highly Conductive Material with Excellent Adsorption Properties for High-Resolution Bio/Nanodetection and Identification. ChemPhysChem 2010, 11, 585-589, 10.1002/cphc.200900743.
- Sukang Bae; Hyeong Keun Kim; Youngbin Lee; Xiangfan Xu; Jae-Sung Park; Yi Zheng; Jayakumar Balakrishnan; Tian Lei; Hye Ri Kim; Young Il Song; et al.Young-Jin KimKwang S. KimBarbaros ÖzyilmazJong-Hyun AhnByung Hee HongSumio Iijima Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nature Nanotechnology 2010, 5, 574-578, 10.1038/nnano.2010.132.
- John F. Dobson; Angela C. White; Angel Rubio; Asymptotics of the Dispersion Interaction: Analytic Benchmarks for van der Waals Energy Functionals. Physical Review Letters 2006, 96, 073201, 10.1103/physrevlett.96.073201.
- Ignat V. Fialkovsky; Valery N. Marachevsky; Dmitri V. Vassilevich; Finite-temperature Casimir effect for graphene. Physical Review B 2011, 84, 35446, 10.1103/physrevb.84.035446.
- Michael Segal; Selling graphene by the ton. Nature Nanotechnology 2009, 4, 612-614, 10.1038/nnano.2009.279.
- Dale A. C. Brownson; Craig E. Banks; Graphene electrochemistry: an overview of potential applications. The Analyst 2010, 135, 2768-2778, 10.1039/c0an00590h.
- Yuxin Liu; Xiaochen Dong; Peng Chen; Biological and chemical sensors based on graphene materials. Chem. Soc. Rev. 2012, 41, 2283-2307, 10.1039/c1cs15270j.
- Ram Sevak Singh; Venkatram Nalla; Wei Chen; Andrew T. S. Wee; Ji. Wei; Laser Patterning of Epitaxial Graphene for Schottky Junction Photodetectors. ACS Nano 2011, 5, 5969-5975, 10.1021/nn201757j.
- Mohanty N., Vikas B.; Graphene-based single-bacterium resolution biodevice and DNA-transistor – interfacing graphene derivatives with nano- and micro-scale biocomponents. Nano Lett. 2008, 8, 4469–4476.
- Bhaskara V Chikkaveeraiah; Alice Soldà; Dharamainder Choudhary; Flavio Maran; James F. Rusling; Ultrasensitive nanostructured immunosensor for stem and carcinoma cell pluripotency gatekeeper protein NANOG. Nanomedicine 2012, 7, 957-965, 10.2217/nnm.11.178.
- Subbiah Alwarappan; Kyle Cissell; Suraj Dixit; Chen-Zhong Li; Shyam Mohapatra; Chitosan-modified graphene electrodes for DNA mutation analysis. Journal of Electroanalytical Chemistry 2012, 686, 69-72, 10.1016/j.jelechem.2012.09.026.
- Murugan Veerapandian; Yeong-Tai Seo; Hyunkyung Shin; Kyusik Yun; Min-Ho Lee; Functionalized graphene oxide for clinical glucose biosensing in urine and serum samples. International Journal of Nanomedicine 2012, 7, 6123-6136, 10.2147/IJN.S38402.
- Lili Cao; Liwei Cheng; Zhengyong Zhang; Yi Wang; Xianxia Zhang; Hui Chen; Baohong Liu; Song Zhang; Jilie Kong; Visual and high-throughput detection of cancer cells using a graphene oxide-based FRET aptasensing microfluidic chip. Lab on a Chip 2012, 12, 4864-4869, 10.1039/c2lc40564d.
- Alessandra Bonanni; Chun Kiang Chua; Guanjia Zhao; Zdeněk Sofer; Dr. Martin Pumera; Inherently Electroactive Graphene Oxide Nanoplatelets As Labels for Single Nucleotide Polymorphism Detection. ACS Nano 2012, 6, 8546-8551, 10.1021/nn301359y.
- Shuang Guo; Danxin Du; Lina Tang; Yong Ning; Qunfeng Yao; Guo-Jun Zhang; PNA-assembled graphene oxide for sensitive and selective detection of DNA. The Analyst 2013, 138, 3216-3220, 10.1039/c3an00266g.
- Matteo Lorenzoni; F. Brandi; Silvia Dante; Andrea Giugni; Bruno Torre; Simple and effective graphene laser processing for neuron patterning application. Scientific Reports 2013, 3, srep01954, 10.1038/srep01954.
- Erqun Song; Dan Cheng; Yang Song; Mingdong Jiang; Jifei Yu; Yunyun Wang; A graphene oxide-based FRET sensor for rapid and sensitive detection of matrix metalloproteinase 2 in human serum sample. Biosensors and Bioelectronics 2013, 47, 445-450, 10.1016/j.bios.2013.03.030.
- Dian-Ming Zhou; Qiang Xi; Man-Fen Liang; Cui-Hua Chen; Li-Juan Tang; Jian-Hui Jiang; Graphene oxide-hairpin probe nanocomposite as a homogeneous assay platform for DNA base excision repair screening. Biosensors and Bioelectronics 2013, 41, 359-365, 10.1016/j.bios.2012.08.053.
- Jiehua Lin; Zhijing Wei; Huihui Zhang; Meijia Shao; Sensitive immunosensor for the label-free determination of tumor marker based on carbon nanotubes/mesoporous silica and graphene modified electrode. Biosensors and Bioelectronics 2013, 41, 342-347, 10.1016/j.bios.2012.08.051.
- Farid Menaa; Functional Graphene-Based Nanobioimaging Platforms: New Powered Real-Time Interfaces. Journal of Molecular Imaging & Dynamics 2013, 2, e103, 10.4172/2155-9937.1000e103.
- Farid Menaa; Menaa Farid; 2-D Graphene and Derivatives- Based Scaffolds in Regenerative Medicine: Innovative Boosters Mimicking 3-D Cell Microenvironment. Journal of Regenerative Medicine 2013, 2, 2, 10.4172/2325-9620.1000e107.
- Farid Menaa; When Pharma Meets Nano or The Emerging Era of Nano-Pharmaceuticals. Pharmaceutica Analytica Acta 2013, 4, 223, 10.4172/2153-2435.1000223.
- F. Schedin; A. K. Geim; S. V. Morozov; E. W. Hill; Peter Blake; M. I. Katsnelson; K. S. Novoselov; Detection of individual gas molecules adsorbed on graphene. Nature Materials 2007, 6, 652-655, 10.1038/nmat1967.
- Shaffique Adam; E. H. Hwang; Victor Galitski; S. Das Sarma; A self-consistent theory for graphene transport. Proceedings of the National Academy of Sciences 2007, 104, 18392-18397, 10.1073/pnas.0704772104.
- Jean-Christophe Charlier; P. C. Eklund; J. Zhu; Andrea C. Ferrari; Electron and Phonon Properties of Graphene: Their Relationship with Carbon Nanotubes. Topics in Applied Physics 2007, 111, 673-709, 10.1007/978-3-540-72865-8_21.
- Chen J.H., Jang C., Williams E.D. et al.; Charged impurity scattering in graphene. Nat. Phys. 2008, 4, 377–381.
- Jian-Hao Chen; Chaun Jang; Shudong Xiao; Masa Ishigami; Michael S. Fuhrer; Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nature Nanotechnology 2008, 3, 206-209, 10.1038/nnano.2008.58.
- Kuzmenko A.B., van Heumen E., Carbone F., et al.; Universal infrared conductance of graphite. Phys. Rev. Lett. 2008, 100, 117401.
- R. R. Nair; P. Blake; A. N. Grigorenko; K. S. Novoselov; T. J. Booth; T. Stauber; N. M. R. Peres; A. K. Geim; Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308-1308, 10.1126/science.1156965.
- Junfeng Liu; A. R. Wright; Chao Zhang; Zhongshui Ma; Strong terahertz conductance of graphene nanoribbons under a magnetic field. Applied Physics Letters 2008, 93, 41106, 10.1063/1.2964093.
- Qiaoliang Bao; Han Zhang; Yu Wang; Zhenhua Ni; Yongli Yan; Ze Xiang Shen; Kian Ping Loh; Ding Yuan Tang; Atomic-Layer Graphene as a Saturable Absorber for Ultrafast Pulsed Lasers. Advanced Functional Materials 2009, 19, 3077-3083, 10.1002/adfm.200901007.
- Han Zhang; Qiaoliang Bao; Dingyuan Tang; Luming Zhao; Kianping Loh; Large energy soliton erbium-doped fiber laser with a graphene-polymer composite mode locker. Applied Physics Letters 2009, 95, 141103, 10.1063/1.3244206.
- Han Zhang; Stéphane Virally; Qiaoliang Bao; Loh Kian Ping; Serge Massar; Nicolas Godbout; Pascal Kockaert; Z-scan measurement of the nonlinear refractive index of graphene. Optics Letters 2012, 37, 1856-1858, 10.1364/ol.37.001856.
- Ulaş KÜRÜM; Okan Öner Ekiz; H. Gul Yaglioglu; Ayhan Elmali; Mustafa Ürel; Hasan Güner; Alpay Koray Mızrak; Bülend Ortaç; Aykutlu Dana; Electrochemically tunable ultrafast optical response of graphene oxide. Applied Physics Letters 2011, 98, 141103, 10.1063/1.3573797.
- Zhiwei Zheng; Chujun Zhao; Shunbin Lu; Yu Chen; Ying Li; Han Zhang; Shuangchun Wen; Microwave and optical saturable absorption in graphene. Optics Express 2012, 20, 23201-23214, 10.1364/oe.20.023201.
- Changgu Lee; Xiaoding Wei; Jeffrey W. Kysar; James Hone; Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385-388, 10.1126/science.1157996.
- N. Mingo; D. A. Broido; Carbon Nanotube Ballistic Thermal Conductance and Its Limits. Physical Review Letters 2005, 95, 096105-096105, 10.1103/physrevlett.95.096105.
- Koichi Saito; Jun Nakamura; Akiko Natori; Ballistic thermal conductance of a graphene sheet. Physical Review B 2007, 76, 115409, 10.1103/physrevb.76.115409.
- Alexander A. Balandin; Suchismita Ghosh; Wenzhong Bao; Irene Calizo; Desalegne Teweldebrhan; Feng Miao; Chun Ning Lau; Superior Thermal Conductivity of Single-Layer Graphene. Nano Letters 2008, 8, 902-907, 10.1021/nl0731872.
- Shanshan Chen; Qingzhi Wu; Columbia Mishra; Junyong Kang; Hengji Zhang; Kyeongjae Cho; Weiwei Cai; Alexander A. Balandin; Rodney S. Ruoff; Thermal conductivity of isotopically modified graphene. Nature Materials 2012, 11, 203-207, 10.1038/nmat3207.
- Qizhen Liang; Xuxia Yao; Wei Wang; Yan Liu; Ching Ping Wong; A Three-Dimensional Vertically Aligned Functionalized Multilayer Graphene Architecture: An Approach for Graphene-Based Thermal Interfacial Materials. ACS Nano 2011, 5, 2392-2401, 10.1021/nn200181e.
- Heyrovska R. 2008; Atomic structures of graphene, benzene and methane with bond lengths as sums of the single, double and resonance bond radii of carbon. Available at: http://arxiv.org/abs/0804.4086
- By Sudhir Husale; Sangeeta Sahoo; A. Radenovic; Floriano Traversi; Paolo Annibale; Andras Kis; ssDNA Binding Reveals the Atomic Structure of Graphene. Langmuir 2010, 26, 18078-18082, 10.1021/la102518t.
- Shenderova O.B., Zhirnov V.V., Brenner D.W.; Carbon nanostructures. Crit. Rev. Sol. State. Mater. Sci. 2002, 27, 227.
- Scheila F. Braga; † Vitor R. Coluci; † Sergio B. Legoas; † Ronaldo Giro; † And Douglas S. Galvão; Ray H. Baughman‡; Structure and Dynamics of Carbon Nanoscrolls. Nano Letters 2004, 4, 881-884, 10.1021/nl0497272.
- Shen H., Zhang L., Liu M., et al.; Biomedical applications of graphene. Theranostics 2012, 2, 283–294.
- Tapas R. Nayak; Henrik Andersen; Venkata S. Makam; Clement Khaw; Sukang Bae; Xiangfan Xu; Pui-Lai R. Ee; Jong-Hyun Ahn; Byung Hee Hong; Giorgia Pastorin; et al.Barbaros Özyilmaz Graphene for Controlled and Accelerated Osteogenic Differentiation of Human Mesenchymal Stem Cells. ACS Nano 2011, 5, 4670-4678, 10.1021/nn200500h.
- Wong Cheng Lee; Candy Haley Y. X. Lim; Hui Shi; Lena A. L. Tang; Yu Wang; Chwee Teck Lim; Kian Ping Loh; Origin of Enhanced Stem Cell Growth and Differentiation on Graphene and Graphene Oxide. ACS Nano 2011, 5, 7334-7341, 10.1021/nn202190c.
- Sung Young Park; Jaesung Park; Sung Hyun Sim; Moon Gyu Sung; Kwang S. Kim; Byung Hee Hong; Seunghun Hong; Enhanced Differentiation of Human Neural Stem Cells into Neurons on Graphene. Advanced Materials 2011, 23, H263-H267, 10.1002/adma.201101503.
- Se-Mi Kang; Tae-Hyung Kim; Jeong-Woo Choi; Cell chip to detect effects of graphene oxide nanopellet on human neural stem cell.. Journal of Nanoscience and Nanotechnology 2012, 12, 5185-5190, 10.1166/jnn.2012.6378.
- G.-Y. Chen; D.W.-P. Pang; S.-M. Hwang; Hsing-Yu Tuan; Yu-Chen Hu; A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials 2012, 33, 418-427, 10.1016/j.biomaterials.2011.09.071.
- Jangho Kim; Kyoung Soon Choi; Yeonju Kim; Ki-Tack Lim; Hoon Seonwoo; Yensil Park; Deok-Ho Kim; Pill-Hoon Choung; Chong-Su Cho; Soo Young Kim; et al.Yun-Hoon ChoungJong Hoon Chung Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. Journal of Biomedical Materials Research Part A 2013, 101, 3520-3530, 10.1002/jbm.a.34659.
- Houben R., Wischhusen J., Menaa F. et al.; Melanoma stem cells: targets for successful therapy?. J Dtsch Dermatol. Ges. 2008, 6, 541–546.
- Bouzid Menaa; Yuya Miyagawa; Masahide Takahashi; Mar Herrero; Vicente Rives; Farid Menaa; Daryl K. Eggers; Bioencapsulation of apomyoglobin in nanoporous organosilica sol-gel glasses: Influence of the siloxane network on the conformation and stability of a model protein. Biopolymers 2009, 91, 895-906, 10.1002/bip.21274.
- Menaa F., Houben R., Eyrich M., et al.; Stem cells, melanoma and cancer stem cells: the good, the bad and the evil?. G Ital. Dermatol. Venereol. 2009, 144, 287–296.
- Menaa F., Menaa A., Menaa B.; Hyaluronic Acid and Derivatives for Tissue Engineering.. Journal of Biotechnology & Biomaterials 2011, S3, 1-7, 10.4172/2155-952x.s3-001.
- M. Perán; María A García; Elena López-Ruiz; Milán Bustamante; Gema Jiménez; Roberto Madeddu; Juan Antonio Marchal; Functionalized Nanostructures with Application in Regenerative Medicine. International Journal of Molecular Sciences 2012, 13, 3847-3886, 10.3390/ijms13033847.
- Cédric Blanpain; Tracing the cellular origin of cancer. Nature 2013, 15, 126-134, 10.1038/ncb2657.
- J. Guerrero; Sylvain Catros; S.-M. Derkaoui; C Lalande; R. Siadous; Reine Bareille; N. Thébaud; L. Bordenave; O. Chassande; C. Le Visage; et al.D. LetourneurJoëlle Amédée Cell interactions between human progenitor-derived endothelial cells and human mesenchymal stem cells in a three-dimensional macroporous polysaccharide-based scaffold promote osteogenesis. Acta Biomaterialia 2013, 9, 8200-8213, 10.1016/j.actbio.2013.05.025.
- Xing Li; Yayun Zhao; Yue Bing; Yaping Li; Ning Gan; Zhiyong Guo; Zhaoxiang Peng; Yabin Zhu; Biotemplated Syntheses of Macroporous Materials for Bone Tissue Engineering Scaffolds and Experiments in Vitro and Vivo. ACS Applied Materials & Interfaces 2013, 5, 5557-5562, 10.1021/am400779e.
- Spoliti M., Iudicone P., Leone R., et al.; In vitro release and expansion of mesenchymal stem cells by a hyaluronic acid scaffoldused in combination with bone marrow. Muscles Ligaments Tendons J 2013, 2, 289–294..
- Vincenzo D'antò; Maria Grazia Raucci; Vincenzo Guarino; Stefano Martina; Rosa Valletta; Luigi Ambrosio; Behaviour of human mesenchymal stem cells on chemically synthesized HA-PCL scaffolds for hard tissue regeneration. Journal of Tissue Engineering and Regenerative Medicine 2013, 10, E147-E154, 10.1002/term.1768.
- Jiang Deng; Rongfeng She; Wenliang Huang; Zhijun Dong; Gang Mo; Bin Liu; A silk fibroin/chitosan scaffold in combination with bone marrow-derived mesenchymal stem cells to repair cartilage defects in the rabbit knee. Journal of Materials Science: Materials in Electronics 2013, 24, 2037-2046, 10.1007/s10856-013-4944-z.
- Nela Buchtová; Gildas Réthoré; Cécile Boyer; Jérôme Guicheux; Frédéric Rambaud; Karine Vallé; Philippe Belleville; Clément Sanchez; Olivier Chauvet; Pierre Weiss; et al.Jean Le Bideau Nanocomposite hydrogels for cartilage tissue engineering: mesoporous silica nanofibers interlinked with siloxane derived polysaccharide. Journal of Materials Science: Materials in Medicine 2013, 24, 1875-1884, 10.1007/s10856-013-4951-0.
- Xinyuan Liu; Sujat Sen; Jingyu Liu; Indrek Kulaots; David Geohegan; Agnes Kane; Alex A. Puretzky; Christopher M. Rouleau; Karren L. More; G. Tayhas R. Palmore; et al.Robert H. Hurt Antioxidant Deactivation on Graphenic Nanocarbon Surfaces. Small 2011, 7, 2775-2785, 10.1002/smll.201100651.
- Liang Yan; Feng Zhao; Shoujian Li; Zhongbo Hu; Yuliang Zhao; Low-toxic and safe nanomaterials by surface-chemical design, carbon nanotubes, fullerenes, metallofullerenes, and graphenes. Nanoscale 2011, 3, 362-382, 10.1039/c0nr00647e.
- Vanesa C. Sanchez; Ashish Jachak; Robert H. Hurt; Agnes B. Kane; Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review. Chemical Research in Toxicology 2011, 25, 15-34, 10.1021/tx200339h.
- Abhilash Sasidharan; Leela S. Panchakarla; Aparna R. Sadanandan; Anusha Ashokan; Parwathy Chandran; Chundayil Madathil Girish; Deepthy Menon; Shantikumar V. Nair; C. N. R. Rao; Manzoor Koyakutty; et al. Hemocompatibility and Macrophage Response of Pristine and Functionalized Graphene. Small 2012, 8, 1251-1263, 10.1002/smll.201102393.
- Grant A Hartung; G. Ali Mansoori; In vivo General Trends, Filtration and Toxicity of Nanoparticles. Journal of Nanomaterials & Molecular Nanotechnology 2013, 2, 3, 10.4172/2324-8777.1000113.
- Ken-Hsuan Liao; Yu-Shen Lin; Christopher W. Macosko; Christy L. Haynes; Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Applied Materials & Interfaces 2011, 3, 2607-2615, 10.1021/am200428v.
- Fred H Gage; Mammalian Neural Stem Cells. Science 2000, 287, 1433-1438, 10.1126/science.287.5457.1433.
- Ferdinando Rossi; Elena Cattaneo; Neural stem cell therapy for neurological diseases: dreams and reality. Nature Reviews Neuroscience 2002, 3, 401-409, 10.1038/nrn809.
- Hossein Baharvand; Narges-Zare Mehrjardi; Maryam Hatami; Sahar Kiani; Mahendra Rao; Mahdi-Montazer Haghighi; Neural differentiation from human embryonic stem cells in a defined adherent culture condition. The International Journal of Developmental Biology 2007, 51, 371-378, 10.1387/ijdb.072280hb.
- Kunlin Jin; XiaoOu Mao; Lin Xie; Veronica Galvan; Bin Lai; Yaoming Wang; Olivia Gorostiza; Xiaomei Wang; David A. Greenberg; Transplantation of Human Neural Precursor Cells in Matrigel Scaffolding Improves Outcome from Focal Cerebral Ischemia after Delayed Postischemic Treatment in Rats. British Journal of Pharmacology 2009, 30, 534-544, 10.1038/jcbfm.2009.219.
- Eric Y. T. Chen; Yung-Chen Wang; Alexander Mintz; Alan Richards; Chi-Shuo Chen; David Lu; Thien Nguyen; Wei-Chun Chin; Activated charcoal composite biomaterial promotes human embryonic stem cell differentiation toward neuronal lineage. Journal of Biomedical Materials Research Part A 2012, 100, 2006-2017, 10.1002/jbm.a.34201.
- Farid Menaa; Stroke in sickle cell anemia patients: A need for multidisciplinary approaches. Atherosclerosis 2013, 229, 496-503, 10.1016/j.atherosclerosis.2013.05.006.
- Gaëtan J.-R. Delcroix; Paul C. Schiller; Jean-Pierre Benoit; Claudia N. Montero-Menei; Adult cell therapy for brain neuronal damages and the role of tissue engineering. Biomaterials 2010, 31, 2105-2120, 10.1016/j.biomaterials.2009.11.084.
- Dreyer D.R., Park S., Bielawski C.W., et al.; The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240.