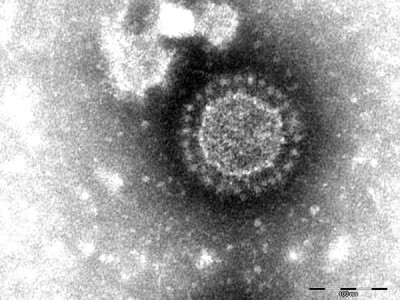
Dalechampia chlorotic mosaic virus (DCMV) is a plant virus that primarily affects species within the genus Dalechampia, particularly Dalechampia spp., which are flowering plants in the family Euphorbiaceae. DCMV is a member of the genus Potyvirus within the family Potyviridae. This virus is known for causing symptoms of chlorotic mosaic, leaf mottling, and stunting in infected plants.
iNaturalist
11 May 2024
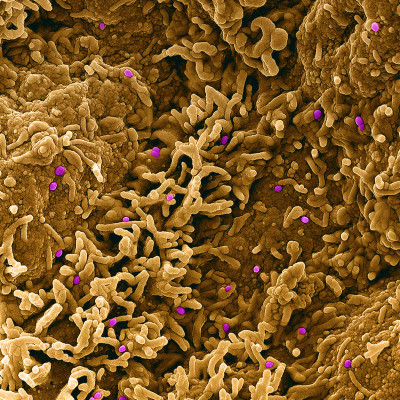
Colorized scanning electron micrograph of monkeypox virus (purple) on the surface of infected VERO E6 cells (tan). Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
NIAID
31 Jan 2024
Virus particles of G. indiensis polydnavirus. The virions are formed by 5 to 10 nucleocapsids enclosed by only one viral envelope. Electron micrograph created by U.S. Department of Agriculture scientists as part of their official duties.
Chen, Y.-P. and Gundersen-Rindal, D.E., Wikimedia Commons
08 Feb 2024
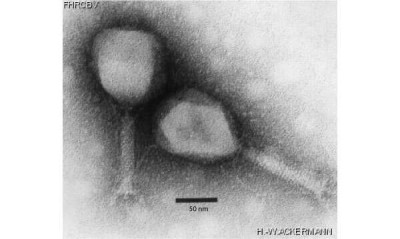
Electron micrograph of two phage particles of Tequatrovirus genus (aka T4likevirus).
ViralZone, SIB Swiss Institute of Bioinformatics, Wikimedia Commons
01 Mar 2024
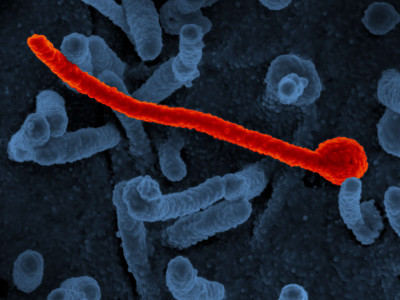
Scanning electron micrograph of Ebola virus Makona (in red) from the West African epidemic shown on the surface of Vero cells (blue).
NIAID, Wikimedia Commons
24 Jan 2024
Negative stain electron micrograph of viral hemorrhagic septicemia virus (VHSV).
Wikimedia Commons, arubha
01 Feb 2024
Symptoms of potato virus y on Nicotiana tabacum.
Wikimedia Commons, R.J. Reynolds Tobacco Company Slide Set, R.J. Reynolds Tobacco Company, Bugwood.org
02 Feb 2024
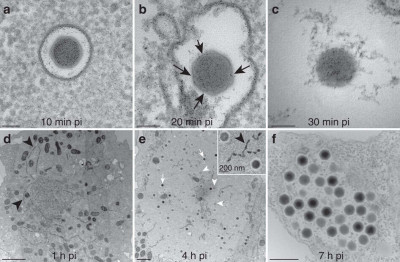
(a) 10 min pi, virions have been engulfed and are in vacuoles. (b) 20 min pi, virions in vacuoles exhibit holes (black arrows) in their external translucent shell and appear more spherical. (c) 30 min pi, virions have entirely lost their external shell and appear as spherical electron-dense nucleoids, which can be seen in vacuoles as well as in the cell cytoplasm (and Fig. 9, black arrows). Scale bar, 100 nm. (d) 1 h pi, electron-dense tubular structures (black arrowheads) appear in the cytoplasm. (e) 4 h pi viral factories (VF) settle in the cell cytoplasm next to the nucleus and the cell organelles are pushed at their periphery (scale bar, 2 μm). The new virions are assembled and the electron-dense mature (white arrows) and immature (white arrowheads) virions are scattered in the same VF. Inset: immature and mature virions inside the VF along with tubular structures (black arrowheads). Scale bar, 200 nm. (f) 7 h pi, the newly synthesized virions are gathered into vacuoles inside the cytoplasm before being released in the extracellular environment (scale bar, 500 nm) [1].
Wikimedia Commons, Elisabeth Fabre, Sandra Jeudy, Sébastien Santini, Matthieu Legendre, Mathieu Trauchessec, Yohann Couté, Jean-Michel Claverie, Chantal Abergel
02 Feb 2024
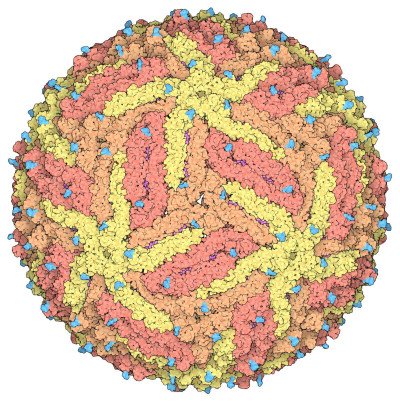
Space-fill drawing of the Zika virus capsid, with the capsid proteins in shades of yellow and orange to show the icosahedral symmetry. The membrane proteins in the under layer (magenta) show through in some places, and the cyan protrusions are attached carbohydrate chains. Drawn by David Goodsell from the cryoEM structure 5ire.
David Goodwill, Wikimedia Commons
07 Feb 2024
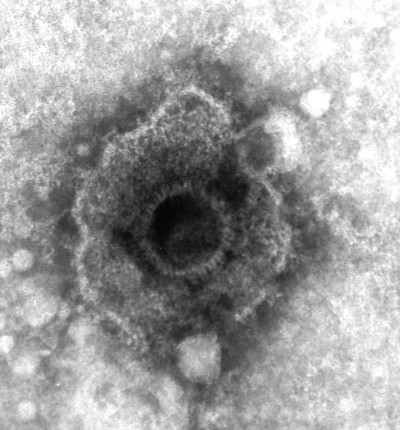
An electron micrograph of a herpesvirus (negative staining).
Graham Beards, Wikimedia Commons
22 Feb 2024
Porcine epidemic diarrhea virus particles seen by negative-stain electron microscopy of fecal samples. Negative staining with 1% phosphotungstic acid. [1]
Dennis Hanke, Maria Jenckel, Anja Petrov, Mathias Ritzmann, Julia Stadler, Valerij Akimkin, Sandra Blome, Anne Pohlmann, Horst Schirrmeier, Martin Beer, and Dirk Höper, Wikimedia Commons
23 Feb 2024
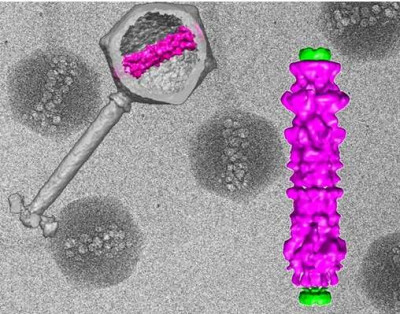
In the background, cryo-electron micrographs of purified viruses with their inner structure bubbling from radiation damage. Overlaid, (left) 3D computer reconstruction of a virus's outer shell and tail in gray, with the inner structure in magenta; (right) blow-up of the inner viral structure in magenta.
National Institutes of Health (NIH), Wikimedia Commons
01 Mar 2024
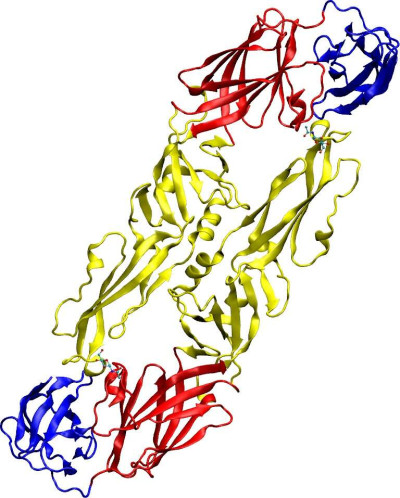
Overview of Tick-borne encephalitis virus envelope protein E. Image prepared with VMD. Based on PDB structure 1SVB.
Wikimedia Commons, Starless
01 Apr 2024
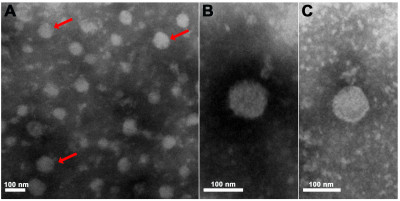
Electron micrographs of purified Pteromalus puparum negative-strand RNA virus 1 (PpNSRV-1) particles from follicular cells. (A) Electron micrographs of purified PpNSRV-1 virions. (B) and (C) are the magnification pictures. Red arrows indicated viral particles. [1]
Wikimedia Commons, Fei Wang, Qi Fang, Beibei Wang, Zhichao Yan, Jian Hong, Yiming Bao, Jens H. Kuhn, John H. Werren, Qisheng Song, Gongyin Ye
24 Apr 2024
Birch leaf roll-associated virus (BLRaV) is a plant virus that primarily infects birch trees (Betula spp.). It belongs to the family Betaflexiviridae and the genus Capillovirus. BLRaV is associated with a disorder known as birch leaf roll, characterized by the rolling or curling of leaves, leaf discoloration, and reduced vigor in infected trees.
iNaturalist
11 May 2024
Dinocampus coccinellae paralysis virus (DcPV) is a virus that infects parasitoid wasps of the species Dinocampus coccinellae, which parasitize ladybird beetles (coccinellids). The primary host of DcPV is the parasitoid wasp Dinocampus coccinellae, which lays its eggs inside ladybird beetle larvae. The virus infects the wasp's reproductive tissues and is transmitted to the ladybird beetle host during oviposition. Once inside the beetle larva, the virus establishes infection and may cause paralysis or other physiological effects.
iNaturalist
11 May 2024
Colorized scanning electron micrograph of Ebola virus particles (red) in extracellular space between infected African green monkey kidney cells.
Wikimedia Commons, NIAID
03 Apr 2024
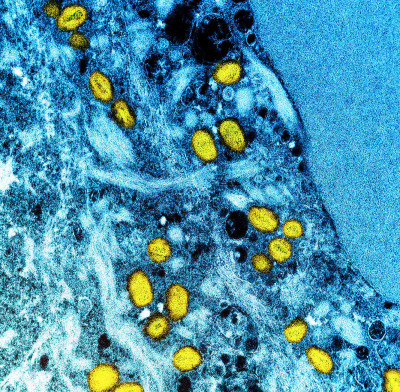
Colorized transmission electron micrograph of monkeypox particles (yellow) found within an infected cell (blue), cultured in the laboratory. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
Wikimedia Commons
09 Apr 2024
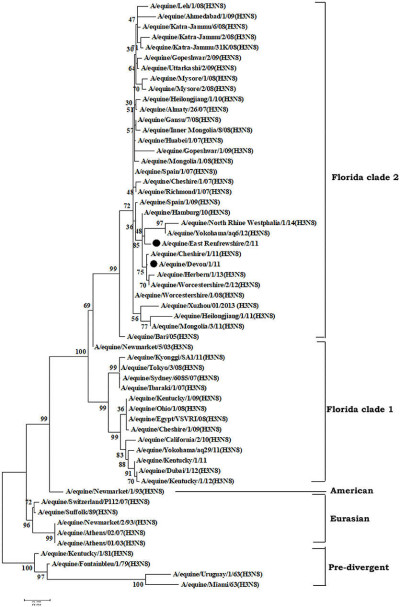
Phylogenetic analysis of hemagglutinin (HA) genes nucleotide sequences from 57 Equine Influenza Viruses (EIVs). The maximum likelihood tree was constructed using stringent T92 + G algorithm which was identified using the find best DNA/protein model tool available in MEGA 6. The reliability of the trees was assessed by bootstrap with 1,000 replications with cut off at 50 are shown in the tree. The phylogram depicts five major clusters of global EIVs. Phylogenetic group's viz., Florida sub-lineage clade 1, Florida sub-lineage clade 2, American, Eurasian and Pre-divergent, are mentioned by bars on the right. The major mutations (I179V and A144V) observed in the Clade 2 viruses of Florida sublineage in recent isolates have been denoted by solid dots. [1]
Wikimedia Commons, Raj K. Singh, Kuldeep Dhama, Kumaragurubaran Karthik, Rekha Khandia, Ashok Munjal, Sandip K. Khurana, Sandip Chakraborty, Yashpal S. Malik, Nitin Virmani, Rajendra Singh, Bhupendra N. Tripathi, Muhammad Munir, and Johannes H. van der Ko
15 Apr 2024
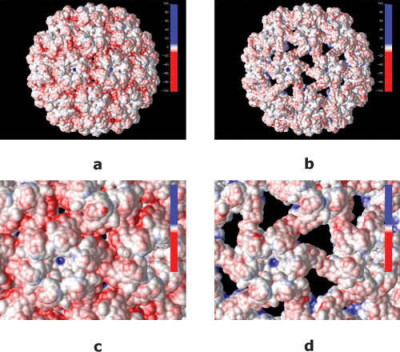
Electrostatic properties of cowpea chlorotic mottle virus and cucumber mosaic virus capsids
Wikimedia Commons, Nathan Andrew Baker
18 Apr 2024
 Encyclopedia
Encyclopedia