
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liang Yu | + 1549 word(s) | 1549 | 2021-05-09 03:28:15 | | | |
| 2 | Lindsay Dong | -509 word(s) | 1040 | 2021-05-18 08:06:18 | | |
Video Upload Options
Zone refining is a technology of deeply purifying metals. Its essence is to use the difference in solubility of impurity elements in the solid and molten state of the main metal to precipitate the impurities or change the distribution of the impurity elements. It provides an effective and easy method for preparing high-purity metals.
1. Introduction
High-purity metals (99.999% (5N) or higher) are widely used in modern electronic information, aerospace, defense. The development trend of modern science and technology requires high-purity or ultra-high-purity of metals, because some important characteristics of metals are affected by the type and amount of impurities in the matrix, and some characteristics are even masked by trace elements [1].
Metals are often purified by vacuum distillation technology [2], ion exchange technology [3], extraction technology [4], electrolytic refining technology [5], zone refining technology [6], and other single or combined technologies. Among them, the zone refining technology has a wide range of applications; in addition, it is simple and easy to control, has no pollution, has a high product purity, and is suitable for the final stage of preparing high-purity metals.
2. Zone Refining Mechanism
2.1. Basic Principles
The distribution of impurity elements in the solid and liquid phase of the bulk metal melt is determined by the thermodynamic properties of the system. Impurities exist as solid solutions in the main metal. Solid solution is generally due to the presence of metal B atoms in the crystal lattice of metal A, thereby forming a solid solution. Due to the presence of trace impurities, the melting point of the metal may decrease or increase. The extent to which the melting point decreases or increases depends on the content of impurities. Decreasing the melting point causes the solid solution to change from the molten state to the solid state, and the impurities migrate from the solid phase to the liquid phase. When the melting point increases, the opposite is true, and the impurities migrate from the liquid phase to the solid phase. For the binary system composed of A and B (the metal A contains impurities B), there is the following relationship [1][7][8]:
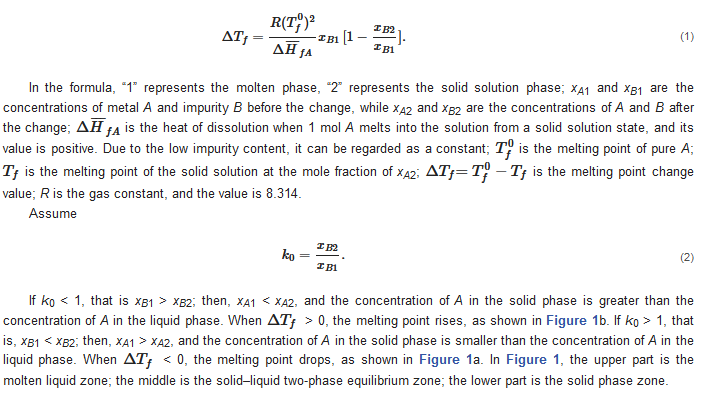
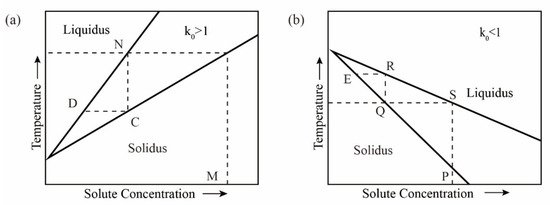

2.2. Analysis of Changes in Impurities during Zone Refining
The specific process of zone refining is shown in Figure 2a. The metal materials to be purified are placed in the tubular furnace; then, install a movable heating ring outside the tube (high frequency heating ring can be used). When using multi-melting zone refining, the advantages of zone refining can be seen. As shown in Figure 2b, a series of closely spaced heaters are used to melt into multiple melting zones in the ingot; after multiple zone refining, the impurity concentration distribution reaches a steady state or limits distribution.
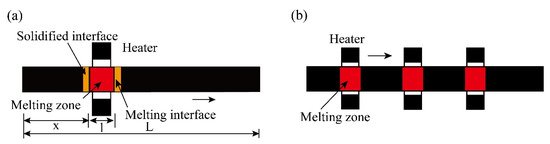
Figure 2. Specific process of zone refining; (a) Single-pass zone refining; (b) Multi-pass melting zone refining.
3. Influencing Factors and Optimization of Zone Refining
Since Pfann [9] published the pioneering work of zone refining, domestic and foreign scholars have conducted a series of research and discussion on the influencing factors of zone refining from a practical and theoretical perspective. These factors are mainly equilibrium distribution coefficient, zone refining rate, melting zone width, diffusion layer thickness, and zone refining times.
3.1. Balanced Distribution Coefficient
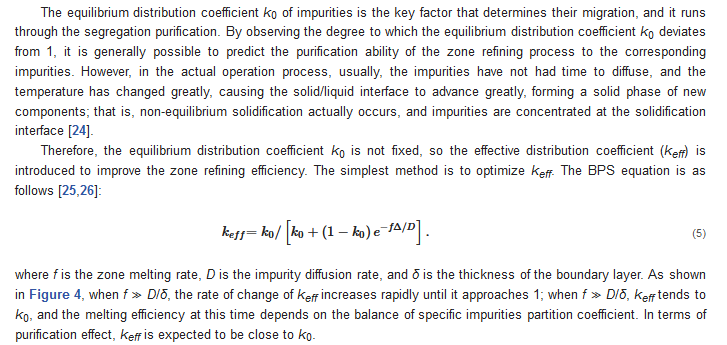
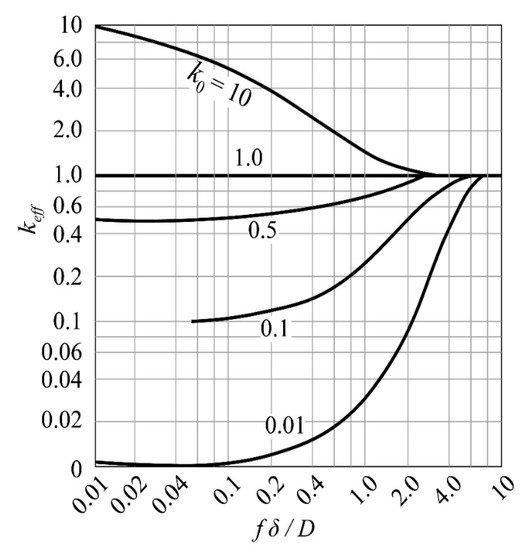

3.2. Zone Refining Rate
The selection of the moving speed of the melting zone (that is, the zone refining rate) directly affects the purification efficiency, which is related to the production cost. Therefore, in the purification process, it is necessary to formulate an appropriate rate according to the characteristics of the material itself. The choice of the optimal refining rate is to strike a balance between the formation of single crystals and the reduction of the degree of segregation [10].
3.3. Length of Melting Zone
The length of the melting zone is affected by many factors, such as thermal field, zone refining speed, crucible thermal conductivity, etc. In zone refining, the purity of the product obtained in the narrow melting zone is higher than that in the wider melting zone, but the impurity concentration of the narrow melting zone increases faster, making it difficult to remove impurities in the melting zone, which must be compensated by extending the purification time [11].
3.4. Zone Refining Scans
The zone refining process needs to be repeated many times. As the number of times increases, the purification effect also increases, but after a certain pass zone melting, the impurity concentration distribution is close to the “limit distribution”, as shown in Figure 5.
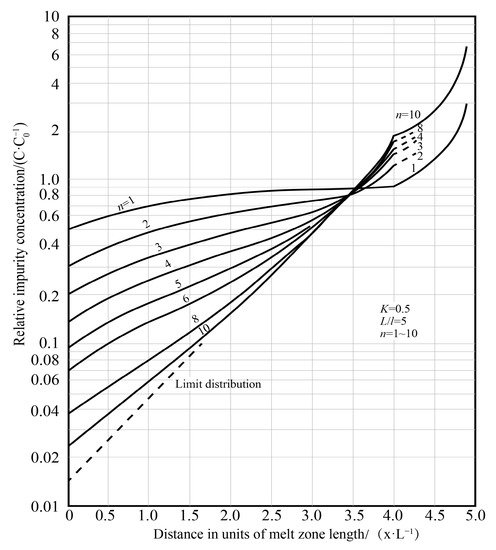
Figure 5. Relationship between impurity concentration and number of zone refining passes [12].
3.5. Application of Current in Impurity Transmission
Applying a current field during the refining process (see Figure 7) can improve the segregation of impurities at the solidification interface through electromigration.
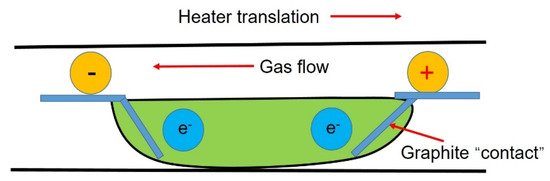
Figure 7. Schematic diagram of the zone refining system under applied current.
3.6. Inclination
During the refining process in the horizontal area, the longitudinal section of the ingot will become tapered due to the mass transfer and even cause the melt to overflow. To avoid this phenomenon, the crucible should be inclined at an angle θ relative to the horizontal direction.
4. Types of Zone Refining
Based on the same principle, there are various zone refining technologies. There are two main types, refining in the suspension area and refining in the horizontal area, as shown in Figure 8.
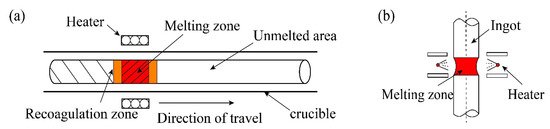
Figure 8. (a) Horizontal zone refining technology; (b) Suspended zone refining technology.
The emergence of refining in the suspended area has greatly promoted the study of refractory metals. This method can not only effectively remove volatile metals and gas impurities in refractory metals, as well as avoid secondary pollution of metals in the refining process, but also effectively control the metal melt flow [13]. Table 4 lists the differences between the two methods.
Table 4. Differences in zone refining technology [13].
| Category | Heating Method | Advantage | Disadvantage | Scale |
|---|---|---|---|---|
| Floating zone refining | Electron beam heating, induction heating, plasma heating, light heating | The ingot does not touch the container, so the product purity is high, and the equipment occupies a small space. | The melting zone is supported by surface tension, so controlling the shape and stability of the melting zone is the key, and the output is low. | Small batch |
| Horizontal zone refining | Induction heating, resistance heating | Simple equipment, continuous purification of multiple melting zones, easy loading and unloading of materials, easy identification of interfaces, and the total length of ingots can be increased or decreased as needed | Large footprint | Batch |
References
- Guo, X.Y.; Tian, Q.H. High Purity Metal. Materials; Metallurgical Industry Press: Beijing, China, 2010.
- Guo, X.; Zhou, Y.; Zha, G. A novel method for extracting metal Ag and Cu from high value-added secondary resources by vacuum distillation. Sep. Purif. Technol. 2020, 242, 116787.
- Virolainen, S.; Suppula, I.; Sainio, T. Continuous ion exchange for hydrometallurgy: Purification of Ag(I)–NaCl from divalent metals with aminomethylphosphonic resin using counter-current and cross-current operation. Hydrometallurgy 2014, 142, 84–93.
- Itam, Z.; Beddu, S.; Mohammad, D. Extraction of metal oxides from coal bottom ash by carbon reduction and chemical leaching. Mater. Today Proc. 2019, 17, 727–735.
- Lu, X.W.; Peng, J.B. Preparation of high purity indium by electrolytic refining method. Min. Metall. 2018, 27, 54–57.
- Zhao, Q.S.; Niu, X.D. Purification of high purity germanium by zone refining. Guangzhou Chem. Ind. 2019, 47, 88–90.
- Zhou, Z.H.; Mo, H.B. Preparation of high purity indium by electrolytic refining-zone melting. Chin. J. Rare Met. 2004, 28, 807–810.
- Hong, W.; Hong, Y.; Dan, W. Making the metal in high solidification by the way of zone refining. Chem. Eng. 2001, 3, 16–17.
- Pfann, W.G. Principles of zone-melting. J. Miner. Met. Mater. Soc. 1952, 4, 747–753.
- Liu, J.; Zee, R.H. Growth of molybdenum-based alloy single crystals using electron beam zone melting. J. Cryst. Growth 1996, 163, 259–265.
- Su, H.; Shen, Z.; Ren, Q. Evolutions of rod diameter, molten zone and temperature gradient of oxide eutectic ceramics during laser floating zone melting. Ceram. Int. 2020, 46, 18750–18757.
- Li, H.G. Principles of Metallurgy; Science Press: Beijing, China, 2005.
- Yin, W.H. New development of high purity and ultra high purity refractory metals. Rare Metal. Mater. Eng. 1991, 3, 1–9.





