
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anjali Singh | + 10600 word(s) | 10600 | 2021-05-07 09:55:25 | | | |
| 2 | Anjali Singh | Meta information modification | 10600 | 2021-05-20 11:23:45 | | | | |
| 3 | Vivi Li | -3075 word(s) | 7525 | 2021-05-21 05:32:07 | | |
Video Upload Options
Prolonged exposure to harmful ultraviolet radiation (UVR) can induce many chronic or acute skin disorders in humans. To protect themselves, many people have started to apply cosmetic products containing UV-screening chemicals alone or together with physical sunblocks, mainly based on titanium–dioxide (TiO2) or zinc-oxide (ZnO2). However, it has now been shown that the use of chemical and physical sunblocks is not safe for long-term application, so searches for the novel, natural UV-screening compounds derived from plants or bacteria are gaining attention. Certain photosynthetic organisms such as algae and cyanobacteria have evolved to cope with exposure to UVR by producing mycosporine-like amino acids (MAAs). These are promising substitutes for chemical sunscreens containing commercially available sunblock filters. The use of biopolymers such as chitosan for joining MAAs together or with MAA-Np (nanoparticles) conjugates will provide stability to MAAs similar to the mixing of chemical and physical sunscreens.
1. Introduction
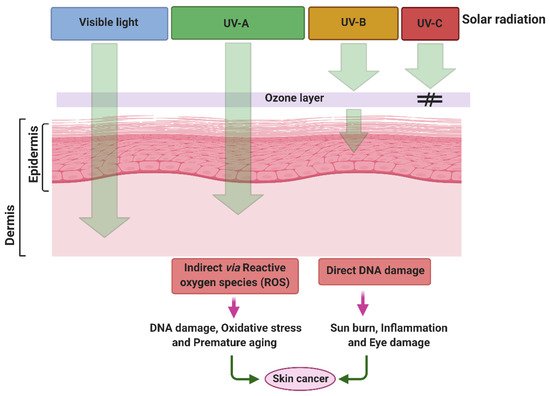
Over recent decades, due to the depletion of the stratospheric ozone layer and by increasing anthropogenic pollution, the levels of UV radiation are increasing gradually over the Earth’s surface. Depending on the wavelength, physical properties and biological activities, UV radiation is arbitrarily divided into three main categories: UV-C radiation (100–280 nm), UV-B (280–320 nm) and UV-A (320–400 nm). UV-A radiation is further divided into two sub-categories, longwave UV-A1 (340–400 nm) and shortwave UV-A2 (320–340 nm). Prior to their reduction by the Earth’s atmosphere, UV-A, UV-B, and UV-C represent 6.3%, 1.5% and 0.5–1.0% of total solar irradiance, corresponding to an intensity of 85.7, 21.7 and 6.4 Wm−2, respectively [1][2]. Among these, highly energetic UV-C radiation and almost 90–95% of UV-B radiation does not reach the ground due to absorption by the stratospheric ozone layer and losses through atmospheric scattering. Solar UV-B radiation reaching the Earth surface represents about 1–10% of total radiation, whereas almost all UV-A radiation (~95%) reaches the ground [1][3][4]. A declining ozone column leads to an increase in the intensity of UV-B reaching the Earth’s surface, and the wavelength composition is also proportionally shifted towards shorter wavelengths, which are more dangerous [1][5]. Such increases in UV-B radiation are most pronounced in the Antarctic, but similar trends have also been recorded at other latitudes [5][6].
It is well documented that excessive exposure to UV radiation has notable negative impacts on the growth, metabolism and reproduction of all living organisms, ranging from animals and plants to microorganisms [7]. Besides exerting several adverse effects on terrestrial vegetation and plankton, UVR can also penetrate deeply into human skin and induce a sequence of skin damaging cascades. Elevated exposure of UVR can be acute or chronic and can cause several skin diseases such as erythema, edema, hyperpigmentation, immune suppression, photo-aging and skin cancer [8][9]. The effect of UV radiation on the skin epidermis depends on the range and intensity of UVR. However, continuous exposure to UV radiation can lead to structural and functional changes in the skin epidermis. It can cause degradation of collagen fibers, lesions and pigmentation, and further promote photo-aging and cancer [10][11]. Several studies have shown that expression of photo-aging-linked genes such as integrin and procollagen are also associated with UV-induced skin damage [12]. Over recent decades, to mitigate the damaging effects of UVR, the use of chemical and inorganic sunscreens has accelerated. However, long-term exposure to these sunscreens has also been shown to cause several skin-associated disorders, leading to skin aging, skin cancer or other neurological disorders [13]. There is, therefore, an urgent need to identify natural and effective sunscreen materials such as MAAs that have the potential to protect against UV-induced damage and can enhance the efficacy of natural sunscreens by forming conjugates with nanoparticles or biopolymers. In response to this need, this review critically summarizes UV-induced skin damage, the use of and problems associated with chemical, physical or inorganic sunscreens, the abundance and properties of MAAs, and the efficacy of MAAs to protect against UV-induced skin damage. This study may provide new insights into our understanding of how MAAs may be a better and safer alternative than the use of cytotoxic sunblocks.
2. Damaging Effects of UVR on Skin
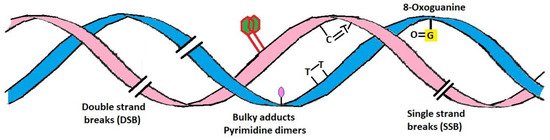
Application of Sunscreen Against UV-Induced Skin Damage
3. Mycosporine-Like Amino Acids
Mycosporine-like amino acids, commonly known as “MAAs” represent a diverse family of more than 40, small <400 Da, water-soluble, colorless UV-absorbing compounds that protect against highly energetic UV photons. They have a unique absorption spectrum with a single, narrow band with an absorption maximum between 309 and 362 nm. Structurally, MAAs are divided into two groups; (i) the mycosporines, which have a single modified amino acid residue connected to a cyclohexenone core, and (ii) MAAs, have two such amino acids substituents [71]. MAAs have a 5-hydroxy-5-hydroxymethyl-cyclohex-1, 2-ene ring structure, and a methoxy-substituent in C2 position. In the MAA structure, the C3 position is always substituted with an amino group, whereas the C1 position can be substituted with either an oxo- or an imino-moiety. In some instances, the term ‘mycosporine’ refers to those with a ketone group at the C1 position, known as oxo-mycosporines or mono-substituted mycosporines.
3.1. Progression of MAAs as UV-Screening Compounds and Their Distribution
3.2. Diversity of MAAs
3.3. Biosynthesis of MAA
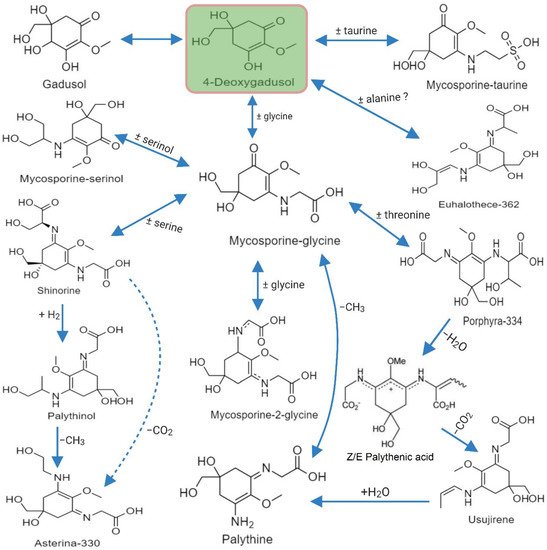
3.4. Methods Employed for Characterization of MAAs
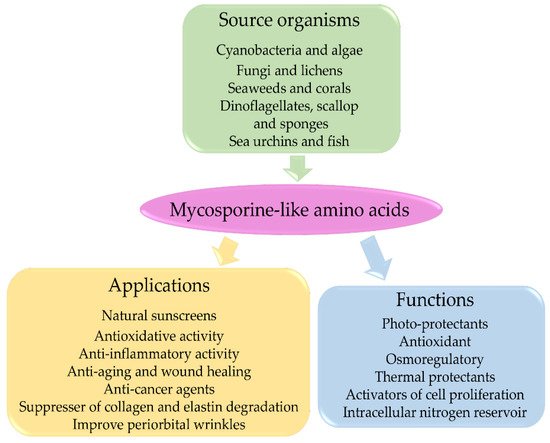
3.5. Mycosporine-Like Amino Acids and Their Applications
MAAs are considered multipurpose metabolites with various functions, including antioxidant and anti-inflammatory activities, accessory pigments in photosynthesis, nitrogen storage, thermal protection, osmotic stress protection, anti-aging, anti-cancer, and wound healing. They have also been widely accepted as UV-A and UV-B photoprotection agents [88]. The evidence supporting these functions of MAAs is mostly indirect and is based on induction of MAA production following stress conditions. MAAs have well-documented applications in cosmetics, toiletries, as UV protectors, and activators of cell proliferation and as suppressors of UV-induced aging in human skin [66][67]. MAAs appear to be promising compounds in artificial sunscreens for future biotechnological research [115]. Some of their properties are briefly discussed below, and Figure 4 shows a diagrammatic representation MAA applications.
3.5.1. Antioxidative Properties of MAAs
3.5.2. Anti-Inflammatory Properties of MAAs
3.5.3. Anti-Aging and Wound Healing Properties of MAAs
| MAAs | Skin Cell Lines | UV Radiation | Efficacy of MAAs | References |
|---|---|---|---|---|
| Palythine | HaCaT human keratinocytes | Solar-simulated radiation (SSR)-5–20 J cm−2 or UV-A radiation-20 J cm−2 | 0.3–10% w/v of MAAs inhibited SSR (20 J cm−2) induced cell death | [68] |
| Porphyra-334 | HaCaT human keratinocytes | UV-radiation | 0.1 mg mL–1 of porphyra-334 increased the survival rates by up to ~88% | [70] |
| Porphyra-334 | Human skin fibroblasts (CCD-986sk) | 10 J cm−2 of UV-A light | 0–40 µM of porphyra-334 were applied, cell viability increased in a dose-dependent manner. 40 µM of porphyra-334 increased cell viability up to 88%; it also helped in wound healing | [117] |
| Mycosporine-Gly | HaCaT human keratinocytes | 23 mW cm−2 between 300–400 nm. Irradiated for 15 min (275 kJ m−2) in UV | COX-2 expression decreased in the presence of a high concentration of mycosporine-Gly (0.3 mM) | [69] |
| Shinorine | HaCaT human keratinocytes | 23 mW cm−2 between 300–400 nm. Irradiated for 15 min (275 kJ m−2) in UV | COX-2 expression decreased in the presence of the lowest concentrations of shinorine (0.03 mM) | [69] |
| Helioguard®365 formulation of porphyra-334 and shinorine | HaCaT human keratinocytes | UV-A (320 nm) with 10 J cm−2 intensity | Concentrations of 0.125% and 0.25% of Helioguard®365 improved cell viability in a dose-dependent manner; 0.25% Helioguard®365 increase cell viability by up to 97.8%. | [123] |
| Mycosporine-Gly, shinorine, and porphyra-334 | Human normal skin fibroblast cell line TIG-114 | UV irradiation with a peak at 302 nm and 0.16 mW cm−2 intensity. | No toxic effect of MAAs, it increased cell proliferative activity to mycosporine-Gly = shinorine > porphyra-334, and 50 mM mycosporine-Gly accelerated growth by approximately 40%. | [75] |
| Algal cell extracts of H. cornea and G. longissima | One murine macrophages RAW264.7, two human cell lines skin fibroblasts HGF, and HaCaT human keratinocytes | Solar simulator with a mercury-xenon lamp (51.4–63.7 W m−2) | The highest SPF 7.5 for G. longissima and 4.8 for H. cornea, were found at a density of 13.9 mg DW of algae per cm−2. Both algal extracts induced the production of TNF-α and IL-6, and they did not show cytotoxicity in human cells. | [121] |
| P. yezoensis cell extract and porphyra-334 | Three different human cell lines Detroit 551, HaCaT, and HFDP cells | One ppm of porphyra-334 increased cell viability by 110.33% and 101.79%, while 200 ppm increased viability by 126.68% and 110.26% in HaCaT and HFDP, respectively. | [119] | |
| Collemin A | HaCaT human keratinocytes | UV-B (200 mJ cm−2 delivered at an irradiance of 3.4 mW s−1) | 3 mg cm−2 collema provide approximately 75% keratinocyte viability against UV-B radiation | [81] |
| Shinorine and porphyra-334 | IRC mouse skin | UV-A 20.81 J cm−2 and UV-B 0.47 J cm−2 | MAAs inhibited hydroxyproline reduction and protected against damage to collagen fibers in photo-aging skin. It also reduced the expression of MMP-1, MMP-3 and TNF-α. | [122] |
| Porphyra-334 and shinorine (11.5:1). | Mouse fibroblasts 3T3 | UV-A (10 J cm−2). | The concentration of 0.1 to 5 µg mL−1 provides concentration dependent protection. Highest protection recorded at 5 µg mL−1 MAAs. | [124] |
3.5.4. Photo-Protective Properties of MAAs
Similarly, Moline et al. [80] showed that the photoprotective activity of yeast involved the synthesis of mycosporine-glutaminol glucoside (MGG). In this work, they analyzed the relationship between MGG production, cell survival after UV-B irradiation, formation of CPDs, photostability and singlet oxygen quenching activity of MGG [80]. Their results showed that CPD accumulation and MGG accumulation were inversely related. The conclusion of their work was that MGG plays an important role as a UV-B photoprotective metabolite in yeasts by protecting against direct DNA damage and probably against indirect damage by singlet oxygen quenching. Likewise, porphyra-334 isolated from Porphyra yezoensis exhibited a protective effect on human skin fibroblasts against exposure to UV-A radiation. Cell viability was increased in a dose dependent manner similar to 40 µM porphyra-334 that increased cell viability by up to 88% [117]. Recently, Suh et al. [70] used porphyra-334 to minimize the UV-induced apoptosis of HaCaT cell lines. Likewise, they also showed that UV-absorbing compounds (M-Gly, shinorine and porphyra-334) modulated gene expression associated with oxidative stress, inflammation, and skin aging caused by UV [69]. Álvarez-Gómez et al. [121] have investigated the effect of algal cell extracts of G. longissima and H. cornea on two human and one murine cell lines and found that they had no cytotoxic effects on human cell lines. Nevertheless, murine cell lines exhibited cytotoxic effects linked to immunomodulatory roles. The algal extracts included five different MAAs: palythine, asterina-330, shinorine, porphyra-334, and palythinol. They also found that the photoprotective capacity of the algal extracts in terms of SPF values showed a gradual increase with extract concentration. Both algal extracts induced the production of TNF-α and IL-6 [121].
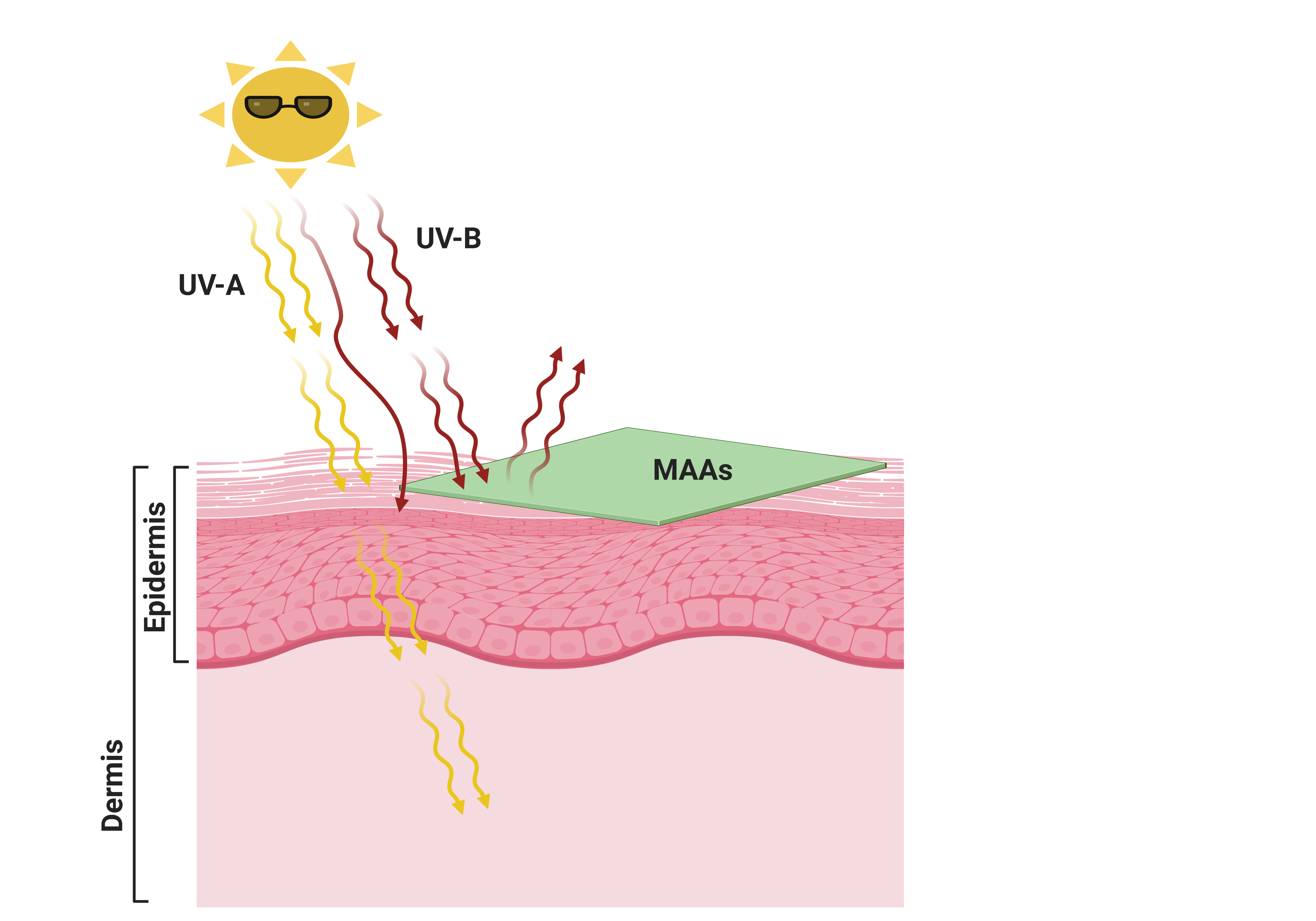 Figure 5. Use of natural eco-friendly mycosporine-like amino acids (MAAs) as a green sunscreen to protect skin against UV-induced skin damage. Created with BioRender (https://biorender.com/ accessed on 15 April 2021).
Figure 5. Use of natural eco-friendly mycosporine-like amino acids (MAAs) as a green sunscreen to protect skin against UV-induced skin damage. Created with BioRender (https://biorender.com/ accessed on 15 April 2021).4. Stability and Enhanced Effectiveness of MAA-Based Sunscreens, and MAA-Conjugates
5. Conclusions
References
- Frederick, J.; Snell, H.; Haywood, E. Solar ultraviolet radiation at the earth’s surface. Photochem. Photobiol. 1989, 50, 443–450.
- Madronich, S.; McKenzie, R.L.; Björn, L.O.; Caldwell, M.M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. J. Photochem. Photobiol. B Biol. 1998, 46, 5–19.
- Frederick, J.E.; Qu, Z.; Booth, C.R. Ultraviolet radiation at sites on the Antarctic coast. Photochem. Photobiol. 1998, 68, 183–190.
- McKenzie, R.L.; Björn, L.O.; Bais, A.; Ilyasd, M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. Photochem. Photobiol. Sci. 2003, 2, 5–15.
- Kerr, J.; McElroy, C. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 1993, 262, 1032–1034.
- Frederick, J.E.; Lubin, D. Solar ultraviolet irradiance at Palmer station, Antarctica. Ultrav. Radiat. Antarct. Meas. Biol. Eff. 1994, 62, 43–52.
- Weihs, P.; Schmalwieser, A.W.; Schauberger, G. UV effects on living organisms. In Environmental Toxicology; Springer: New York, NY, USA, 2013; pp. 609–688.
- Crutzen, P.J. Ultraviolet on the increase. Nature 1992, 356, 104–105.
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986.
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular mechanisms of UV-induced apoptosis and its effects on skin residential cells: The implication in UV-based phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435.
- Reichrath, J.; Reichrath, S. Hope and challenge: The importance of ultraviolet (UV) radiation for cutaneous vitamin D synthesis and skin cancer. Scand. J. Clin. Lab. Investig. 2012, 72, 112–119.
- Bosset, S.; Bonnet-Duquennoy, M.; Barre, P.; Chalon, A.; Kurfurst, R.; Bonte, F.; Schnebert, S.; Le Varlet, B.; Nicolas, J. Photoageing shows histological features of chronic skin inflammation without clinical and molecular abnormalities. Br. J. Dermatol. 2003, 149, 826–835.
- Preston, D.; Stern, R. Nonmelanoma skin cancer. N. Engl. J. Med. 1992, 327, 1649–1662.
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841.
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248.
- Bruls, W.A.; Slaper, H.; van Der Leun, J.C.; Berrens, L. Transmission of human epidermis and stratum corneum as a function of thickness in the ultraviolet and visible wavelengths. Photochem. Photobiol. 1984, 40, 485–494.
- Meinhardt, M.; Krebs, R.; Anders, A.; Heinrich, U.; Tronnier, H. Wavelength-dependent penetration depths of ultraviolet radiation in human skin. J. Biomed. Opt. 2008, 13, 044030.
- Britt, A.B. Repair of DNA damage induced by ultraviolet radiation. Plant. Physiol. 1995, 108, 891.
- Sinha, R.P.; Häder, D.-P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236.
- Lindahl, T.; Wood, R.D. Quality control by DNA repair. Science 1999, 286, 1897–1905.
- Mitchell, D.L.; Karentz, D. The induction and repair of DNA photodamage in the environment. In Environmental UV Photobiology; Springer: Boston, MA, USA, 1993; pp. 345–377.
- Sancar, A. DNA excision repair. Annu. Rev. Biochem. 1996, 65, 43–81.
- Matsumura, Y.; Ananthaswamy, H.N. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308.
- Kemp, M.G.; Spandau, D.F.; Travers, J.B. Impact of age and insulin-like growth factor-1 on DNA damage responses in UV-irradiated human skin. Molecules 2017, 22, 356.
- Marrot, L.; Meunier, J.-R. Skin DNA photodamage and its biological consequences. J. Am. Acad. Dermatol. 2008, 58, S139–S148.
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124.
- Tl, D.; Mahler, V. The epidemiology of skin cancer. Br. J. Derm. 2002, 146, 1–6.
- Rittié, L.; Kansra, S.; Stoll, S.W.; Li, Y.; Gudjonsson, J.E.; Shao, Y.; Michael, L.E.; Fisher, G.J.; Johnson, T.M.; Elder, J.T. Differential ErbB1 signaling in squamous cell versus basal cell carcinoma of the skin. Am. J. Pathol. 2007, 170, 2089–2099.
- Glanz, K.; Buller, D.B.; Saraiya, M. Reducing ultraviolet radiation exposure among outdoor workers: State of the evidence and recommendations. Environ. Health 2007, 6, 1–11.
- Kaidbey, K.H.; Agin, P.P.; Sayre, R.M.; Kligman, A.M. Photoprotection by melanin—A comparison of black and Caucasian skin. J. Am. Acad. Dermatol. 1979, 1, 249–260.
- Muthumani, T.; Sudhahar, V.; Mukhopadhyay, T. Review on Sunscreens and Sun Protection factor. Res. J. Top. Cosmet. Sci. 2015, 6, 55–65.
- Jansen, R.; Osterwalder, U.; Wang, S.Q.; Burnett, M.; Lim, H.W. Photoprotection: Part II. Sunscreen: Development, efficacy, and controversies. J. Am. Acad. Dermatol. 2013, 69, 867.e1–867.e14.
- Osterwalder, U.; Herzog, B. Chemistry and properties of organic and inorganic UV filters. Sunscreens Photoprot. 2009.
- Shaath, N.A. The chemistry of ultraviolet filters. In Principles and Practice of Photoprotection; Springer: Cham, Switzerland, 2016; pp. 143–157.
- Chatelain, E.; Gabard, B. Photostabilization of Butyl methoxydibenzoylmethane (Avobenzone) and Ethylhexyl methoxycinnamate by Bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a New UV Broadband Filter. Photochem. Photobiol. 2001, 74, 401–406.
- Nguyen, U.; Schlossman, D. Stability study of avobenzone with inorganic sunscreens. In Proceedings of the Kobo Products Poster Presentation, SSC New York Conference, New York, NY, USA, 14 December 2001.
- Matta, M.K.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Florian, J.; Oh, L.; Bashaw, E.; Zineh, I.; Sanabria, C. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: A randomized clinical trial. JAMA 2019, 321, 2082–2091.
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259.
- Wang, S.Q.; Burnett, M.E.; Lim, H.W. Safety of oxybenzone: Putting numbers into perspective. Arch. Dermatol. 2011, 147, 865–866.
- Fourtanier, A.; Moyal, D.; Seité, S. Sunscreens containing the broad-spectrum UVA absorber, Mexoryl® SX, prevent the cutaneous detrimental effects of UV exposure: A review of clinical study results. Photodermatol. Photoimmunol. Photomed. 2008, 24, 164–174.
- Brand, R.M.; Pike, J.; Wilson, R.M.; Charron, A.R. Sunscreens containing physical UV blockers can increase transdermal absorption of pesticides. Toxicol. Ind. Health 2003, 19, 9–16.
- Sarveiya, V.; Risk, S.; Benson, H.A. Liquid chromatographic assay for common sunscreen agents: Application to In Vivo assessment of skin penetration and systemic absorption in human volunteers. J. Chromatogr. B 2004, 803, 225–231.
- Kim, T.H.; Shin, B.S.; Kim, K.-B.; Shin, S.W.; Seok, S.H.; Kim, M.K.; Kim, E.J.; Kim, D.; Kim, M.G.; Park, E.-S. Percutaneous absorption, disposition, and exposure assessment of homosalate, a UV filtering agent, in rats. J. Toxicol. Environ. Health Part A 2014, 77, 202–213.
- Krause, M.; Klit, A.; Blomberg Jensen, M.; Søeborg, T.; Frederiksen, H.; Schlumpf, M.; Lichtensteiger, W.; Skakkebaek, N.; Drzewiecki, K. Sunscreens: Are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int. J. Androl. 2012, 35, 424–436.
- De Groot, A.C.; Roberts, D.W. Contact and photocontact allergy to octocrylene: A review. Contact Dermat. 2014, 70, 193–204.
- Park, C.-B.; Jang, J.; Kim, S.; Kim, Y.J. Single-and mixture toxicity of three organic UV-filters, ethylhexyl methoxycinnamate, octocrylene, and avobenzone on Daphnia magna. Ecotoxicol. Environ. Saf. 2017, 137, 57–63.
- Zhang, Q.Y.; Ma, X.Y.; Wang, X.C.; Ngo, H.H. Assessment of multiple hormone activities of a UV-filter (octocrylene) in zebrafish (Danio rerio). Chemosphere 2016, 159, 433–441.
- Zgadzaj, A.; Skrzypczak, A.; Welenc, I.; Ługowska, A.; Parzonko, A.; Siedlecka, E.; Sommer, S.; Sikorska, K.; Nałęcz-Jawecki, G. Evaluation of photodegradation, phototoxicity and photogenotoxicity of ofloxacin in ointments with sunscreens and in solutions. J. Photochem. Photobiol. B Biol. 2015, 144, 76–84.
- Frank Gerberick, G.; Ryan, C.A. Contact photoallergy testing of sunscreens in guinea pigs. Contact Dermat. 1989, 20, 251–259.
- Nash, J.F.; Tanner, P.R. Relevance of UV filter/sunscreen product photostability to human safety. Photodermatol. Photoimmunol. Photomed. 2014, 30, 88–95.
- Darbre, P.D. Environmental oestrogens, cosmetics and breast cancer. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 121–143.
- Janjua, N.R.; Mogensen, B.; Andersson, A.-M.; Petersen, J.H.; Henriksen, M.; Skakkebæk, N.E.; Wulf, H.C. Systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. J. Investig. Dermatol. 2004, 123, 57–61.
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.; Gruetter, M.; Herzog, I.; Reolon, S.; Ceccatelli, R. Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology 2004, 205, 113–122.
- Klammer, H.; Schlecht, C.; Wuttke, W.; Schmutzler, C.; Gotthardt, I.; Köhrle, J.; Jarry, H. Effects of a 5-day treatment with the UV-filter octyl-methoxycinnamate (OMC) on the function of the hypothalamo-pituitary–thyroid function in rats. Toxicology 2007, 238, 192–199.
- Rodríguez, E.; Valbuena, M.C.; Rey, M.; Porras de Quintana, L. Causal agents of photoallergic contact dermatitis diagnosed in the national institute of dermatology of Colombia. Photodermatol. Photoimmunol. Photomed. 2006, 22, 189–192.
- Szwarcfarb, B.; Carbone, S.; Reynoso, R.; Bollero, G.; Ponzo, O.; Moguilevsky, J.; Scacchi, P. Octyl-methoxycinnamate (OMC), an ultraviolet (UV) filter, alters LHRH and amino acid neurotransmitters release from hypothalamus of immature rats. Exp. Clin. Endocrinol. Diabetes 2008, 116, 94–98.
- Egambaram, O.P.; Kesavan Pillai, S.; Ray, S.S. Materials science challenges in skin UV protection: A review. Photochem. Photobiol. 2020, 96, 779–797.
- Schlossman, D. Sunscreen Technologies for Foundations and Lipsticks. Nice (France); Kobo Products Inc.: South Plainfield, NJ, USA, 2001.
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247.
- Mohammed, Y.H.; Holmes, A.; Haridass, I.N.; Sanchez, W.Y.; Studier, H.; Grice, J.E.; Benson, H.A.; Roberts, M.S. Support for the safe use of zinc oxide nanoparticle sunscreens: Lack of skin penetration or cellular toxicity after repeated application in volunteers. J. Investig. Dermatol. 2019, 139, 308–315.
- Smijs, T.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112.
- Wu, M.-S.; Sun, D.-S.; Lin, Y.-C.; Cheng, C.-L.; Hung, S.-C.; Chen, P.-K.; Yang, J.-H.; Chang, H.-H. Nanodiamonds protect skin from ultraviolet B-induced damage in mice. J. Nanobiotechnol. 2015, 13, 1–12.
- Zenerino, A.; Boutard, T.; Bignon, C.; Amigoni, S.; Josse, D.; Devers, T.; Guittard, F. New CeO2 nanoparticles-based topical formulations for the skin protection against organophosphates. Toxicol. Rep. 2015, 2, 1007–1013.
- Abe, A.; da Cunha, R.; Salomon, P.; Valença, S.; de Brito-Gitirana, L.; Junior, E. Nanoecotoxicological Effects of a Sunscreen Formulation based on TiO2 Nanoparticles on Microalgae from Guanabara Bay (Rio De Janeiro, Brazil). MOJ Polym. Sci. 2017, 1, 99–107.
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646.
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: Potential health and beauty ingredients. Mar. Drugs 2017, 15, 326.
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Pharmacogn. Rev. 2011, 5, 138.
- Lawrence, K.; Gacesa, R.; Long, P.; Young, A. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2018, 178, 1353–1363.
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187.
- Suh, S.-S.; Oh, S.K.; Lee, S.G.; Kim, I.-C.; Kim, S. Porphyra-334, a mycosporine-like amino acid, attenuates UV-induced apoptosis in HaCaT cells. Acta Pharm. 2017, 67, 257–264.
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656.
- Babele, P.K.; Singh, G.; Singh, A.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. UV-B radiation and temperature stress-induced alterations in metabolic events and defense mechanisms in a bloom-forming cyanobacterium Microcystis aeruginosa. Acta Physiol. Plant. 2017, 39, 1–11.
- Singh, A.; Tyagi, M.B.; Kumar, A. Cyanobacteria growing on tree barks possess high amount of sunscreen compound mycosporine-like amino acids (MAAs). Plant. Physiol. Biochem. 2017, 119, 110–120.
- Singh, G.; Babele, P.K.; Kumar, A.; Srivastava, A.; Sinha, R.P.; Tyagi, M.B. Synthesis of ZnO nanoparticles using the cell extract of the cyanobacterium, Anabaena strain L31 and its conjugation with UV-B absorbing compound shinorine. J. Photochem. Photobiol. B Biol. 2014, 138, 55–62.
- Oyamada, C.; Kaneniwa, M.; Ebitani, K.; Murata, M.; Ishihara, K. Mycosporine-like amino acids extracted from scallop (Patinopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells. Mar. Biotechnol. 2008, 10, 141–150.
- Kogej, T.; Gostinčar, C.; Volkmann, M.; Gorbushina, A.A.; Gunde-Cimerman, N. Mycosporines in extremophilic fungi—Novel complementary osmolytes? Environ. Chem. 2006, 3, 105–110.
- Andre, G.; Pellegrini, M.; Pellegrini, L. Algal Extracts Containing Amino Acid Analogs of Mycosporin Are Useful as Dermatological Protecting Agents against Ultraviolet Radiation. Patent No. FR2803201, 6 July 2001.
- Callone, A.I.; Carignan, M.; Montoya, N.G.; Carreto, J.I. Biotransformation of mycosporine like amino acids (MAAs) in the toxic dinoflagellate Alexandrium tamarense. J. Photochem. Photobiol. B Biol. 2006, 84, 204–212.
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; De Gálvez, M.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169.
- Moliné, M.; Arbeloa, E.M.; Flores, M.R.; Libkind, D.; Farías, M.E.; Bertolotti, S.G.; Churio, M.S.; van Broock, M.R. UVB photoprotective role of mycosporines in yeast: Photostability and antioxidant activity of mycosporine-glutaminol-glucoside. Radiat. Res. 2011, 175, 44–50.
- Torres, A.; Hochberg, M.; Pergament, I.; Smoum, R.; Niddam, V.; Dembitsky, V.M.; Temina, M.; Dor, I.; Lev, O.; Srebnik, M. A new UV-B absorbing mycosporine with photo protective activity from the lichenized ascomycete Collema cristatum. Eur. J. Biochem. 2004, 271, 780–784.
- Oda, Y.; Zhang, Q.; Matsunaga, S.; Fujita, M.J.; Sakai, R. Two new mycosporine-like amino acids LC-343 and mycosporine-ethanolamine from the micronesian marine sponge Lendenfeldia chondrodes. Chem. Lett. 2017, 46, 1272–1274.
- Ferrier-Pages, C.; Richard, C.; Forcioli, D.; Allemand, D.; Pichon, M.; Shick, J.M. Effects of temperature and UV radiation increases on the photosynthetic efficiency in four scleractinian coral species. Biol. Bull. 2007, 213, 76–87.
- Adams, N.; Shick, J. Mycosporine-like amino acids prevent UVB-induced abnormalities during early development of the green sea urchin Strongylocentrotus droebachiensis. Mar. Biol. 2001, 138, 267–280.
- Newman, S.J.; Dunlap, W.C.; Nicol, S.; Ritz, D. Antarctic krill (Euphausia superba) acquire a UV-absorbing mycosporine-like amino acid from dietary algae. J. Exp. Mar. Biol. Ecol. 2000, 255, 93–110.
- Rosic, N.N. Mycosporine-like amino acids: Making the foundation for organic personalised sunscreens. Mar. Drugs 2019, 17, 638.
- Mulkidjanian, A.Y.; Junge, W. On the origin of photosynthesis as inferred from sequence analysis. Photosynth. Res. 1997, 51, 27–42.
- Oren, A.; Gunde-Cimerman, N. Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 2007, 269, 1–10.
- Oren, A. Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiol. J. 1997, 14, 231–240.
- Cockell, C.S. Ultraviolet radiation, evolution and the π-electron system. Biol. J. Linn. Soc. 1998, 63, 449–457.
- Böhm, G.A.; Pfleiderer, W.; Böger, P.; Scherer, S. Structure of a novel oligosaccharide-mycosporine-amino acid ultraviolet A/B sunscreen pigment from the terrestrial cyanobacterium Nostoc commune. J. Biol. Chem. 1995, 270, 8536–8539.
- Garcia-Pichel, F. Solar ultraviolet and the evolutionary history of cyanobacteria. Orig. Life Evol. Biosph. 1998, 28, 321–347.
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345.
- Garcia-Pichel, F.; Castenholz, R.W. Occurrence of UV-absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl. Environ. Microbiol. 1993, 59, 163–169.
- Shick, J.M.; Dunlap, W.C. Mycosporine-like amino acids and related gadusols: Biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu. Rev. Physiol. 2002, 64, 223–262.
- Wada, N.; Sakamoto, T.; Matsugo, S. Multiple roles of photosynthetic and sunscreen pigments in cyanobacteria focusing on the oxidative stress. Metabolites 2013, 3, 463–483.
- Sommaruga, R.; Garcia-Pichel, F. UV-absorbing mycosporine-like compounds in planktonic and benthic organisms from a high-mountain lake. Arch. Hydrobiol. 1999, 144, 255–269.
- Rastogi, R.; Madamwar, D.; Incharoensakdi, A. Sun-screening bioactive compounds mycosporine-like amino acids in naturally occurring cyanobacterial biofilms: Role in photoprotection. J. Appl. Microbiol. 2015, 119, 753–762.
- Osborn, A.R.; Almabruk, K.H.; Holzwarth, G.; Asamizu, S.; LaDu, J.; Kean, K.M.; Karplus, P.A.; Tanguay, R.L.; Bakalinsky, A.T.; Mahmud, T. De novo synthesis of a sunscreen compound in vertebrates. eLife 2015, 4, e05919.
- Shinzato, C.; Shoguchi, E.; Kawashima, T.; Hamada, M.; Hisata, K.; Tanaka, M.; Fujie, M.; Fujiwara, M.; Koyanagi, R.; Ikuta, T. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 2011, 476, 320–323.
- Carignan, M.O.; Cardozo, K.H.; Oliveira-Silva, D.; Colepicolo, P.; Carreto, J.I. Palythine–threonine, a major novel mycosporine-like amino acid (MAA) isolated from the hermatypic coral Pocillopora capitata. J. Photochem. Photobiol. B Biol. 2009, 94, 191–200.
- Portwich, A.; Garcia-Pichel, F. Biosynthetic pathway of mycosporines (mycosporine-like amino acids) in the cyanobacterium Chlorogloeopsis sp. strain PCC 6912. Phycologia 2003, 42, 384–392.
- Geraldes, V.; de Medeiros, L.S.; Lima, S.T.; Alvarenga, D.O.; Gacesa, R.; Long, P.F.; Fiore, M.F.; Pinto, E. Genetic and biochemical evidence for redundant pathways leading to mycosporine-like amino acid biosynthesis in the cyanobacterium Sphaerospermopsis torques-reginae ITEP-024. Harmful Algae 2020, 35, 177–187.
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446.
- Conde, F.R.; Churio, M.S.; Previtali, C.M. Experimental study of the excited-state properties and photostability of the mycosporine-like amino acid palythine in aqueous solution. Photochem. Photobiol. Sci. 2007, 6, 669–674.
- Singh, S.P.; Klisch, M.; Sinha, R.P.; Häder, D.-P. Genome mining of mycosporine-like amino acid (MAA) synthesizing and non-synthesizing cyanobacteria: A bioinformatics study. Genomics 2010, 95, 120–128.
- Gao, Q.; Garcia-Pichel, F. Microbial ultraviolet sunscreens. Nat. Rev. Microbiol. 2011, 9, 791–802.
- La Barre, S.; Roullier, C.; Boustie, J. Mycosporine-like amino acids (MAAs) in biological photosystems. In Outsatnding Marine Molecules; Wiley-VCH: Weinheim, Germany, 2014; pp. 333–360.
- Cardozo, K.H.; Vessecchi, R.; Carvalho, V.M.; Pinto, E.; Gates, P.J.; Colepicolo, P.; Galembeck, S.E.; Lopes, N.P. A theoretical and mass spectrometry study of the fragmentation of mycosporine-like amino acids. Int. J. Mass Spectrom. 2008, 273, 11–19.
- Whitehead, K.; Hedges, J.I. Electrospray ionization tandem mass spectrometric and electron impact mass spectrometric characterization of mycosporine-like amino acids. Rapid Commun. Mass Spectrom. 2003, 17, 2133–2138.
- Nazifi, E.; Wada, N.; Yamaba, M.; Asano, T.; Nishiuchi, T.; Matsugo, S.; Sakamoto, T. Glycosylated porphyra-334 and palythine-threonine from the terrestrial cyanobacterium Nostoc commune. Mar. Drugs 2013, 11, 3124–3154.
- Furusaki, A.; Matsumoto, T.; Tsujino, I.; Sekikawa, I. The crystal and molecular structure of palythine trihydrate. Bull. Chem. Soc. Jpn. 1980, 53, 319–323.
- Uemura, D.; Katayama, C.; Wada, A.; Hirata, Y. Crystal and molecular structure of palythene possessing a novel 360 nm chromophore. Chem. Lett. 1980, 9, 755–756.
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute configuration of mycosporine-like amino acids, their wound healing properties and in vitro anti-aging effects. Mar. Drugs 2020, 18, 35.
- Derikvand, P.; Llewellyn, C.A.; Purton, S. Cyanobacterial metabolites as a source of sunscreens and moisturizers: A comparison with current synthetic compounds. Eur. J. Phycol. 2017, 52, 43–56.
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar. Drugs 2019, 17, 222.
- Ryu, J.; Park, S.-J.; Kim, I.-H.; Choi, Y.H.; Nam, T.-J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803.
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39.
- Kim, S.Y.; Cho, W.K.; Kim, H.-I.; Paek, S.H.; Jang, S.J.; Jo, Y.; Choi, H.; Lee, J.H.; Moh, S.H. Transcriptome Profiling of Human Follicle Dermal Papilla Cells in response to Porphyra-334 Treatment by RNA-Seq. Evid. Based Complement. Altern. Med. 2021, 2021, 6637513.
- Becker, K.; Hartmann, A.; Ganzera, M.; Fuchs, D.; Gostner, J.M. Immunomodulatory effects of the mycosporine-like amino acids shinorine and porphyra-334. Mar. Drugs 2016, 14, 119.
- Álvarez-Gómez, F.; Korbee, N.; Casas-Arrojo, V.; Abdala-Díaz, R.T.; Figueroa, F.L. UV photoprotection, cytotoxicity and immunology capacity of red algae extracts. Molecules 2019, 24, 341.
- Rui, Y.; Zhaohui, Z.; Wenshan, S.; Bafang, L.; Hu, H. Protective effect of MAAs extracted from Porphyra tenera against UV irradiation-induced photoaging in mouse skin. J. Photochem. Photobiol. B Biol. 2019, 192, 26–33.
- Schmid, D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmet 2006, 9, 1–4.
- Schmid, D.; Schürch, C.; Zülli, F.; Nissen, H.-P.; Prieur, H. Mycosporine-like amino acids: Natural UV-screening compounds from red algae to protect the skin against photoaging. Söfw. J. 2003, 129, 38–42.
- De la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB photoprotective capabilities of topical formulations containing mycosporine-like amino acids (MAAs) through different Biological Effective Protection Factors (BEPFs). Mar. Drugs 2019, 17, 55.
- Fernandes, S.C.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.N.; Labidi, J.; Bulone, V. Exploiting mycosporines as natural molecular sunscreens for the fabrication of UV-absorbing green materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564.
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014.
- Helioguard 365 A Natural UVA-Screening Compound from Sea Algae to Protect the Skin against Photo-Aging. Available online: (accessed on 14 April 2021).
- Colabella, F.; Moliné, M.; Libkind, D. UV sunscreens of microbial origin: Mycosporines and mycosporine-like aminoacids. Recent Pat. Biotechnol 2015, 8, 179–193.




