The unique properties of graphene make graphene (and its derivatives) a valuable nanomaterial for 2D or 3D stem cells culture maintenance and differentiation. To the best of our knowledge, this is the first review worldwide that besides provide clues and insights on the way we can much better manage and study stem cells especially for tissue engineering, regenerative medicine, transplantation, orthopedic surgery.
- Graphene
- tissue engineering
- Stem cells
- Cells nanoculture
- Regenerative medicine
- Nanomedicine
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
Definition
The discovery of the interesting intrinsic properties of graphene, a two-dimensional nanomaterial, has boosted further research and development for various types of applications from electronics to biomedicine. During the last decade, graphene and several graphene-derived materials, such as graphene oxide, carbon nanotubes, activated charcoal composite, fluorinated graphenes and three-dimensional graphene foams, have been extensively explored as components of biosensors or theranostics, or to remotely control cell–substrate interfaces, because of their remarkable electro-conductivity. To date, despite the intensive progress in human stem cell research, only a few attempts to use carbon nanotechnology in the stem cell field have been reported. Interestingly,most of the recent in vitro studies indicate that graphene-based nanomaterials (i.e. mainly graphene, graphene oxide and carbon nanotubes) promote stem cell adhesion, growth, expansion and differentiation. Although cell viability in vitro is not affected, their potential nanocytoxicity (i.e. nanocompatibility and consequences of uncontrolled nanobiodegradability) in a clinical setting using humans remains unknown. Therefore, rigorous internationally standardized clinical studies in humans that would aim to assess their nanotoxicology are requested.
1. Introduction
Nanomaterials encompass a variety of materials with nanoscale structural features, including nanoparticles, nanofibres, nanosurfaces and nanocomposites. As nanomaterials become increasingly more sophisticated in their range of physical properties, e.g. two-dimensional (2D) surfaces, three-dimensional (3D) structures, variable porosity, stiffness, biocompatibility and biodegradability, their diversity of use for medical applications continues to expand. Both physical and chemical properties of biomaterials are now more readily altered, providing opportunities to improve efficacy and safety [1](Huebsch and Mooney, 2009).
Since Langer and Vacanti [2](1993) proposed the combined use of stem cells (SCs), nanomaterial-based scaffolds and inductive factors as the basis for tissue engineering (TE), researchers have been able to fabricate increasingly complex tissue/organ constructs and some are used clinically today as standard treatment for a variety of conditions. Scaffolds are processed in order to produce 3D structures, with appropriate shape, size, architecture and physical properties tailored to fulfil specific functions. In other words, TE products are designed to mimic tissue architecture and responses. Therefore, key scaffold requirements are biocompatibility, controlled porosity and permeability, suitable mechanical and degradation kinetics properties comparable to those of the targeted tissue and, additionally, support for cell attachment and proliferation by the addition of nanotopography to the biomaterial surface [3][4](Chung and King, 2011; Carletti et al., 2011).
TE and regenerative medicine (RM) represent areas of increasing interest, due to the major progress in cell and organ transplantation, as well as advances in materials science and engineering. Isolated from a variety of embryonic, fetal and adult tissues, SC populations consequently differ in their ease of in vitro culture, proliferation rates and capacity to form specialized cell types. Moreover, their unique, pluripotent characteristics related to the differentiation into derivatives of all germ layers in vitro, ex vivo and in vivo, regeneration (i.e. high self-renewal capacity), development, remodelling, and replenishment of aged and diseased tissues, make them leading candidates in TE research and RM (e.g. the treatment of currently incurable diseases).
Conceptually, SCs can be divided into two major types:
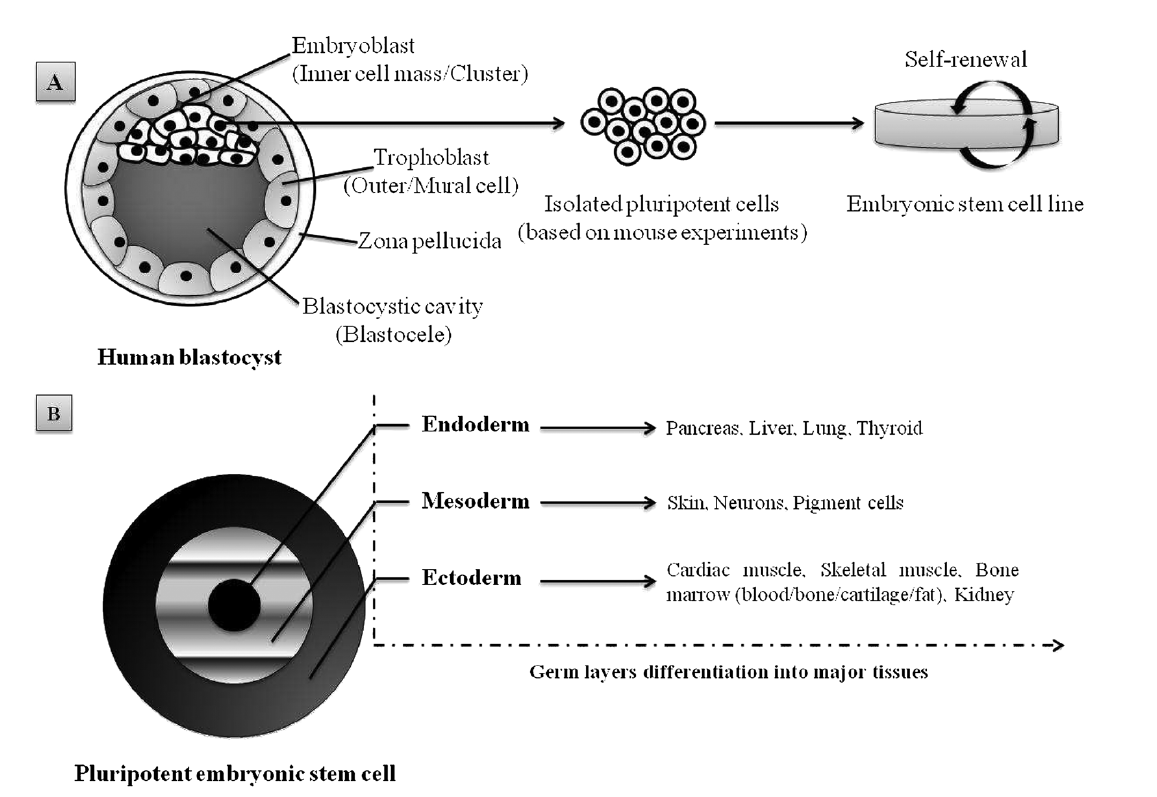
1. Embryonic stem cells (ESCs), derived from a very early embryo and adult stem cells, can be found in postnatal tissues (e.g. bone marrow, adipose tissue, umbilical cord) [5](Nelson et al., 2006). In 1981, the first ESCs derived from mice were isolated and grown in culture [6](Evans and Kaufman, 1981) and almost two decades later the isolation of human ESCs was reported by Thomson et al. [7](1998). ESCs are considered pluripotent notably due to their ability to self-renew (Figure 1A) and differentiate in various cells lineages (Figure 1B). The pluripotency of ESCs can be demonstrated by either: (a) injection of ESCs into immune-deficientmice to produce teratomas containing cells expressing markers of each of the three primary germ layers, endoderm, ectoderm and mesoderm [7][8](Martin, 1981; Thomson et al., 1998); (b) injection of ESCs into a mouse blastocyst to form a chimeric mouse and subsequent assessment of offspring to confirm incorporation of these cells into the germline [9](Bradley et al., 1984). Evidence suggests that human ESC-derived cell populations display low immunogenicity and could, potentially, be transplanted with minimal immune suppression [10][11][12](Drukker et al., 2002; Fändrich et al., 2002; Li et al., 2004). However, the conditions required for maintaining pluripotency and self-renewal of mESCs and hESCs in vitro are quite different, and thus studies in one animal ESC line are not always transferable to another. In addition to ethical and political concerns, their clinical application is severely limited by their lack of accessibility and the difficulties that impede purification and manipulation techniques, as well as concerns related to the risk of teratoma formation [13](Fan et al., 2011).
Figure 1. Schematic representation of pluripotent human ESC lines isolated from the inner cell mass of a blastocyst-stage embryo: (A) appropriate culture maintenance of ESCs allows them to undergo self-renewal, proliferation with retention of the SC state; (B) alternatively, upon stimulus (e.g. growth factors, electrostatic interactions/electrical pulses), ESCs can differentiate into any cell type of the three germ layers (i.e. endoderm, mesoderm or ectoderm). Adapted from Kingham and Oreffo [14](2013), with permission.
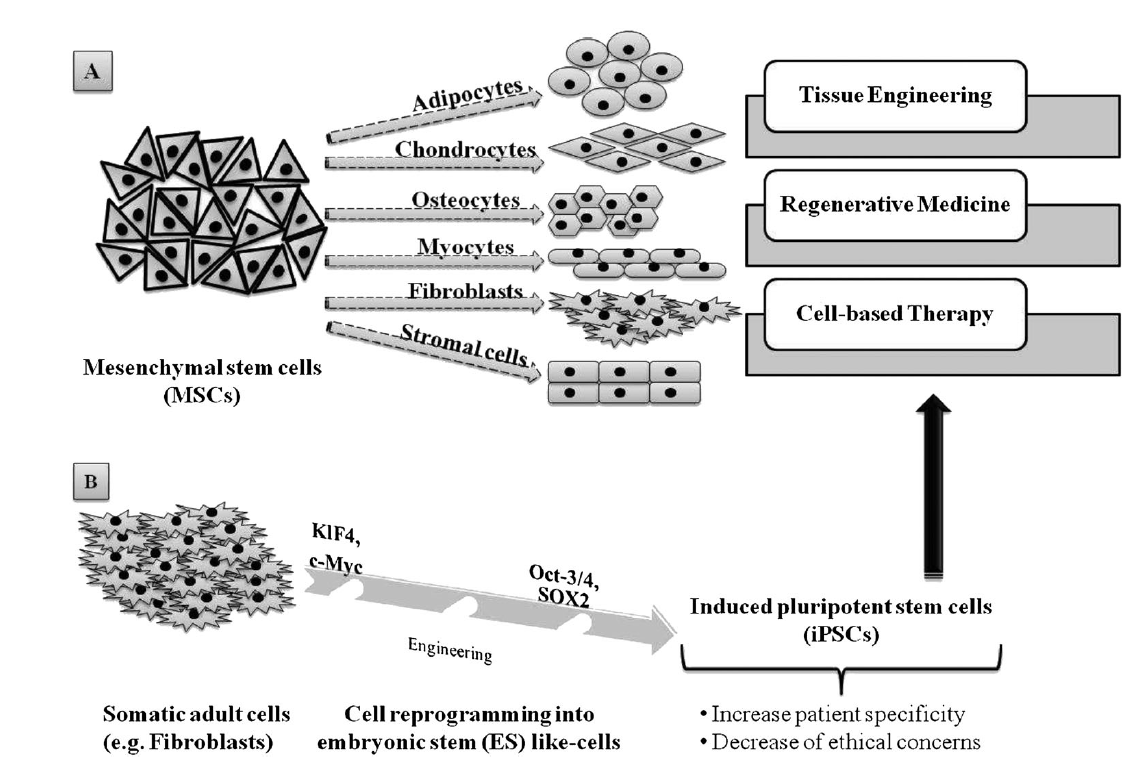
2. Adult SCs, which are ethically preferable to ESCs but, unlike the latter, their sources are somewhat lineagerestricted in humans. Also, their isolation can prove complex and can be painful for the patient, besides their capacity to self-renew that makes their expansion in vitro a significant challenge. However, mesenchymal stem cells (MSCs), one of the many types of adult SCs, display interesting features that make them suitable for tissue regeneration and cell therapy, such as versatility in changing their phenotype during differentiation, and ease of isolatation and culture (Pittenger [15]et al., 1999). Because MSCs of multiple adult vertebrate species originate from extraembryonic mesoderm, their capacity to differentiate into adipogenic, chondrogenic, osteogenic, myogenic and fibroblastic lineages (Figure 2A) has been extensively studied [13][15][16](Pittenger et al., 1999; Fan et al., 2011; Iancu et al., 2011). Similarly to human ESCs, MSCs and human ESC-derived MSCs are also able to provide immune-suppressive properties [17][18][19](Yen et al., 2009; Ghannam et al., 2010; Shahrokhi et al., 2014) which are important to consider when it comes toTE, RM and global cell-based therapy. Interestingly, adult somatic cell-derived ESC-like induced pluripotent stem cells (iPSCs; Figure 2B) are increasingly being investigated as a patient-specific alternative to human ESCs, with less controversy. Importantly, Yamanaka and colleagues demonstrated that mouse fibroblasts could be reprogrammed to mouse ESC-like cells by the expression of four mouse ESC-specific transcription factor genes (i.e. Klf4, c-Myc, Oct-3/4 and Sox2) [20][21](Takahashi and Yamanaka, 2006; Okita et al., 2007). Similarly, adult human fibroblasts have been genetically manipulated to form human iPSCs [22][23](Takahashi et al., 2007; Nakagawa et al., 2008). Subsequently, further reports have described iPSCs formed from non-pluripotent, somatic adult cells, and additional strategies have been developed to limit genetic manipulation or to incorporate reprogramming factor-free methods [24](O’Malley et al., 2009). The high degree of similarity between iPSCs and ESCs, while meanwhile enhancing patient specificity and lowering ethical concerns about iPSCS, undeniably constitutes a new hope for stem cell-based therapy and, therefore, for TE and regenerative therapies [25][26][27](Condic and Rao, 2008; Amabile and Meissner, 2009; Lee et al., 2009).
Figure 2. Adult stem cells capabilities and applications: (A) mesenchymal stem cells (MSCs) are able to differentiate into various cell lineages, making them highly valuable in tissue engineering, regenerative medicine and cell-based therapy; (B) somatic adult cells, such as fibroblasts, can be genetically reprogrammed using four transcription factors, Klf4, c-Myc, Oct-3/4 and SOX2, in order to produce induced pluripotent stem cells (iPSCs), which constitute a consequent alternative source for TE, RM and and cell-based therapy.
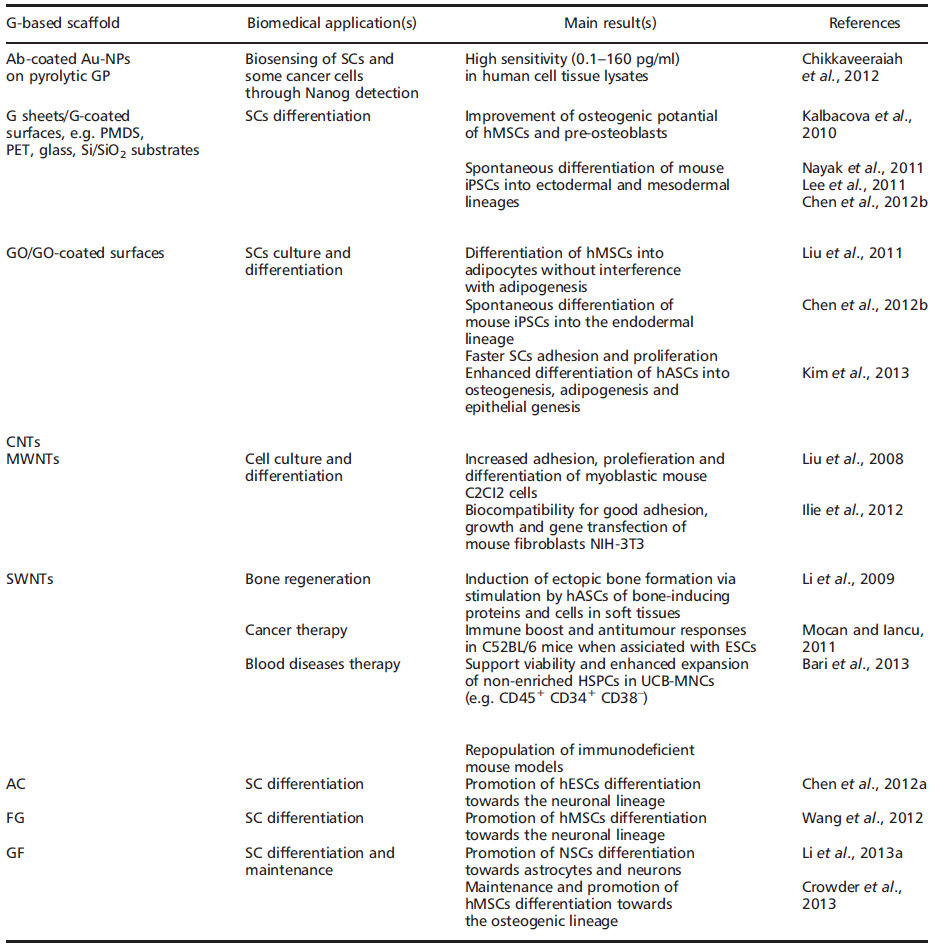
In fact, regardless of stemcell type, current focus remains on SC expansion, maintenance of the SC state, differentiation and, ultimately, transplantation and clinical applications. In some ways, nanomaterials such as graphene (G) and derivatives may hold the key for future advances in TE and RM (Table 1).
G (also known as free-standing 2D crystals or single atom-thick crystallites) is an allotrope of pure carbon with atoms arranged in a regular hexagonal pattern, similar to graphite (GP) but in a single planar sheet (i.e. a flat monolayer) (Figure 3A) of sp2-bonded carbon atoms that are densely/tightly packed in a 2D honeycomb crystal lattice [28][29][30](Geim and Novoselov, 2007; Geim and MacDonald, 2007; Geim, 2009). It can be wrapped up into 0D fullerenes, rolled into 1D nanotubes or stacked into 3D-GP [30](Geim and Novoselov, 2007).
Figure 3. SEM images of graphene foams at low magnification: (A) 2D graphene foam; paper models and SEM images showing stacks of (a) flat graphene sheets, (b) heavily wrinkled sheets and (c) crumpled graphene balls; (B) 3D graphene foam. Reproduced with permission from Luo et al. [31](2013) and Li et al. [32](2013a), respectively
The name ‘graphene'’ was coined to describe single-layer carbon foils [33][34][35](Boehm et al., 1962a, 1962b, 1994) and is a derivative of 3D-GP, for which the electronic properties were first studied by Wallace [36][37](Wallace, 1947; Avouris et al., 2007). In 1987, the term ‘graphene'’ was used to describe single sheets/layers of GP (i.e. monolayer GP, also known as atomic plane structure of GP; [38]Mouras et al., 1987), fullerenes including carbon nanotubes (CNTs) [39](Saito et al., 1992), epitaxial G [40](Forbeaux et al., 1998) and polycyclic aromatic hydrocarbons [41](Wang et al., 2000). Almost a decade ago, Geim and Novoselov [30](2007) demonstrated the possibility of isolating G by a ‘Scotch tape'’ technique from bulk GP; the latter was then defined as a stack ofmulti-G sheets [42](Novoselov et al., 2004). The ‘Scotch tape'’ method (also called micro-mechanical exfoliation) is defined as the cleavage/alleviation of GP in the presence of silicon dioxide (SiO2), which could be used as a ‘back gate'’ electrode to vary the charge density in the extracted, nearly-neutral G (also known as a zero-gap semiconductor) [42](Novoselov et al., 2004). The process consists of using adhesive tape to repeatedly split GP crystals into increasingly thinner pieces (i.e. 0.01 thousandths of an inch) in a silicon wafer [42][43](Rutherford and Dudman, 2002; Novoselov et al., 2004). From 2004, electronic properties have been increasingly studied, and scaling up of innovative procedures to produce G sheets, e.g. by exfoliation by the dry deposition or drawing method (Geim and Novoselov, [28][29][30]2007; Geim and MacDonald, 2007; Geim, 2009), by layer-by-layer (LbL) self-assembly [44][45](Guo et al., 2009; Park et al., 2011a), by epitaxial growth in GP or metals as substrates [46][47][48][49](Riedl et al., 2009; Sutter, 2009; Pletikosić et al., 2009; Song et al., 2010), notably via chemical vapour deposition (CVD) (Bae [50]et al., 2010), by a carbon dioxide reduction method [44](Guo et al., 2009) or from GP sonication [51][52](Dobson et al., 2006; Fialkovsky et al., 2011), has allowed companies to sell good quality G inexpensively [53][50](Segal, 2009; Bae et al., 2010). In 2010, the Nobel Prize in Physics was awarded to Geim and Novoselov for groundbreaking experiments regarding G (http://www.nobelprize.org). Since then, the isolation of free-standing G sheets [54][55](Brownson and Banks, 2010; Liu et al., 2012) has caused widespread attention and immense excitement amongst scientists, because of its large potential in industry (e.g. for innovative biosensors, functionalized carbon nanoconstructs) [44][45][56](Guo et al., 2009; Park et al., 2011a; Singh et al., 2011) and theranostic broad applications (e.g. oncology, regenerative medicine) [57][58][59][60][61][62][63][64][65][66][67][68][69][70](Mohanty and Vikas, 2008; Chikkaveeraiah et al., 2012; Alwarappan et al., 2012; Veerapandian et al., 2012; Cao et al., 2012; Bonanni et al., 2012; Guo et al., 2013; Lorenzoni et al., 2013; Song et al., 2013; Zhou et al., 2013; Lin et al., 2013; Menaa, 2013a, 2013b, 2013e). Indeed, G displays extraordinary physicochemical properties, e.g. electronic [30][71][72][73][74][75](Geim and Novoselov, 2007; Schedin et al., 2007; Adam et al., 2007; Charlier et al., 2008; Chen et al., 2008a, 2008b), optical (Kuzmenko et al., 2008; Nair et al., 2008; Liu et al., 2008; Bao et al., 2009; Zhang et al., 2009, 2012; Kürüm et al., 2011; Zheng et [76][77][78][79][80][81][82][83]al., 2012), mechanical [84](Lee et al., 2008) and thermal [85][86][87][88][89](Mingo and Broido, 2005; Saito et al., 2007; Balandin et al., 2008; Chen et al., 2012c; Qizhen et al., 2011), in addition to being small (i.e. carbon–carbon bond length about 0.142 nm; interplanar spacing of G sheets about 0.335 nm), light (i.e. about 0.77 mg/m2), strong, flexible, cost-effective and ecological [90][91](Heyrovska, 2008; Husale et al., 2010). However, it is worth noting that ab initio calculations showed that a G sheet is thermodynamically unstable if its size is ca. < 20 nm, certainly because of G´ s lower-energy state [92][93](Shenderova et al., 2002; Braga et al., 2004). Eventually, G´s modifiable chemistry, large surface area, atomic thickness and molecular gate-tunable structure make antibody-functionalized G sheets excellent candidates for cells (e.g. mammalian, microbial) and molecular (e.g. blood biomarkers) detection, as well as for the development of innovative theranostic tools (Mohanty and Vikas, 2008; Shen et al., 2012; Alwarappan et al., 2012; Chikkaveeraiah et al., 2012; Veerapandian et al., [57][58][59][60][61][62][63][64][65][66][67][68][94]2012; Cao et al., 2012; Bonanni et al., 2012; Lorenzoni et al., 2013; Guo et al., 2013; Zhou et al., 2013; Lin et al., 2013; Song et al., 2013; Menaa, 2013a) (Table 1). Interestingly, recent findings have shown that G-based devices and methods can be also used to detect SCs as well as facilitate growth, maintenance and differentiation [69][95][96][97][98][99][100](Nayak et al., 2011; Lee et al., 2011; Park et al., 2011b; Kang et al., 2012; Chen et al., 2012b; Kimet al., 2013; Menaa, 2013b). G and derivatives, e.g. graphene oxide (GO) and CNTs, might then be of high importance for SC-based therapies, such as bone regeneration, and oncology, such as the detection and isolation of ‘cancer SCs'’ (Table 1). They also represent valuable alternatives to other nanobiomaterials, e.g. silica and/or polysaccharide-based scaffolds (Houben et al., 2008; Menaa et al., 2009a, [14][101][102][103][104][105][106][107][108][109][110][111][112]2009b, 2011a; Perán et al., 2012; Blanpain, 2013; Kingham and Oreffo, 2013; Guerrero et al., 2013; Li et al., 2013b; Spoliti et al., 2013; D’Antò et al., 2013; Deng et al., 2013; Buchtová et al., 2013). Nevertheless, whether G and derivatives provide better properties in terms of efficacy and safety than other nanomaterials for biomedical and pharmaceutical applications, such as TE and RM, SCs culture and maintenance, drug delivery and bio-imaging, is not yet known, therefore careful, comparative and comparable studies are needed. Indeed, if G and its derivatives offer exceptional physicochemical properties and are relatively safe in vitro, one should also be aware of their potential cell and systemic nanotoxicity in vivo unless data clearly prove acceptable human safety (Liu et al., 2011; Yan et al., 2011; Sanchez [113][114][115][116][117]et al., 2012; Sasidharan et al., 2012; Hartung and Mansoori, 2013). Overall, the cell/tissue biocompatibility and biodegradability of G and derivatives may depend on: (a) their concentration and time of incubation under in vitro, ex vivo or in vivo conditions (Sasidharan [116][117]et al., 2012; Hartung and Mansoori, 2013); (b) their surface-area design, e.g. chemical surface functionalization, such as covalent attachment of amines to increase the dispersability and/or non-linear optical performance of chemically converted/ modified G [113][114](Liu et al., 2011; Yan et al., 2011); (c) their exposure environment, which may or may not lead to G sheets aggregation (Liao [118]et al., 2011); and (d) the exposure route/mode of interaction with cells, i.e. suspension vs adherent cell types [118](Liao et al., 2011).
2. Graphene and derivatives: speeding up stem cell research?
Current TE approaches combine different scaffold materials with living cells to provide biological substitutes that can repair and eventually improve tissue functions (Menaa et al., 2009a, 2011a; Perán et al., 2012; Guerrero et [102][104][105][107][108][109][110][111][112]al., 2013; Li et al., 2013b; Spoliti et al., 2013; D’Antò et al., 2013; Deng et al., 2013; Buchtová et al., 2013). Indeed, several available natural and synthetic nanomaterials are useful for transplantation of SCs and their specific differentiation into muscle, bone and cartilage (Menaa et [102][104][105][107][108][109][110][111][112]al., 2009a, 2011a; Perán et al., 2012; Guerrero et al., 2013; Li et al., 2013b; Spoliti et al., 2013; D’Antò et al., 2013; Deng et al., 2013; Buchtová et al., 2013). One of the key objectives for bone regeneration therapy to be successful is to direct the proliferation of SCs and accelerate their differentiation in a controlled manner through the use of growth factors and osteogenic inducers [14][104](Menaa et al., 2011a; Kingham and Oreffo, 2013).
The culture of bone marrow-derivedMSCs, as well as the control of their differentiation towards a different tissue lineage, represents a very important part of TE, where cells are combined with artificial scaffolds to regenerate tissues [95][96][107][109][110][111](Nayak et al., 2011; Lee et al., 2011; Guerrero et al., 2013; Spoliti et al., 2013; D’Antò et al., 2013; Deng et al., 2013).
Further, neural stemcells (NSCs)-based therapy provides a promising approach for neural regeneration. Indeed, NSCs represent a self-renewing and multipotent cell population in the central nervous system (CNS) which exhibits promising prospects in developing cell therapies for neural regeneration [119][120](Gage, 2000; Rossi and Cattaneo, 2002). For the success of clinical application of NSCs, a scaffold is required to provide 3D cell-growth microenvironments and appropriate synergistic cell-guidance cues, and so, in this context, 3D graphene foams (3D-GFs) (Figure 3B) would be a better option than 2D-GFs (Figure 3A) or 2D-G derivatives, such as GO (Figure 4) [32](Li et al., 2013a). Besides, transplantation of biomaterial scaffolds encasing either human ESCs [121][122][123][124](Baharvand et al., 2007; Jin et al., 2010; Chen et al., 2012a; Menaa, 2013d) or adult SCs (Delcroix et al., 2010; [124][125]Menaa, 2013d) has been proposed as a clinical therapy for various neurological lesions and disorders, such as spinal cord injury, cerebral ischaemia and stroke. Eventually, iPSCs hold great promise as a cell source for RM; however their culture, maintenance of pluripotency and induction of differentiation remain challenging [99](Chen et al., 2012b). In light of recent developments, artificially synthesized carbon-based biomaterials/carbon allotropes, such as G, GO and CNTs, have demonstrated feasibility in supporting stem cell attachment and differentiation [32][95][96][97][98][99][100][123](Nayak et al., 2011; Lee et al., 2011; Park et al., 2011b; Chen et al., 2012a, 2012b; Kang et al., 2012; Kim et al., 2013; Li et al., 2013a) and so have already found a wide variety of applications in biomedicine. Nevertheless, despite the recent progress in human SC research, only a few attempts to use carbon nanotechnology in the TE and RM fields have been reported. Also, the applicability of carbon nanotechnology is significantly hampered by evidence of nanotoxic effects on multiple cell types, which tends to be minimized with appropriate surface designs, i.e. surface physical or chemical functionalizations (Yan [114]et al., 2011). Nonetheless, an emergent drive for an innovative carbonaceous biomaterial calls for a safer platform with comparable advantageous characteristics.
Figure 4. A proposed schematic (Lerf–Klinowski model) of graphene oxide structure. The variations of the model indicate ambiguity regarding (A) the presence or (B) the absence of carboxylic acids at the periphery of the basal plane of the graphitic platelets of GO. Reproduced with permission from Dreyer et al. [126].(2010)
3. Conclusions and perspectives
The mechanical properties of G, such as high elasticity, flexibility and adaptability to flat or irregular surfaces, are suitable for the structural reinforcement of biocompatible films, hydrogels such as polyvinyl alcohol (PVA) and poly(methyl methacrylate) (PMMA), and other scaffold materials, e.g. polysaccharides such as chitosan and alginate, frequently used for TE and RM. Although mostly in its initial stage, the research on biomedical applications of G has seen exciting and encouraging advances. Nevertheless, some challenges must be overcome, such as: (a) a better understanding of cellular interactions with G and derivatives, especially the cellular uptake mechanism. This might facilitate the development of more efficient G or Gderivatives-based nanoplatforms for biosensing, drug delivery, cell culture and maintenance, among other applications; (b) the relative nanotoxicity of G and derivatives. Preliminary results indicate that the physicochemical properties, e.g. flat shape, surface charges, of G and derivatives are closely related to their cytotoxicity, e.g. in vitro induction of cellular oxidative stress and DNA damage, and affect the in vivo biodistribution and fate. Thus, before clinical applications, a systematic comparative study, e.g. a deep meta-analysis, is highly desired to address the relative safety concerns (subtracting false-negative and -positive effects) of G and derivatives. Eventually, the research on G and derivatives-based scaffold materials for cell culture is a relatively new direction that deserves special attention. Indeed, studies in this field so far have demonstrated that G and derivatives are able to accelerate the growth, proliferation and differentiation of SCs, therefore holding great promise in TE, RM and other biomedical fields.We strongly believe that the trend of this emerging field will continue and even speed up in the future.