
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcin Miszczyk | + 533 word(s) | 533 | 2021-03-29 08:15:12 | | | |
| 2 | Rita Xu | Meta information modification | 533 | 2021-05-10 08:19:44 | | |
Video Upload Options
Ventricular tachycardia is a type of arrhythmia (heart rhythm disorder) characterized by fast heart rate (exceeding 100 beats per minute), originating from abnormal electrical impulse localized in heart ventricles. The electrophysiology-guided noninvasive cardiac radioablation, also known as STAR (stereotactic arrhythmia radioablation), is an emerging treatment method of non-invasive treatment for ventricular tachycardia persistent after conventional methods of ablation.
1. What is STAR?
Ventricular arrhythmias, including ventricular tachycardias (VT), are a major cause of sudden cardiac death (SCD) [1], which globally contributes to 4.25 million deaths every year [2].
The guidelines-driven management of VTs consists of antiarrhythmic drugs (AADs), placement of implantable cardiac defibrillators (ICDs), and the ablation of the arrhythmogenic substrate [3][4][5]. Despite the significant clinical improvement, results of conventional radiofrequency ablation (RFA) strategy can be limited especially in case of challenging anatomy and subepicardial substrate location. Post-RFA recurrence rate ranges from 12–17% at 1 year follow-up [6], and, although rarely directly associated with procedural complications (0.6%), RFA is associated with up to ~5% short-term mortality in ischemic VT cases [7].
A need to deliver better care for patients makes VT ablations one of the most dynamically growing areas in the field of cardiac electrophysiology (EP) [8]. The increasing number of patients with refractory VT leveraged by the implementation of new technologies has already improved clinical results [9]. Specifically, stereotactic arrhythmia radioablation (STAR) aims to reduce the burden of arrhythmia [10][11][12] through a combination of 3D electrophysiological mapping (EAM), noninvasive myocardial scar imaging (computed tomography (CT), positron emission tomography (PET), cardiac magnetic resonance (cMR) [13][14]), and noninvasive delivery of ablative radiation doses [15], as presented in Figure 1. STAR faces everyday clinical challenges associated with the extension of indications for VT ablations, including advanced ischemic and nonischemic cardiomyopathies [16][17][18]. As a routine step of the STAR methodology, cardiac imaging showed that a substantial number of VT patients have fibrotic scars located intramurally and epicardially, thus not being accessible with endocardial ablation approaches [19]. New insights confirm the necessity to co-register anatomical and functional data reassuring integration of imaging techniques with electrophysiological studies.
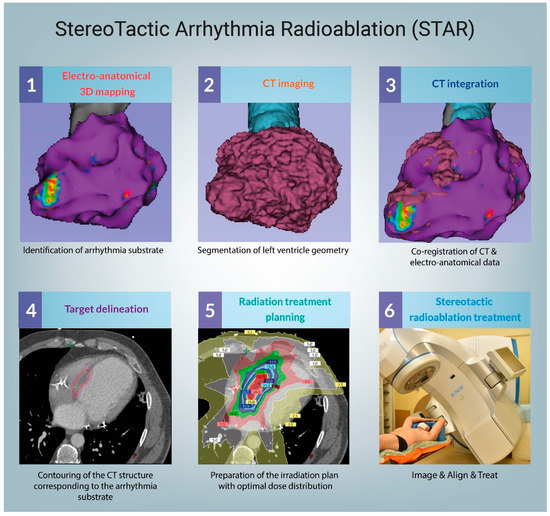
Figure 1. Procedural workflow. CT–computed tomography.
2. Unique challenges
For the majority of radiotherapeutic history, the collaboration with cardiologists mainly focused on the subject of damage control of radiation sequelae, including late (i.e., coronary disease) and early (i.e., ICD damage, thrombosis) toxicity. The introduction of curative radiosurgery into a strictly cardiological field caused a major paradigm shift and forced the development of many new and innovative strategies, including the necessity to integrate the EP maps with the data available in the radiotherapy treatment planning systems, as well as develop strategies for the delivery of high, curative doses to the heart.
3. Current status
The future of STAR is still undetermined. Despite many promising results, the methodology of this emerging treatment method is significantly different between studies, including a significant lack of agreement in terms of the treatment volumes. Despite the favorable early toxicity profile, studies have shown clinically relevant late toxicities at significantly later timepoints than the follow-up available in the majority of the reports. Nevertheless, considering a significant amount of promising data, we believe that the development of optimal STAR workflow is possible and could help to address a specific and clinically important problem of the post-ablation VT recurrences.
References
- Tang, P.T.; Shenasa, M.; Boyle, N.G. Ventricular arrhythmias and sudden cardiac death. Card. Electrophysiol. Clin. 2017, 9, 693–708.
- Srinivasan, N.T.; Schilling, R.J. Sudden cardiac death and arrhythmias. Arrhythmia Electrophysiol. Rev. 2018, 7, 111.
- Shivkumar, K. Catheter ablation of ventricular arrhythmias. N. Engl. J. Med. 2019, 380, 1555–1564.
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A report of the american college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. Heart Rhythm 2018, 15, e190–e252.
- Dukkipati, S.R.; Koruth, J.S.; Choudry, S.; Miller, M.A.; Whang, W.; Reddy, V.Y. Catheter ablation of ventricular tachycardia in structural heart disease: Indications, strategies, and outcomes-Part II. J. Am. Coll. Cardiol. 2017, 70, 2924–2941.
- Fernandez-Armenta, J.; Soto-Iglesias, D.; Silva, E.; Penela, D.; Jáuregui, B.; Linhart, M.; Bisbal, F.; Acosta, J.; Fernandez, M.; Borras, R.; et al. Safety and outcomes of ventricular tachycardia substrate ablation during sinus rhythm: A prospective multicenter registry. JACC Clin. Electrophysiol. 2020.
- Santangeli, P.; Frankel, D.S.; Tung, R.; Vaseghi, M.; Sauer, W.H.; Tzou, W.S.; Mathuria, N.; Nakahara, S.; Dickfeldt, T.M.; Lakkireddy, D.; et al. Early mortality after catheter ablation of ventricular tachycardia in patients with structural heart disease. J. Am. Coll. Cardiol. 2017, 69, 2105–2115.
- Palaniswamy, C.; Kolte, D.; Harikrishnan, P.; Khera, S.; Aronow, W.S.; Mujib, M.; Mellana, W.M.; Eugenio, P.; Lessner, S.; Ferrick, A.; et al. Catheter ablation of postinfarction ventricular tachycardia: Ten-year trends in utilization, in-hospital complications, and in-hospital mortality in the United States. Heart Rhythm 2014.
- Arenal, Á.; Hernández, J.; Calvo, D.; Ceballos, C.; Atéa, L.; Datino, T.; Atienza, F.; González-Torrecilla, E.; Eídelman, G.; Miracle, Á.; et al. Safety, long-term results, and predictors of recurrence after complete endocardial ventricular tachycardia substrate ablation in patients with previous myocardial infarction. Am. J. Cardiol. 2013.
- Cuculich, P.S.; Schill, M.R.; Kashani, R.; Mutic, S.; Lang, A.; Cooper, D.; Faddis, M.; Gleva, M.; Noheria, A.; Smith, T.W.; et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N. Engl. J. Med. 2017.
- Robinson, C.G.; Samson, P.P.; Moore, K.M.S.; Hugo, G.D.; Knutson, N.; Mutic, S.; Goddu, S.M.; Lang, A.; Cooper, D.H.; Faddis, M.; et al. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation 2019, 139, 313–321.
- Neuwirth, R.; Cvek, J.; Knybel, L.; Jiravsky, O.; Molenda, L.; Kodaj, M.; Fiala, M.; Peichl, P.; Feltl, D.; Januška, J.; et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. EP Eur. 2019, 21, 1088–1095.
- Dickfeld, T.; Tian, J.; Ahmad, G.; Jimenez, A.; Turgeman, A.; Kuk, R.; Peters, M.; Saliaris, A.; Saba, M.; Shorofsky, S.; et al. MRI-guided ventricular tachycardia ablation integration of late gadolinium-enhanced 3D scar in patients with implantable cardioverter- defibrillators. Circ. Arrhythmia Electrophysiol. 2011.
- Cochet, H.; Komatsu, Y.; Sacher, F.; Jadidi, A.S.; Scherr, D.; Riffaud, M.; Derval, N.; Shah, A.; Roten, L.; Pascale, P.; et al. Integration of merged delayed-enhanced magnetic resonance imaging and multidetector computed tomography for the guidance of ventricular tachycardia ablation: A pilot study. J. Cardiovasc. Electrophysiol. 2013.
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010.
- Pappone, C.; Ciconte, G.; Manguso, F.; Vicedomini, G.; Mecarocci, V.; Conti, M.; Giannelli, L.; Pozzi, P.; Borrelli, V.; Menicanti, L.; et al. Assessing the malignant ventricular arrhythmic substrate in patients with brugada syndrome. J. Am. Coll. Cardiol. 2018.
- Talib, A.K.; Takagi, M.; Shimane, A.; Nakano, M.; Hayashi, T.; Okajima, K.; Kentaro, M.; Fukada, K.; Kowase, S.; Kurosaki, K.; et al. Efficacy of endocardial ablation of drug-resistant ventricular fibrillation in brugada syndrome. Circ. Arrhythma Electrophysiol. 2018.
- Igarashi, M.; Nogami, A.; Kurosaki, K.; Hanaki, Y.; Komatsu, Y.; Fukamizu, S.; Morishima, I.; Kaitani, K.; Nishiuchi, S.; Talib, A.K.; et al. Radiofrequency catheter ablation of ventricular tachycardia in patients with hypertrophic cardiomyopathy and apical aneurysm. JACC Clin. Electrophysiol. 2018.
- Bogun, F.M.; Desjardins, B.; Good, E.; Gupta, S.; Crawford, T.; Oral, H.; Ebinger, M.; Pelosi, F.; Chugh, A.; Jongnarangsin, K.; et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy. utility for identifying the ventricular arrhythmia substrate. J. Am. Coll. Cardiol. 2009.




