
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hongjie Zhang | + 9071 word(s) | 9071 | 2021-05-06 05:21:40 | | | |
| 2 | Camila Xu | -59 word(s) | 9012 | 2021-05-07 05:20:15 | | |
Video Upload Options
Cyclopeptides, also known as cyclic peptides, are polypeptides formed from amino acids arranged in a cyclic ring structure.
1. Introduction
Cancer has become an enormous burden in society and a leading cause of death in the world. Apart from aging, the risk factors of modern daily life such as smoking, unhealthy diet and mental stress also play significant roles in the growing incidence of cancer [1]. According to the World Health Organization (WHO), nearly 10 million of people were killed by cancer in 2020. New cancer cases reached to 18 million in 2018, and are expected to grow to up to 27.5 million in 2040 [2][3]. Conventional therapeutic approaches for the treatment of cancer include chemotherapy, radiotherapy and surgical resection. In recent years, advancement in cancer treatment has been applied with some novel therapies and choices for patients to improve their survival chances, such as immunotherapy, targeted therapy, hormone therapy, stem cell transplant and precision medicine [4]. However, low specificity to cancer cells, high toxicities to normal tissues as well as drug resistance are often the most problematic concerns [5][6][7]. Therefore, studies on the development of new drugs and therapies for cancer with higher efficacy are urgent and crucial.
From ancient times, taking Chinese Medicine as an example, people have been familiar with medicinal properties of natural resources and the benefits of using them to treat diseases [8]. Hence, due to their extraordinary chemical diversity with different origins, natural products are of great potential to be developed as new drugs for cancer prevention and treatment [9]. Natural cyclopeptides, due to their special chemical properties and therapeutic potency have attracted great attention from both academic researchers and pharmaceutical companies in recent years.
Cyclopeptides, also known as cyclic peptides, are polypeptides formed from amino acids arranged in a cyclic ring structure [10]. Most natural cyclopeptides are found to be assembled with 4–10 amino acids. However, some natural cyclopeptides can contain dozens of amino acids [11][12][13][14]. In the past 20 years, hundreds of ribosomally synthesized cyclopeptides have been isolated from a variety of living creatures with various biological activities. In fact, there are two biosynthetic mechanisms of cyclopeptides—ribosomal and non-ribosomal pathways. The ribosomal pathway merely requires the 21 basic proteinogenic amino acids for the synthesis of cyclopeptides, whereas the non-ribosomal pathway depends on non-ribosomal peptide synthetases (NRPSs) in incorporating functional groups, such as hydroxyl and N-methyl groups, to the existing peptides [15][16]. A large number of cyclopeptides have been isolated and demonstrated to deliver a wide spectrum of bioactivities. Very often, they can be served as antibiotics (e.g., microcin 25 from Escherichia coli) [17], protease inhibitors (e.g., SFTI-1 from Helianthus annuus) [18], boosting agents for the innate immune system (e.g., θ-defensins from Macaca mulatta) [19], anticancer agents (e.g., chlamydocin from Diheterospora chlamydosporia) [20] and so on. According to previous reports, cyclization is an important process for peptide stabilization [21]. Compared with linear peptides, cyclic peptides may have a better biological potency due to the rigidity of their conformation [22]. Moreover, the rigidity of cyclopeptides may also increase the binding affinity to their target molecules, allowing enhanced receptor selectivity as rigid structures often decrease the interaction entropy to a negative Gibbs free energy. Furthermore, cyclopeptides are resistant to exopeptidases because they contain no amino/carbonyl termini in their skeletons, so that they exert more stable binding capability than linear peptides do. Owing to the absence of the amino/carbonyl termini, the less flexible backbone of cyclopeptides even enhances their resistance to endopeptidases, and therefore, cyclopeptides are considered as useful biochemical tools with high specificity [23]. In particular, when serving as an anticancer agent, the cyclic structure of the peptide eliminates the effect of charged termini and thus increases membrane permeability [24]. As a result, cyclopeptides can penetrate to the tumors without much hurdle while exhibiting their anti-proliferative effect [25].
Overall, the structural rigidity, biochemical stability, binding affinity and membrane permeability are the predominant advantages of cyclopeptides in comparison of linear peptides and other chemicals for therapeutic purposes [10]. Owing to these exceptional characteristics, natural cyclopeptides are considered as promising lead compounds for the development of novel anticancer drugs. With the efforts from researchers around the world, a great number of cyclopeptides with anticancer effects have been identified from various living entities.
2. Marine-Derived Anticancer Cyclopeptides
Marine organisms are the vast source of many chemical products because of the diversity of species and their abundance [26]. In fact, the marine environment though somehow mysterious, promotes the fruitful development of a great number of natural products, which may possess novel structures, chemical properties and biological effects [27]. The marine-derived cyclopeptides have received great attention due to their high potency in anti-microbial, anti-inflammatory and anticancer activities [28][29][30][31]. This section mainly discusses the discovery of marine-derived cyclopeptides with anticancer properties in the last 20 years.
2.1. Anticancer Cyclopeptides Derived from Sponge
As sessile marine filter feeders, sponges possess decent abilities to defense against foreign invaders such as viruses, bacteria and fungi [32]. Marine sponges belong to the phylum Porifera, which is the source of many bioactive marine natural products [33]. It is estimated that about 5000 different new compounds have been discovered from sponges each year [32]. Among them, cyclopeptides are an important portion of the sponge-derived compounds that are accompanied with broad biological effects including anticancer activities [34]. The structures of these sponge-derived natural cyclopeptides compounds are shown in Figure 1 and their in vitro activities against different cancer cells are listed in Table 1.
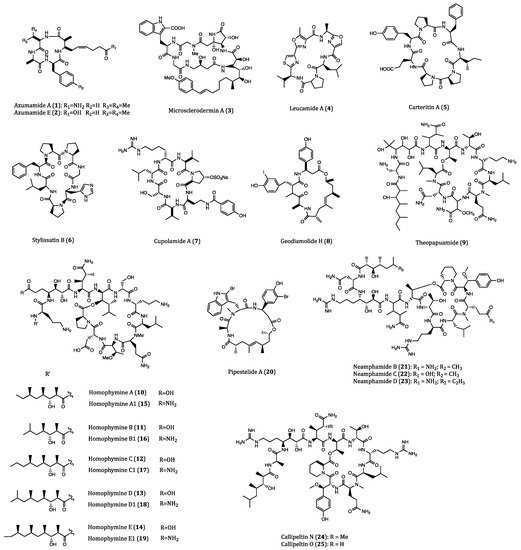
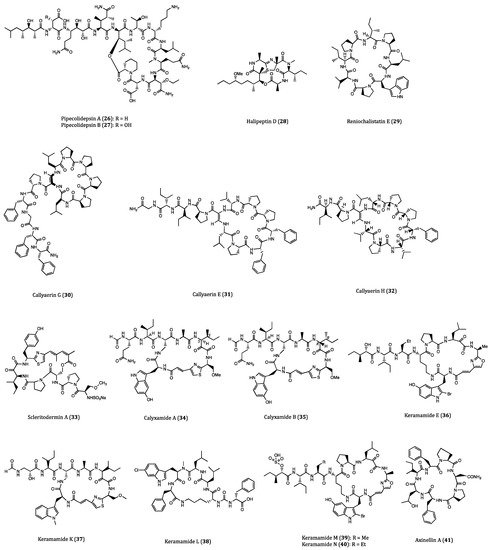
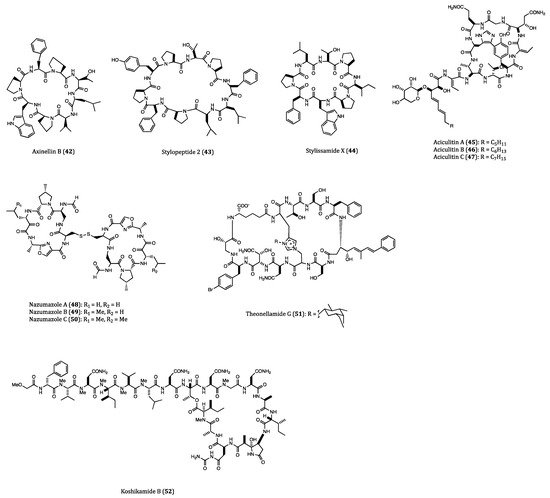
Figure 1. Structures of cyclopeptides 1–52 derived from sponges.
Azumamides A (1) and E (2) were two cyclotetrapeptides isolated from the marine sponge Mycale izuensis. These two compounds have been demonstrated to exhibit potent inhibitory activities against histone deacetylase (HDAC) enzymes [35]. It is widely acknowledged that overexpression of HDACs may lead to the occurrence of heterochromatin and thus different types of cancers [36]. In consequence, HDAC inhibitors are suggested with a role in cancer growth inhibition, and their anticancer mechanisms are mainly associated with their induction of apoptotic events in cancer cells [37]. According to a previous study, azumamide A showed anti-proliferative effect on K562 (human bone marrow chronic myelogenous leukemia) cells with IC50 values of 0.045 μM, and cytostatic effect on WiDr (human colon cancer) and K562 cells with IC50 values of 5.8 and 4.5 μM, respectively [35]. Furthermore, azumamides A and E obtained from total synthesis were demonstrated to exert significant zinc-binding affinity to HDACs, which may contribute to the rational design for synthesizing potent anticancer agents [38][39].
Microsclerodermin A (3), a cyclic hexapeptide, first isolated from the lithistid sponge Microscleroderma herdmani, was found to be a potent antifungal agent [40][41]. This compound was also reported to have anti-proliferative activities against lung, leukemia and pancreatic cancer cells. In human pancreatic adenocarcinoma cells (AsPC-1), the level of phosphorylated NFκB was significantly reduced by the incubation with microsclerodermin A (IC50 = 2.3 µM) whereas apoptosis was strongly induced. In the human pancreatic adenocarcinoma cells BxPC-3 (IC50 = 0.8 µM) and human pancreatic epithelioid carcinoma PANC-1 cells (IC50 = 4.0 µM), microsclerodermin A exhibited remarkable inhibitions at low IC50 values. In human umbilical vein endothelial cells (HUVECs), this cyclopeptide was shown to have no effect of angiogenesis [42]. The above biological investigations demonstrated that microsclerodermin A is a good anticancer lead compound for further development.
Leucamide A (4) is a cytotoxic cyclic heptapeptide with potent cytotoxicity, which was first purified from the Australian marine sponge Leucetta microraphis. In the study of Kehraus and co-workers, this peptide was found to exhibit inhibitory activities against human mucin-producing gastric cells (HM02), and human liver cancer cells (HepG2 and Huh7) with GI50 values of 8.5, 9.7 and 8.3 µM, respectively [43]. In the consecutive year, the total synthesis of leucamide A was accomplished, and provided a viable approach to the sufficient supply of this compound for further comprehensive investigations [44].
Afifi et al. isolated a new cyclic heptapeptide, named carteritin A (5), from the marine sponge Stylissa carteri. Carteritin A exhibited cytotoxicities against human colorectal (HCT116), mouse macrophage (RAW264) and human cervical (HeLa) cancer cells with IC50 values of 1.3, 1.5 and 0.7 µM, respectively [45].
Stylissatin B (6), a novel cytotoxic cycloheptapeptide, was obtained from the sponge Stylissa massa. This compound displayed inhibitory effects on HCT116, HepG2, gastric (BGC823), lung (NCI-H1650), ovarian (A2780) and breast (MCF7) human cancer cells with IC50 values ranging 2.3 to 10.6 µM [46]. These results implicated that significant structural modification is needed to improve the moderate activity of Stylissatin B.
Derived from the sponge Theonella cupola, cupolamide A (7) was found to be a biologically active cyclic heptapeptide. This compound was cytotoxic against mouse lymphoma cells (P388) with an IC50 value of 7.5 µM but no cytotoxic against thrombin. Moreover, the resemblance of cupolamide A to γ-aminobutyric acid (GABA) unveils its potential effect on the central nervous system, which requires further investigation [47].
Geodiamolide H (8), a cyclic depsipeptide isolated from the Brazilian sponge Geodia corticostylifera, was firstly identified as a neurotoxic and hemolytic agent [48]. This compound showed a potent anti-proliferative activity against the human ductal carcinoma T47D (EC50 = 38.36 nM) and MCF7 (EC50 = 89.96 nM) cells via altering the cytoskeleton of cancer cells by actin depolymerization. However, no such deleterious effect was observed in normal cell lines even when higher concentrations was used (e.g., 136 nM) [49]. Subsequently, the results of another study further demonstrated that geodiamolide H exhibited significant inhibitory effects on the migration and invasion of breast cancer cells (Hs578T) that were poorly differentiated and highly aggressive [50]. These studies revealed geodiamolide H as a potential lead compound for further development of therapeutic use.
Theopapuamide (9), isolated from the lithistid sponge Theonella swinhoei, was also a new cytotoxic depsipeptide with a cyclic ring structure. This cyclopeptide exhibited significant cytotoxicity against human leukemia (CEM-TART) (EC50 = 0.5 µM) and HCT116 (EC50 = 0.9 µM) cells. When compared with other depsipeptide with HIV-inhibitory activities, the β-methoxytyrosine residue of this compound appears to be of great significance for its anti-HIV biological effect [51].
A group of structurally similar cyclodepsipeptides named homophymines A-E (10–14) and A1-E1 (15–19) isolated from the sponge Homophymia sp., were found to be potent anti-proliferative agents against a wide panel of cancer cell lines with IC50 values in the range of 2–100 nM. Among these cell lines, human prostate (PC3) and ovarian (OV3) human cancer cell lines were the most sensitive to this group of cyclopeptides. Furthermore, the overexpression of P-glycoprotein (P-gp) proteins did not affect the intracellular concentrations of homophymines [52]. Apart from cytotoxic activities, homophymine A also exhibited cytoprotective activity against HIV-1 infection (IC50 = 75 nM) [53]. In the family of homophymins, their general chemical structures are complicated but structural differences among them are subtle. Because of their significant anticancer potential, further structure activity relationship (SAR) assessment, bioactivity evaluation and mechanism of action studies should be performed.
Jaspamide is an early-discovered cyclic depsipeptide from the sponge Jaspis splendens, which delivered pronounced inhibitory activities against breast and prostate cancer cells [31]. Pipestelide A (20), a cyclodepsipeptide derived from the marine sponge Pipestela candelabra, was biosynthetically related to jaspamide. When subjected to cytotoxicity assay, this compound showed cytotoxicity against the human nasopharyngeal epidermoid carcinoma (KB) cells with an IC50 value of 0.1 µM, which was less potent than jaspamide [54].
Three cytotoxic cyclodepsipeptides, neamphamides B-D (21–23), were purified from the Australian sponge Neamphius huxleyi. These three compounds were cytotoxic to human lung (A549), cervical (HeLa) and prostate (LNCaP and PC3) cancer cells with IC50 values varying from 91 to 230 nM. Nevertheless, at sub-nanomolar concentrations (100 nM for 48 h and 72 h), neaphamide D was observed to induce proliferation in human lung cancer A549 cells. Therefore, these neamphamindes should be discreetly utilized [55]. Apart from the cytotoxic effects, neaphamide D also showed a great antimicrobial potency against the growth of Mycobacterium smegmatis and M. bovis BCG [56].
Collected in Solomon Islands, the sponge Asteropus sp. yielded two new derivatives of callipeltin A, which were named cyclic depsipeptides callipeltins N (24) and O (25). Their cytotoxicities against human melanoma (A2058), colorectal (HT29), and breast (MCF7) cancer cells as well as non-malignant MRC-5 fibroblast cells were accompanied with IC50 values ranging from 0.16 to 0.21 µM for callipeltins N and ranging from 0.48 to 2.08 µM for callipeltins O. The slight difference of cytotoxicities between malignant cancer cells and non-malignant cells indicated that these two compounds can provide minimal specificity.Structure-wise, the biological potencies of the two compounds was highly related to the positions of the methylGln group [57].
Pipecolidepsins A (26) and B (27), two head-to-side-chain cyclodepsipeptides with significant anticancer potential, were isolated from the sponge Homophymia lamellosa. Both compounds were tested for their cytotoxic effects against A549, HT29 and human breast cancer (MDA-MB-231) cells. The results demonstrated that pipecolidepsin B (GI50 = 0.04, 0.01 and 0.02 µM, respectively) was more cytotoxic than pipecolidepsin A (GI50 = 0.6, 1.12 and 0.7 µM, respectively), though pipecolidepsin B contains only one more hydroxy group. The SAR assessment further revealed that the replacement of residue at C-3 was an important step for gaining higher hydrophilic properties in pipecolidepsin B [58], whereas the γ-amino acid in pipecolidepsin A was the major functional group for its cytotoxicity [59], While exerting cytotoxic activity, pipecolidepsin A was shown to induce intense membrane damage and necrotic cell death [60]. Due to the early discovery of pipecolidepsin A, a total solid-phase synthesis of this compound had been previously accomplished [61]. Considering the great anticancer potential of pipecolidepsin B, the further understanding of its biological property, total synthetic strategy as well as other pharmacological actions is of high research interest.
Halipeptin D (28) was a cyclic depsipeptide isolated from the sponge Leiosella cf. arenifibrosa by Faulkner and Manam [62]. This natural product was reported to exhibit significant cytotoxicity against HCT116 cells (IC50 = 7 nM) and BMS ODCP (oncology diverse cell panel) with an average IC50 value of 420 nM. However, the synthetic halipeptin D and other halipeptins (e.g., halipeptins A-C) did not show potent cytotoxicities. Based on the distinct results, it is reasonable to assume that the previous naturally derived compound might have been contaminated with other cytotoxic agents during harvest [63]. The discrepancy of cytotoxicity between the synthetic and naturally derived compounds is not surprising as such happens somehow. As a result, biological properties of halipeptin D remained uncertain and required further investigations.
The cyclic peptide reniochalistatin E (29) was isolated from the marine sponge Reniochalina stalagmitis by Zhan and co-workers. Differed from other congeners (reniochalistatins A-D) of the same origin, this compound showed in vitro cytotoxicity against RPMI-8226 (human myeloma) and MGC-803 (human gastric carcinoma) cells with IC50 values of 4.9 and 9.7 μM, respectively [64]. The subsequent total synthesis of reniochalistatin E provided some inspiring methods for the synthetic strategies of cyclopeptides with similar structures [65].
From the freeze-dried Indonesian sponge Callyspngia aerizusa, a cyclic peptide called callyaerin G (30) was purified. Biological studies showed that this compound was cytotoxic to mouse lymphoma cells (L5178Y) and HeLa cells with ED50 values of 0.41 and 4.17 μM, respectively [66]. Callyaerins E (31) and H (32), were purified using another bioassay-guided fractionation of the same sponge Callyspngia aerizusa. Callyaerins E and H exhibited strong anti-proliferative activities against L5178Y cells with ED50 values of 0.39 and 0.48 µM, respectively. Similar to the three compounds above, other proline-rich callyaerins yielded from the same sponge also showed high antimicrobial activities [67]. Accordingly, the biological significance of proline residue in cyclic peptides becomes a trendy research topic. The mechanism of callyaerins in biological actions definitely deserves further investigation.
Obtained from the lithistid sponge Scleritoderma nodosum, scleritodermin A (33) was a cyclic peptide with anticancer potential. By means of cell viability assay, this compound was demonstrated to exert cytotoxicity in various cancer cell lines including HCT116, HCT116/VM46 (human vinblastin-resistant colon cancer cell), A2780 and SKBR3 (human breast cancer cells) with IC50 values of 1.9, 5.6, 0.94 and 0.67 µM, respectively. Regarding its molecular mechanism, scleritodermin A induced G2/M phase arrest and inhibited tubulin polymerization [68]. By using different moieties and fragments, the total synthesis of scleritodermin A had been accomplished [69][70], and consequently provided a sufficient supply of this compound for further comprehensive studies of its other biological properties and the synthesis of analogs or derivatives.
Calyxamides A (34) and B (35), two novel cyclic peptides with bioactivities, were first isolated from the marine sponge Discodermia calyx in Japan. When incubated with leukemia P388 cells, both compounds yielded moderate cytotoxicities with IC50 values of 3.9 and 0.9 µM, respectively. Subsequently, the analysis of the 16S rDNA sequence indicated that the biosynthesis of calyxamides A and B were possibly related to Candidatus Entotheonella sp. inhabiting in the Theonella genus [71]. Hence, further biological studies of these compounds would be relied on the bulky isolation from their natural source.
A group of compounds named keramamides were yielded from the Okinawa marine sponge Theonella sp., which possess a variety of bioactivities. Keramamide E (36) was reported to display inhibitory activities against mouse lymphocytic leukemia (L1210) and KB cells with IC50 values of 1.42 and 1.38 µM, respectively [72]. Subsequent explorations on another Theonella sponge led to the purification of keramamides K (37) and L (38). Keramamide K contains a (1-Me)Trp residue, which is rarely seen in any naturally derived compound whilst keramamide L is a natural cyclopeptide possessing a MeCtrp residue. These two special cyclopeptides exhibited strong cytotoxicity against L1210 (IC50 = 0.77 and 0.5 µM, respectively) and KB (IC50 = 0.45 and 0.97 µM, respectively) cells [73]. Keramamides M (39) and N (40) were two other congeners from the Theonella sponge that were discovered by the same research team. Both cyclic peptides contained a sulfate ester, which is also a rare residue to be found in compounds derived from marine sponge. Keramamides M and N showed moderate cytotoxicities against L1210 (IC50 = 2.02 and 2.33 µM, respectively) and KB (IC50 = 5.05 and 6.23 µM, respectively) cells [74]. The total synthesis of different keramamides had been completed by the same research group [75]. From the results of structural elucidation, some intriguing constituents of these cyclic peptides exhibited anticancer potentials against some other cancer cell lines. In order to highlight the aspect of their anticancer effect, more types of cancer cell lines and mechanism of their biological actions should be exploited.
The marine sponge Axinella carteri collected near Vanuatu islands yielded two proline-containing cyclopeptides, which were called axinellins A (41) and B (42). Both compounds were found to display moderate cytotoxicity against non-small lung cancer cells (NSLC-N6) with IC50 values of 16.7 and 29.8 µM, respectively [76]. The first synthesis of axinellin A was achieved shortly after its discovery. However, the synthetic compound did not show cytotoxicity as the naturally occurring peptide did [77]. Therefore, further investigation should be performed for clarifying such discrepancy on cytotoxicities between the synthetic and the natural axinellin A.
The proline-rich cyclopeptide stylopeptide 2 (43) was isolated from the marine sponge Stylotella sp. in Papua New Guinea. According to the study of Brennan et al., this compound inhibited 23% growth of BT-549 and 44% growth of Hs578T human breast cancer cells in the National Cancer Institute one-dose (10−5 M) 60-cell-line assay [78]. However, further dose-dependent cell viability assays should be carried out for a more accurate evaluation of its cytotoxicity and anticancer potential.
Stylissamide X (44) was a cyclic proline-rich octapeptide discovered in the Indonesian marine sponge of Stylissa species. Although only little inhibitory activity was observed in the viability assay of HeLa cells, stylissamide X induced significant anti-migration effects on the same cell line in the wound-healing assay [79]. As a new member of the stylissamides family, the total synthetic approach of it became extremely important for securing a good supply of materials for further comprehensive chemical and biological studies [80]. More systemic cell viability assays and detailed evaluation of its anti-migration properties would be of similar importance for the mode of action studies of this type of compounds.
Aciculitins A-C (45–47) were three bicyclic peptides extracted from the lithistid sponge Aciculites orientalis with an unusual histidino-tyrosin bridge. They were also the first glycopeptidolipids obtained from a marine origin. In regards to their biological activities, aciculitins A-C were cytotoxic against HCT116 (IC50 = 0.37 µM) and inhibitory to the growth of bacterium Candida albicans. Comparing with other compounds of the same origin, aciculitins A-C possess a histidine residue which is responsible for their predominant bioactivities [81].
Nazumazoles A-C (48–50) were three novel bicyclic pentapeptides isolated from a mixture derived from the marine sponge Theonella swinhoei. The mixture of nazumazoles A-C showed cytotoxic activities against P388 cells with an IC50 value of 0.83 µM. The reduction of either the ketone or thiol group in the nazumazoles led to a significant decrease of the cytotoxicity of the mixture [82]. Based on the results above, separation of individual nazumazoles A-C is undoubtedly needed to validate the anticancer potential of this type of compounds.
Theonellamide G (51) was a novel bicyclic glycopeptide isolated from the Red Sea Sponge Theonella swinhoei. When compared the positive anticancer control etoposide (2.0 µM), theonellamide G merely yielded a moderate cytotoxicity against HCT116 with an IC50 value of 6.0 µM. However, this compound showed a potent antifungal activity against the wild and amphotericin B-resistant strains of Candida albicans [83]. These results added some fresh insights into the diverse biological activities of this natural compound, which may inspire further studies on the pharmacological actions and SAR for this class of compounds.
Koshikamide B (52) was a 17-residue cyclic peptide lactone purified from a marine sponge of the genus Theonella. This compound was the first natural product containing the constitution of Nδ-carbamoylasparagine. Importantly, the 2-(3-amino-2-hydroxy-5-oxopyrrolidin-2-yl) propionic acid (AHPP) residue of koshikamide B was a unique moiety in peptide lactones. Koshikamide B exhibited cytotoxicity in HCT116 and P388 cancer cells with IC50 values of 3.62 and 0.22 µM, respectively [84].
2.2. Anticancer Cyclopeptides Derived from Ascidians/Tunicates
Ascidians are also known as tunicates. They are found in high-current fields, and firmly fixed to rocks. About 3000 species of ascidians have been reported so far [85]. As more and more compounds were isolated from the ascidians, the US National Cancer Institute (NCI) estimated that about 1% of the marine natural products (MNPs) showed anticancer activities [86]. This section focuses on the ascidians derived cyclopeptides with promising anticancer potential. The structures of these compounds are present in Figure 2 and their activities are listed in Table 1.
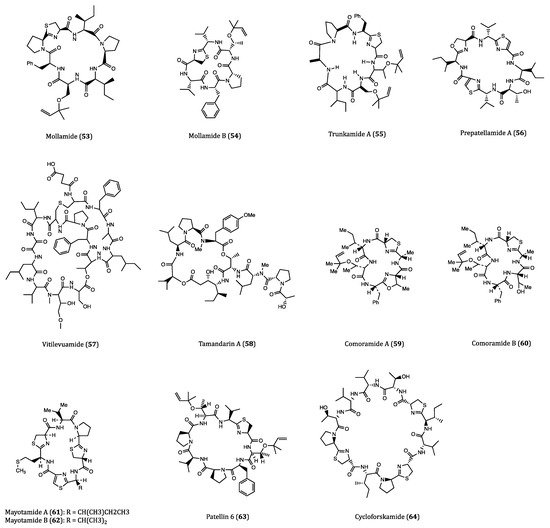
Figure 2. Structures of cyclopeptides (53–64) derived from ascidians/tunicates.
Mollamide (53) was a cyclopeptide isolated from the ascidian Didemnum molle. This compound displayed a moderate cytotoxicity against several cancer cell lines, with IC50 values of 1.24 μM against P388 cells, and 3.1 μM against A549, HT29 and monkey kidney CV1 cells. The anti-proliferative property of mollamide was plausibly associated with its ability to inhibit RNA synthesis in the cancer cells [87][88]. Another research study on the same Indonesian tunicate Didemnum molle led to the isolation of a novel congener, which was a cyclic hexapeptide called mollamide B (54). Mollamide B (100 μM) displayed significant growth inhibition against human lung carcinoma (H460), MCF7 and glioblastoma (SF-268) cells by 29%, 44% and 42%, respectively. Other than its anticancer activities, mollamide B also exhibited notable anti-HIV (HIV-1, EC50 = 48.7 μM) and anti-malarial (Plasmodium falciparum, IC50 = 2.87 μM) properties [89]. For the future perspective of its anticancer potential, the specificity of mollamide B acting against different cancer cell lines should be investigated.
Trunkamide A (55) is an analog of mollamide with a similar cyclopeptide structure that is naturally produced by ascidians of Lissoclinum sp. Compared to mollamide, trunkamide A was reported to have a more promising antitumor activity [90]. Due to its significant in vitro cytotoxicity against several human-derived cancer cell lines including A549, P388, HT29 and MEL28 (human melanoma) with IC50 values ranging from 0.6 to 1.19 μM, trunkamide A had been evaluated for its antitumor efficacy in several preclinical trials [91].
Fu et al. purified a novel cyclic peptide called prepatellamide A (56), from Lissoclinum patella. Although the structure of this peptide had been determined, its biological properties were incompletely understood. The IC50 value of prepatallamide A against P388 cells was 6.57 µM [92]. Other than this piece of result, no further information on prepatallamide A could be obtained. Therefore, the bioactivity studies of this compound are desperately needed.
Vitilevuamide (57), another ascidian derivative with a bicyclic structure, was found in two species of ascidians, Didemnum cuculiferum and Polysyncranton lithostrotum. It displayed potent cytotoxicity against HCT116 cells, with an IC50 value of 6.24 nM. In A549, SKMEL-5 (human melanoma) and A498 (human kidney carcinoma) cells, vitilevuamide exhibited a less potent anti-proliferative effect as its IC50 values were determined to be 0.12, 0.31 and 3.12 μM, respectively. In addition, the compound showed a percentage increase in the mean lifespan (%ILS) of 70 at the concentration of 30 μg/mL in a P388-xenograft in vivo experiment in mice, which indicated its significant anticancer potential [93]. Regarding its anticancer mechanisms, vitilevuamide inhibited tubulin polymerization and induced cell cycle arrest at the G2/M phase [94]. As the supply of the naturally derived vitilevuamide is limited, further evaluations on this compound are largely hindered. Due to its complex structure, identification of its interactive site(s) with tubulin and methods of chemical synthesis are in slow progress.
Tamandarin A (58) was first isolated from a Brazilian marine ascidian of the family Didemnidae. This compound was cytotoxic to three cancer cell lines, BX-PC3 (pancreatic carcinoma), DU145 (prostate carcinoma) and UM-SCC-10B (laryngeal squamous cell carcinoma) with IC50 values of 1.69, 1.29 and 0.94 nM, respectively. Didemnin B, another renowned tunicate-derived cyclodepsipeptide, had been promoted into clinical trials several years ago owing to its significant anticancer potential [95]. With slightly lower IC50 values than didemnin B, tamandarin A can also be a good anticancer candidate [96][97]. Although its mechanism of action remains unclear, the structural similarity between tamandarin A and didemnin B may represent their similar mechanisms of action in cancer cells, which warrants it for further biological investigations.
Rudi’s research group found two groups of cyclic hexapeptides, comoramides A and B (59–60) and mayotamides A and B (61–62), which were isolated from the ascidian Didemnum molle collected at different spots. Cytotoxicity screening revealed that these two groups of compounds showed cytotoxic activities against A549, HT29 and MEL28 cell lines with IC50 values ranging from 7.22 to 14.97 µM [98].
Patellin 6 (63) was a cytotoxic cyclic hexapeptide isolated from the colonial ascidian Lissoclinum patella. Although patellins 1-5 exhibited no cytotoxicity, patellin 6 was highly cytotoxic to P388, A549, HT29 and CV1 cells with the IC50 values around 2.08 µM. In addition, patellin 6 inhibited the activity of topoisomerase II with an IC50 value of 2.6 µM [99].
Cycloforskamide (64) was a macrocyclic dodecapeptide isolated from the sea slug Pleurobranchus forskalii collected in Japan. This compound showed cytotoxic effect against P388 cells with an IC50 value of 5.8 µM. In addition, from an ecological perspective, cycloforskamide is presumed to chelate toxic metals and plays a detoxification role [100].
2.3. Anticancer Cyclopeptides Derived from Mollusks
As the largest marine phylum and the secondary largest phylum of invertebral animals, mollusks possess highly diverse anatomical structures while having various behavior and habitats [101]. Surprisingly, a great amount of enzymes, polysaccharides, lipids and peptides with broad therapeutic uses have been discovered from mollusks. No wonder mollusks are considered a great resource for the discovery of bioactive compounds [102]. Many previous studies reported that mollusk-derived compounds showed significant anticancer properties, and many of them are indeed cyclopeptides. This section lists a wide range of mollusk-derived cyclopeptides with decent anticancer activities. The structures of these compounds are shown in Figure 3 and their activities are listed in Table 1.
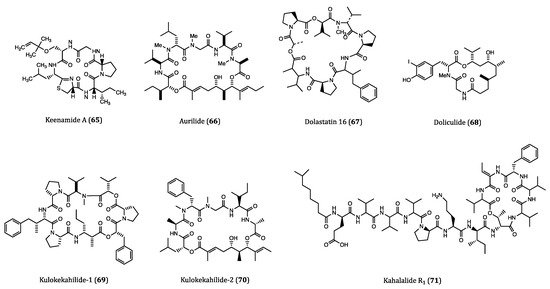
Figure 3. Structures of cyclopeptides (65–71) derived from ascidians/tunicates.
Keenamide A (65) is a cyclic hexapeptide isolated from Pleurobranchus forskali. This compound elicited anti-proliferative activities against tumor cell lines A549, P388, MEL20 (uveal melanoma) and HT29 with IC50 values ranging from 4.03 to 8.05 μM; however, its molecular mechanisms remained elusive [103].
Aurilide (66) is a 26-membered cyclic peptide extracted from the internal organs of sea hare Dolabella auricularia. It exhibited significant cytotoxicity against HeLa S3 tumor cells with an IC50 value of 0.013 µM. In the NCI-60 assay, this compound showed a strong inhibitory activity against the ovarian, renal as well as prostate cancer cells. However, no significant antitumor effect was observed in the subsequent in vivo experiments in xenograft mice. In regards to its antitumor mechanism, aurilide promoted microtubule stabilization rather than inducing a direct tubulin interaction [104]. Sato et al. found that aurilide could selectively bind to prohibitin-1 (PHB1) in mitochondria and activate proteolytic processing of OPA1, resulting in mitochondrial apoptosis [105]. However, further studies are needed to clarify the obvious diverging results obtained from the in vitro and in vivo tests of aurilide. In addition, detailed studies of mechanism and SAR are also needed to develop this type of cyclopeptides as druggable anticancer agents.
Dolastatin 16 (67) is a cyclodepsipeptide originated from the sea hare Dolabella auricularia in Papua New Guinea. When tested against NCI cancer cell lines, this compound exhibited strong inhibitory activities against H460 (GI50 = 1.09 nM), colon adenocarcinoma KM20L2 (GI50 = 1.37 nM), glioblastoma SF-295 (GI50 = 5.92 nM) and SKMEL-5 (GI50 = 3.75 nM) cells. Moreover, dolastatin 16 also showed comparable anti-proliferative effects against five human leukemia cell lines [106]. Although studies on the molecular mechanisms or pharmacological actions of dolastatin 16 are rather limited, this highly potent bioactive compound is worthy for further development into an anticancer agent.
Doliculide (68), a potent cytotoxic cyclodepsipeptide, was previously isolated from the Japanese sea hare Dolabella auricularia. This compound showed very potent cytotoxicity against HeLaS3 cells with an IC50 value of 1.62 nM [107]. It is worth noting that the first total synthesis of doliculide was accomplished soon after its isolation [108]. Actin is an abundant protein in many eukaryotic cells, and it is also an important target in many cancer studies. In order to target actin polymerization, Matcha et al. developed an efficient strategy for synthesizing (-)-doliculide, which was proven with great actin binding ability [109]. When applied to breast cancer cell lines MCF7 and MDA-MB-231, the synthetic doliculide showed a decent anti-proliferative activity with IC50 values of 93 and 77 nM, respectively. Induction of apoptotic events and migratory impairment were also observed in both cell lines when incubated with doliculide [110]. Collectively, the above experimental results unveiled the high anticancer potential of doliculide.
Kulokekahilide-1 (69) was a cyclic depsipeptide purified from the cephalaspidean mollusk Philinopsis speciosa. This compound showed cytotoxic activity against P388 cells with an IC50 value of 2.2 µM [111]. Kulokekahilide-2 (70), a much more potent cytotoxic cyclic depsipeptide than 69 was isolated from the same origin two years later. The IC50 values of kulokekahilide-2 in P388, human ovarian cancer (SK-OV-3), human breast cancer (MDA-MB-435) and rat myoblast (A-10) cells were determined to be 4.2, 7.5, 14.6 and 59.1 nM, respectively [112]. Due to the potent anticancer potential, kulokekahilide-2 and its derivatives were totally synthesized in subsequent studies. According to the SAR assessments and cytotoxicity tests performed by Umehara et al., the IC50 values of the synthesized kulokekahilide-2 against A549, K562 and MCF7 cells were found to be 0.0021 nM, 0.0031 nM and 0.22 nM. These low IC50 values indicated that this compound was of great anticancer potential. Interestingly, the cyclic structure of this peptide accompanied by the chirality at the 21 position largely contributes to its super potent cytotoxicity [113]. According to the NCI COMPARE analysis the mechanisms of action of kulokekahilide-2 appeared rather different from those of the conventional anticancer agents such as aurilide (66), palauamide (182) and lagunamide A (152) [114]. Nevertheless, kulokekahilide-2 and its derivatives are considered good anticancer candidates for testing in vivo efficacies in future human clinical trials. In the long run, further studies on specific mechanism of action, exploration of new derivatives as well as structural modifications would be of high significance for the development of kulokekahilide-2 as therapeutic agent for cancer treatment.
Kahalalide F is an intensively studied cyclic peptide that was first isolated from Elysia rufescens in 1993 [115]. Owing to its remarkable anticancer potential, several phase II clinical trials for kahalalide F are ongoing whilst a few had been completed [116]. As an analog of kahalalide F, kahalalide R1 (71) is also a cyclic depsipeptide, which was reported to be isolated from a species of sea slug E. grandifolia in 2006. Both kahalalide R1 and kahalalide F were tested for their cytotoxicities against MCF7 cells, and the results showed that they had comparable IC50 values of 0.14 and 0.22 µM, respectively. Furthermore, kahalalide R1 also exhibited anti-proliferative activity in mouse lymphoma L1578Y cell line with an IC50 value of 4.26 nM. The anti-proliferative potency of kahalalide R1 against cancer cells was almost the same potent as kahalalide F [117]. Nevertheless, further in vivo and preclinical studies on kahalalide R1 are urgently needed for a thorough validation of its anticancer potential.
2.4. Anticancer Cyclopeptides Derived from Marine Algae
In the plant kingdom, marine algae are the most ancient members responsible for maintaining the stability of the marine ecosystem though they are merely simple organisms possessing chlorophyll. Many bioactive compounds have also been derived from marine algae, therefore these microorganisms have attracted a lot of attention in the recent years for the exploration of algal products [118]. The structures of renowned bioactive algal compounds are provided in Figure 4 and the activities of these compounds are listed in Table 1.
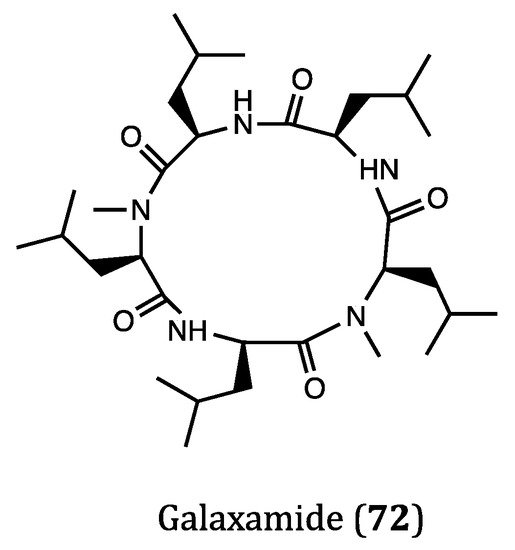
Figure 4. Structure of cyclopeptide (72) derived from marine algae.
Galaxamide (72) was a representative algae-derived cyclic pentapeptide discovered in marine algae Galaxaura filamentosa, which showed moderate inhibitory activities against GRC-1 (human renal cell carcinoma, IC50 = 7.18 μM) and HepG2 (IC50 = 7.81 μM) cell lines [119]. In the study of Lunagariya et al. [120], they investigated the mechanism of action of galaxamide on MCF7 cells in detail. Their results demonstrated that galaxamide induced apoptosis via the oxidative stress-mediated signaling pathway, by which the mitochondrial membrane potential was disrupted in response to the overwhelmed production of reactive oxygen species (ROS). Further, cell cycle arrest at G1 phase was also observed after the treatment with galaxamide due to ROS production, which contributed much to the apoptosis of MCF7 cells. Several synthetic analogs of galaxamide were found to be more potent than the natural scaffold, thus this type of compounds is worthy for further studies as novel candidates for the treatment of breast cancer.
Table 1. Marine-derived anticancer cyclopeptides.
| Name | Biological Source | Anticancer Activity | Reference |
|---|---|---|---|
| Anticancer Cyclopeptides Derived from Sponge | |||
| Azumamide A (1) | Mycale izuensis | HDAC inhibitory activity against K562 cells (IC50 = 0.045 μM); Cytostatic effects on WiDr (IC50 = 5.8 μM) and K562 (IC50 = 4.5 μM) cells | [35][36][37][38][39] |
| Azumamide E (2) | Mycale izuensis | HDAC inhibitory activity against K562 cells (IC50 = 0.045 μM) | [35][36][37][38][39] |
| Microsclerodermin A (3) | Microscleroderma herdmani | Induction of apoptosis in AsPC-1 (IC50 = 2.3 µM), BxPC-3 (IC50 = 0.8 µM) and PANC-1 (IC50 = 4.0 µM) cells. | [40][41][42] |
| Leucamide A (4) | Leucetta microraphis | Inhibitory activities to HM02 (GI50 = 8.5 µM), HepG2 (GI50 = 9.7 µM) and Huh7 (GI50 = 8.3 µM) cells. | [43][44] |
| Carteritin A (5) | Sponge, Stylissa carteri | Cytotoxicity against HCT116 (IC50 = 1.3 µM), RAW264 (IC50 = 1.5 µM) and HeLa (IC50 = 0.7 µM) cells | [45] |
| Stylissatin B (6) | Stylissa massa | Inhibitory effects on HCT116, HepG2, BGC823, NCI-H1650, A2780 and MCF7 cells. (IC50 = 2.3 to 10.6 µM) | [46] |
| Cupolamide A (7) | Theonella cupola | Cytotoxicity against P388 (IC50 = 7.5 µM) cell. | [47] |
| Geodiamolide H (8) | Geodia corticostylifera | Anti-proliferative activitiy against T47D (EC50 = 38.36 nM) and MCF7 (EC50 = 89.96 nM) cells | [48][49][50] |
| Theopapuamide (9) | Theonella swinhoei | Cytotoxicity against CEM-TART (EC50 = 0.5 µM) and HCT116 (EC50 = 0.9 µM) cells. | [51] |
| Homophymines A-E (10–14) & A1-E1 (15–19) | Homophymia sp. | Anti-proliferative against several cancer cell lines (IC50 = 2 to 100 nM), among which PC3 and OV3 are the most sensitive. | [52][53] |
| Pipestelide A (20) | Pipestela candelabra | Cytotoxicity against KB (IC50 = 0.1 µM) cell. | [54] |
| Neamphamides B-D (21–23) | Neamphius huxleyi | Cytotoxicity against A549, LNCaP and PC3 cells. (IC50 = 91 to 230 nM) | [55][56] |
| Callipeltins N (24) and O (25) | Asteropus sp. | Cytotoxicity against A2058, HT29, MCF7 and MRC-5 cells. (IC50 = 0.16 to 0.21 µM and 0.48 to 2.08 µM, respectively) | [57] |
| Pipecolidepsins A (26) and B (27) | Homophymia lamellosa | Cytotoxicity against A549 (GI50 = 0.6 and 0.04 µM, respectively), HT29 (GI50 = 1.12 and 0.01 µM, respectively) and MDA-MB-231 (GI50 = 0.7 and 0.02 µM, respectively) cells. | [58][59][60][61] |
| Halipeptin D (28) | Leiosella cf. arenifibrosa | Cytotoxicity against HCT116 cell line (IC50 = 7 nM) and BMS ODCP with an average IC50 value of 420 nM. | [62][63] |
| Reniochalistatin E (29) | Reniochalina stalagmitis | Cytotoxicity against RPMI-8226 (IC50 = 4.9 μM) and MGC-803 (IC50 = 9.7 μM) cells. | [64][65] |
| Callyaerin G (30) | Callyspngia aerizusa | Cytotoxicity against L5178Y (ED50 = 4.1 mM) and HeLa (ED50 = 41.8 mM) cells. | [66] |
| Callyaerins E (31) and H (32) | Callyspngia aerizusa | Cytotoxicity against L5178Y (ED50 = 0.39 and 0.48 μM, respectively). | [67] |
| Scleritodermin A (33) | Scleritoderma nodosum | Cytotoxicity against HCT116 (IC50 = 1.9 μM), HCT116/VM46 (IC50 = 5.6 μM), A2780 (IC50 = 0.94 μM) and SKBR3 (IC50 = 0.67 μM) cells. | [68][69][70] |
| Calyxamides A (34) and B (35) | Discodermia calyx | Cytotoxicity against P388 (IC50 = 3.9 and 0.9 μM, respectively) cell. | [71] |
| Keramamide E (36) | Theonella sp. | Cytotoxicity against L1210 (IC50 = 1.42 µM) and KB (IC50 = 1.38 µM) cells. | [72] |
| Keramamides K (37) and L (38) | Theonella sp. | Cytotoxicity against L1210 (IC50 = 0.77 and 0.5 µM, respectively) and KB (IC50 = 0.45 and 0.97 µM, respectively) cells. | [73] |
| Keramamides M (39) and N (40) | Theonella sp. | Cytotoxicity against L1210 (IC50 = 2.02 and 2.33 µM, respectively) and KB (IC50 = 5.05 and 6.23 µM, respectively) cells. | [74][75] |
| Axinellins A (41) and B (42) | Axinella carteri | Cytotoxicity against NSLC-N6 (IC50 = 16.7 and 29.8 µM, respectively) cell. | [76][77] |
| Stylopeptide 2 (43) | Stylotella sp. | Inhibition of 23% BT-549 cell growth and 44% Hs578T cell growth at one dose (10−5 M). | [78] |
| Stylissamide X (44) | Stylissa sp. | Anti-migration effects on HeLa cell. | [79][80] |
| Aciculitins A-C (45–47) | Aciculites orientalis | Cytotoxicity against HCT116 (IC50 = 0.37 µM) cell. | [81] |
| Nazumazoles A-C (48–50) | Theonella swinhoei | Cytotoxicity against P388 (IC50 = 0.83 µM) cell. | [82] |
| Theonellamide G (51) | Theonella swinhoei | Cytotoxicity against HCT116 (IC50 = 6.0 µM) cell. | [83] |
| Koshikamide B (52) | Theonella sp. | Cytotoxicity against HCT116 (IC50 = 3.62 µM) and P388 (IC50 = 0.22 µM) cells. | [84] |
| Anticancer Cyclopeptides Derived from Ascidians/Tunicates | |||
| Mollamide (53) | Ascidian, Didemnum molle | Cytotoxicity against P388 (IC50 = 1.24 µM), A549 (IC50 = 3.1 µM), HT29 (IC50 = 3.1 µM) and CV1 (IC50 = 3.1 µM) cells. | [87][88] |
| Mollamide B (54) | Tunicate, Didemnum molle | Growth inhibition of H460, MCF7 and SF-268 cells. | [89] |
| Trunkamide A (55) | Ascidian, Lissoclinum sp. | Cytotoxicity against A549, P388, HT29 and MEL28 cells. (IC50 = 0.6 to 1.19 µM) | [90][91] |
| Prepatellamide A (56) | Ascidian, Lissoclinum patella | Cytotoxicity against P388 cells. (IC50~6.57 µM) | [92] |
| Vitilevuamide (57) | Ascidian, Didemnum cuculiferum | Cytotoxicity against HCT116 (IC50 = 6.24 nM), A549 (IC50 = 0.12 µM), SKMEL-5 (IC50 = 0.31 µM) and A498 (IC50 = 3.12 µM) cells. | [93][94] |
| Tamandarin A (58) | Ascidian, Didemnidae sp. | Cytotoxicity against BX-PC3 (IC50 = 1.69 nM), DU145 (IC50 = 1.29 nM), UM-SCC-10B (IC50 = 0.94 nM) cells. | [95][96][97] |
| Comoramides A-B (59–60) & Mayotamides A-B (61–62) | Ascidian, Didemnum molle | Cytotoxicity against A549, HT29 and MEL28 cells. (IC50 = 7.22 to 14.97 µM) | [98] |
| Patellin 6 (63) | Ascidian, Lissoclinum patella | Cytotoxicity against P388, A549, HT29 and CV1 cells. (IC50~2.08 µM) | [99] |
| Cycloforskamide (64) | Sea slug, Pleurobranchus forskalii | Cytotoxicity against P388 cell (IC50 = 5.8 µM). | [100] |
| Anticancer Cyclopeptides Derived from Mollusks | |||
| Keenamide A (65) | Mollusk, Pleurobranchus forskali | Anti-proliferative activity against A549, P388, MEL20 and HT29 cells. (IC50 = 4.03 to 8.05 µM) | [103] |
| Aurilide (66) | Sea hare, Dolabella auricularia | Cytotoxicity against HeLa S3 (IC50 = 0.013 µM) cell. Inhibitory activity against ovarian, renal and prostate cancer cells in NCI 60 cell lines. | [104][105] |
| Dolastatin 16 (67) | Sea hare, Dolabella auricularia | Cytotoxicity against H460 (GI50 = 1.09 nM), KM20L2 (GI50 = 1.37 nM), SF-295 (GI50 = 5.92 nM) and SKMEL-5 (GI50 = 3.75 nM) cells. Anti-proliferative effects against 5 human leukemia cell lines | [106] |
| Doliculide (68) | Sea hare, Dolabella auricularia | Cytotoxicity against HeLa S3 (IC50 = 1.62 nM) cell. | [107][108][109][110] |
| Kulokekahilide-1 (69) | Mollusk, Philinopsis speciosa | Cytotoxicity against P388 (IC50 = 2.2 µM) cell. | [111] |
| Kulokekahilide-2 (70) | Mollusk, Philinopsis speciosa | Cytotoxicity against P388 (IC50 = 4.2 nM), SK-OV-3 (IC50 = 7.5 nM), MDA-MB-435 (IC50 = 14.6 nM) and A-10 (IC50 = 59.1 nM) cells. | [112][113][114] |
| Kahalalide R1 (71) | Sea slug, Elysia grandifolia | Cytotoxicity against MCF7 (IC50 = 0.14 µM), L1578Y (IC50 = 4.26 nM) cells. | [117] |
| Anticancer Cyclopeptides Derived from Marine Algae | |||
| Galaxamide (72) | Algae, Galaxaura filamentosa | Inhibitory activity against GRC-1 (IC50 = 7.18 μM) and HepG2 (IC50 = 7.81 μM) cells. | [119][120] |
3. Terrestrial Plant-Derived Anticancer Cyclopeptides
Plants are undeniably an important source for natural products. Nowadays, traditional plant-based medicines are still prevalently used for healthcare and medical treatment around the world. Over 28,000 species of plants have been recorded with differenct therapeutic purposes [121]. Therefore, plants constituents have long been regarded as the mainstream materials for drug discovery. In particular, over one third of current anticancer drugs are derived from plant natural products [122]. In this section, the recently isolated plant-derived cyclopeptides and their anticancer properties are discussed. The structures of these compounds are shown in Figure 5 and their activities are listed in Table 2.
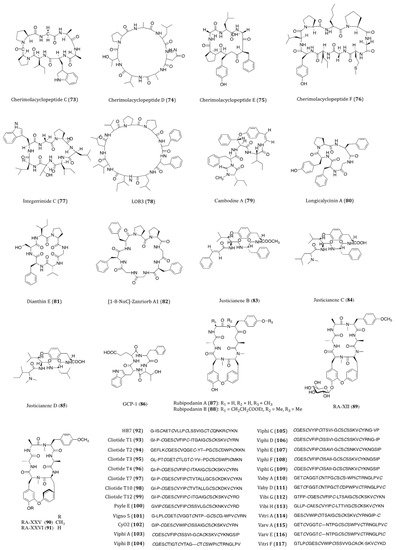
Figure 5. Structures of cyclopeptides (73–117) derived from terrestrial plant.
Cherimolacyclopeptides C-F (73–76) were cyclic heptapeptides obtained from the seeds of Annona cherimola in 2004 and 2005. All four compounds exhibited significant in vitro cytotoxicity against KB cells with IC50 values ranging from 0.017 to 0.97 μM [123][124][125]. Although these compounds showed anticancer potential, no further screening on other cancer cell lines or pharmacological investigations of these congeners have been reported thereafter.
Integerrimide C (77), a novel cycloheptapeptide, has been recently isolated from the latex of Jatropha integerrima. The cytotoxicity bioassay indicated its inhibitory effect against KB cells was associated with an IC50 value of 1.7 μM [126]. The discovery of integerrimide C was actually the continuation of studies on the latex of J. integerrima. Previously reported cyclopeptides integerrimides A and B were also the isolates of this particular species and exhibited moderate inhibitory activities against human melanoma (IPC-298) cell proliferation (up to 40% at 50 μM) and human pancreatic carcinoma (Capan II) cell migration (30% and 20% at 50 μM, respectively) [127].
Linoorbitides (LOBs) are a group of cyclopeptides found in flaxseed oil. Actually, flaxseed and its oil have been considered and utilized as anticancer products for many years [128]. In order to investigate whether flaxseed-derived LOBs are cytotoxic to cancer cells, Denis et al. [129]. tested the cytotoxicity of four cyclopeptides derived from flaxseed against A375 (melanoma), SKBR3 and MCF7 cells. Their study showed that LOB3 (78) possessed the highest in vitro potency among the test compounds, yet the minimum concentration of LOBs in serum had to be reached about 400–500 μg/mL for the delivery of its effectiveness. Such concentration is unlikely to be used as a drug through oral administration in humans. Nevertheless, as topical medication, LOB3 could be a potential agent for the treatment of melanoma and other skin cancers. Although flaxseed is well known for its health benefits against cancer, searching for specific compounds derived from flaxseed with high potency is still under way.
Cambodine A (79) was a novel 14-membered ring cyclopeptide extracted from the root barks of Ziziphus cambodiana. The in vitro bioassay showed that it was moderately cytotoxic against the BC-1 (lymphoma) cells with an IC50 value of 11.1 µM, whilst no toxicity was shown against the non-cancerous Vero cells [130].
The species Dianthus superbus is an important anti-inflammatory and diuretic herb in traditional Chinese medicine. Longicalycinin A (80), a cytotoxic cyclopeptide, was first isolated from the extract of D. superbus several years ago. The natural compound showed a growth inhibitory activity against HepG2 cancer cells with an IC50 value of 22.13 µM [131]. This compound had been successfully synthesized by the solid-phase methodology. When acting against in vitro Dalton’s lymphoma ascites (DLA) and Enrlich’s ascites carcinoma (EAC) cells, CTC50 values were determined to be 2.62 and 6.17 µM, respectively [132]. In the study of Tehrani et al., they demonstrated that the synthesized linear and cyclic disulfide heptapeptides of longicalycinin A showed similar inhibitory effect against HepG2 (IC50 = 16.97 and 16.91 µM, respectively) and HT29 (IC50 = 20.38 and 27.68 µM, respectively) cells. However, considering their vulnerability and proapoptotic actions against normal cells (skin fibroblast cells), the linear disulfide heptapeptides were more tempting for promotion as novel anticancer agents [133]. Although longicalycinin A and its analogs can be totally synthesized, the mechanism of actions as well as the in vivo actions of these compounds are yet to be thoroughly studied.
Similar to longicalycinin A, dianthin E (81) is another cyclic peptide isolated from Dianthus superbus. With the IC50 values of >33.3 µM against Hep3B (human hepatic carcinoma), MCF7, A549 and MDA-MB-231 cells, this compound exhibited a selective in vitro cytotoxic activity against HepG2 cells (IC50 = 3.51 µM) [134].
Orbitides are a group of plant cyclic peptides possessing a signature short chain of 5 to 11 residues, they are biosynthesized by ribosomes and characterized by their N-to-C amide bonds, but not disulfide bonds [135]. [1-8-NαC]-Zanriorb A1 (82) was a novel orbitide isolated from the leaves of Zanthoxylum riedelianum with proapoptotic effects. This compound was found to be cytotoxic against Jurkat leukemia T cells with an IC50 value of 218 nM. Regarding its molecular mechanism, it induced remarkable cell death, which would be partially inhibited by the caspase inhibitor Z-VAD-FMK. Moreover, it also induced apoptosis via reducing the levels of mitochondrial membrane potential (Ψmit) and deactivating caspase-3 [136].
Three novel cyclopeptide alkaloids, justicianenes B-D (83–85) were isolated from the Justicia procumbens L. These three compounds were evaluated their cytotoxicity against human breast cancer MCF-7, cervix carcinoma HeLa, and lung cancer A549 and H460. However, only justicianene D displayed weak cyctotoxicity against MCF-7 cells with IC50 of 90 μM [137].
Seven novel ginseng cyclopeptides (GCPs) were isolated and showed anti-proliferative activities in gastric cancer SGC-7901 cells, in which GCP-1 (86) showed the most potency with IC50 value of 37.8 μM. Regarding its molecular mechanism, it induces apoptosis by activating the caspases and regulating thioredoxin (Trx)-dependent pathways, including signal-regulating kinase 1 (ASK1), mitogen-activated protein kinases (MAPKs)-p38 and JNK pathways [138].
Cyclotides are the macrocyclic cysteine-rich peptides derived from plants, and featured with three disulfide bonds, 28 to 37 amino acids and a head-to-tail cyclized backbone in a knotted arrangement [139]. The typical structure of cyclotides makes them broadly bioactive and remarkably stable against enzymatic and thermal degradation [140].
In traditional Chinese medicine, the roots and rhizomes of Rubia plants have been used for thousands of years to treat menoxenia, contusion, rheumatism and tuberculosis [141]. Rubiaceae-type cyclopeptides (RAs) are referred to as natural cyclopeptides derived from Rubia. Up to now, 56 RAs have been isolated from Rubia plants. Due to their characteristic bicyclic structures and significant anticancer activities, RAs have gained much attention in recent years [142]. Rubipodanin A (87), a novel cyclic hexapeptide obtained from the roots and rhizomes of R. podantha, is the first naturally identified N-desmonomethyl RA. This compound was tested to be cytotoxic against three cancer cell lines including HeLa, A549 and SGC-7901 (human gastric cancer cell), with IC50 values ranging from 3.80 to 7.22 μM. However, when compared to the previously known cyclopeptide RA-V, in which two N-methyl groups are present in its backbone skeleton, the cytotoxicity of rubipodanin A was considered much weaker. In regards to its mechanisms, rubipodanin A exhibited a down-regulating effect on the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, which largely contributed to its anticancer activity [143]. Derived from the same plant species, another new RA with potent cytotoxicity was designated rubipodanin B (88). This compound showed IC50 values of 1.47 μM, 0.69 μM and 3.37 μM in MDA-MB-231, SW620 (human colorectal adenocarcinoma) and HepG2 cells respectively. Nevertheless, the anticancer activity of rubipodanin B was not associated with any down-regulation of the NF-κB signaling pathway [144]. Another cyclopeptide compound from RA family is RA-XII (89), which derived from R. yunnanensis, showed inhibitory effects on tumor growth and metastasis IC50 value of 606 nM and 96 nM on human breast cancer 4T1 cells for 24 and 48 h, respectively [145][146][147]. RA-XII also exerts antitumor activity by supressing autophagy via activating Akt-mTOR and NF-κB pathways in colocrectal cancer SW620 and HT29 cells [148]. RA-XXV (90) and RA-XXVI (91) are two new bicyclic hexapeptides, which isolated from the roots of R. cordifolia L. RA-XXV showed cytotoxic activity against human promyelocytic leukemia HL-60 and human colorectal carcinoma HCT 116 cells with IC50 of 62 nM and 28 nM, respectively. RA-XXVI showed cytotoxic activity agains HL-60 and HCT 116 cells with with IC50 of 66 nM and 51 nM, respectively [149]. To further determine RA compounds underlying mechanism, a wide screening of its molecular targets is desperately needed.
The intensive studies of famous traditional Chinese herb Hedyotis biflora had led to the isolation of HB7 (92), which is considered a potential anticancer cyclotide. By means of MTT assay, HB7 showed in vitro cytotoxicity against four pancreatic cancer cell lines BxPC3, Capan2, MOH-1 and PANC1 with IC50 values of 0.68, 0.45, 0.33 and 0.36 µM, respectively. At a non-toxic concentration of 0.05 µM, HB7 even inhibited cellular migration and invasion of Capan2 cells. When extrapolated to the in vivo xenograft mouse models, this cyclotide was observed to significantly suppress tumor growth without exhibiting any obvious organ injury or toxicity. Based on the results above, the cytotoxicity of HB7 was plausibly related to the different net charges of the cyclotide [150]. Because of its decent anticancer potential, the specific underlying mechanism of action of HB7 deserves further investigation.
Cliotides T1-T4 (93–96) were four novel cyclotides isolated from the tropical plant Clitoria ternatea, belonging to the Fabaceae family. With the prevalent distribution in almost every tissue of C. ternate, the extraction of these four cyclotides is rather efficient. These cyclotides have been confirmed to be heat-stable cysteine-rich peptides. When incubated with HeLa cells, all these four compounds yielded significant cytotoxicity with IC50 values ranging from 0.6 to 8.0 µM. Among the four, T1 and T4 were the most potent ones (IC50 = 0.6 µM) [151]. Cliotides T2 (94), T4 (96), T7 (97), T10 (98) and T12 (99) also exhibited significant cytotoxicity towards A549 cells (IC50 values ranging from 0.21 to 7.59 µM) as well as paclitaxel-resistant A549 cells (IC50 values ranging from 0.45 to 7.92 µM). Intriguingly, the IC50 values of these five test compounds were decreased by two to four folds when incubated with paclitaxel [152]. Nevertheless, the chemosensitizing ability of the cliotides became evidenced in drug-resistant cell lines, which deserves further studies on the aspects of compound charge status and in vivo efficacy. Apart from anticancer potential, cliotides, T1 to T4 in particular, also exhibited notable antimicrobial properties, particularly E. coli [152].
Among the cyclotides isolated from Psychotria leptothyrsa var. longicarpa, psyle E (100) was the most cytotoxic one. The IC50 value of psyle E against human lymphoma cell line U937-GTB was determined to be 0.76 µM. According to the reported SAR assessment, the linear structure of psyle E was required to maintain its potent cytotoxicity [153].
Vigno 5 (101) was a cyclotide discovered in Viola ignobilis [154]. This compound showed its in vitro cytotoxicity against HeLa cells with an IC50 value around 2.5 µM. Due to the complexity of its chemical structure and technical limitation, only the primary structure of vigno 5 had been elucidated so far. In the study of Esmaeili el al., vigno 5 induced apoptosis in a dose-dependent manner accompanied by nuclear shrinkage, DNA fragmentation, caspase activation and the cleavage of PARP. The vigno 5 induced-apoptosis was found to be caspase-dependent, explicitly caspase-3. To the induction of apoptotic events and mitochondrial dysfunction, a decreased level of anti-apoptotic Bcl-2 and an increased level of pro-apoptotic Bax were observed in the vigno 5-treated HeLa cells [155].
Cycloviolacin O2 (CyO2, 102), a potent cytotoxic cyclotide, was first isolated from the plant Viola odorata L. (Violaceae). The presence of glutamic acid in cycloviolacin O2 contributed much to its cytotoxic activity against U937 GTB (human lymphoma) cells with an IC50 value of 0.75 μM [156]. Besides, CyO2 also exhibited concentration-dependent cytotoxicities towards CCRF-CEM (leukemia cell line), NCI-H69 (small cell lung cancer) and HT29 cells with IC50 values of 0.3, 1.2 and 5.3 μM, respectively [157]. Disruption of the U937 cell membranes by CyO2 further indicated its membrane-disrupting activity while exerting cytotoxicity [158]. Gerlach et al. even demonstrated that CyO2 induced pore formation specifically in highly proliferating tumor cells [159]. In addition, CyO2 was also reported to exhibit bioactivities against gram-negative bacteria and HIV-1 virus via multipleregulatory mechanisms [153][160]. However, another study on the evaluation of toxicity and antitumor activity of CyO2 in mice showed that its antitumor effects were little or even absent at sublethal doses. It is worth noting that quick lethality of CyO2 was observed at 2 mg/kg whilst no abnormal signs were seen at the dose of 1.5 mg/kg in the experimental mice [157]. Thus, there is a great disparity between the results of the in vitro and in vivo experiments. For further investigation of this cyclotide, problems such as low in vivo efficacy need to be addressed.
Characterized by the macrocyclic backbones, cyclotides viphis A-G (103–109) were isolated from Viola philippica. These eight compounds were cytotoxic to human melanoma (MM96L), HeLa and human gastric adenocarcinoma (BGC-823) cells and human normal fibroblast cells (HFF-1). When applied at 3.24 and 3.17 µM, viphis D-E showed no activity against BGC-823 cell; however all these eight cyclotides displayed cytotoxicities against many cancer cell lines with IC50 values ranging from 1.03 to 15.5 µM. From the results of SAR assessments, hydrophobicity and glutamic acid residue of loop 1 are suggested of great importance for their cytotoxic activities. Further biochemical assays revealed that these viphis could be influenced by minimal sequential changes for alterations of their bioactivities [161].
Vabys A (110) and D (111) were two cyclotides derived from Viola abyssinica, a plant growing at an altitude over 3400 m. Both compounds contain charged residues; however different net charges lead to different bioactivities. Vabys A and D exhibited cytotoxicities against U-937 lymphoma cells in a dose-dependent manner, with IC50 values of 2.6 and 7.6 µM, respectively [162].
Isolated from the alpine violet Viola biflora, vibis G (112) and H (113) were two bracelet cyclotides. These two compounds showed similar cytotoxic potency (IC50 = 0.96 and 1.6 µM, respectively) against lymphoma U-937 GTB cells [163].
A bioactivity-guided fractionation of Viola tricolor led to the isolation of a cluster of highly similar cyclotides, among which vitri A (114), varv A (115) and varv E (116) were found to be cytotoxic agents. Two human cancer cell lines, U-937 GTB (IC50 = 0.6, 6 and 4 µM, respectively) and myeloma RPMI-8226/s (IC50 = 1, 3 and 4 µM, respectively) were used in the cytotoxicity assays. Sequence determination and SAR analysis demonstrated that differences in the net charges and cationic amino acid residues in these cyclotides are extremely crucial for their cytotoxicities [164]. Assessment of the cytotoxic activities against human malignant glioblastoma (U251), MDA-MB-231, A549, DU145 and human hepatoma (BEL7402) cell lines showed the inhibitory activities of vitri A (114) with IC50 values ranging from 0.97 to 1.91 µM and vitri F (117) with IC50 values ranging from 0.85 to 1.97 µM. Importantly, the distribution of highly hydrophobic residues on the surface was highlighted for the cytotoxic activities of these cyclotides [165]. Undeniably, plant-derived cyclotides are the intriguing candidates for drug development as anticancer therapeutics. Owing to the abundant source of cyclotides, V. tricolor warrants further studies for the identification of new bioactive compounds and their anticancer mechanism of actions.
Table 2. Terrestrial plant-derived anticancer cyclopeptides.
|
Name |
Biological Source |
Anticancer Activity |
Reference |
|---|---|---|---|
|
Cherimolacyclopeptides C-F (73–76) |
Seeds of Annona cherimola |
Cytotoxicity against KB cell. (IC50 = 0.017 to 0.97 µM) |
|
|
Integerrimide C (77) |
Latex of Jatropha integerrima |
Cytotoxicity against KB (IC50 = 1.7 µM) cell. |
[126] |
|
LOB3 (78) |
Flaxseed oil |
Cytotoxicty against A375, SKBR3 and MCF7 cells. |
|
|
Cambodine A (79) |
Root bark of Ziziphus cambodiana |
Cytotoxicity against BC-1 (IC50 = 11.1 µM) cell. |
[130] |
|
Longicalycinin A (80) |
Dianthus superbus |
Growth inhibitory activity against HepG2 (IC50 = 22.13 μM) cell. |
|
|
Dianthin E (81) |
Dianthus superbus |
Cytotoxicity against HepG2 (IC50 = 3.51 μM) cell. |
[134] |
|
[1-8-NαC]-Zanriorb A1 (82) |
Leaves of Zanthoxylum riedelianum |
Cytotoxicity against Jurkat leukemia T cell (IC50 = 218 nM). |
[136] |
|
Justicianenes B-D (83–85) |
Justicia procumbens L. |
Justicianene D displayed cytotoxicity against MCF-7 cells (IC50 = 90 μM). |
[137] |
|
GCP-1 (86) |
Ginseng |
Cytotoxicity against SGC-7901 cells (IC50 = 37.8 μM). |
[138] |
|
Rubipodanin A (87) |
Roots and rhizomes of Rubia podantha |
Cytotoxicity against HeLa, A549 and SGC-7901 cells. (IC50 = 3.80 to 7.22 μM) |
[143] |
|
Rubipodanin B (88) |
Rubia podantha |
Cytotoxicity against MDA-MB-231 (IC50 = 1.47 μM), SW620 (IC50 = 0.69 μM) and HepG2 (IC50 = 3.37 μM) cells. |
[144] |
|
RA-XII (89) |
Rubia yunnanensis |
Inhibitory effects on 4T1 ((IC50 = 606 nM and 96 nM for 24 and 48 h, respectively), SW260 and HT29 cells. |
|
|
RA-XXV (90) |
Roots of Rubia cordifolia L. |
Cytotoxicity against HL-60 ((IC50 = 62 nM) and HCT116 cells ((IC50 = 28 nM). |
[149] |
|
RA-XXVI (91) |
Roots of Rubia cordifolia L. |
Cytotoxicity against HL-60 ((IC50 = 66 nM) and HCT116 cells ((IC50 = 51 nM). |
[149] |
|
HB7 (92) |
Hedyotis biflora |
Cytotoxicity against BxPC3 (IC50 = 0.68 μM), Capan2 (IC50 = 0.45 μM), MOH-1 (IC50 = 0.33 μM) and PANC1 (IC50 = 0.36 μM) cells. |
[150] |
|
Cliotides T1-T4 (93–96), cliotide T7 (97), cliotide T10 (98), cliotide T12 (99) |
Clitoria ternatea |
Cliotides T1-T4: cytotoxicity against HeLa (IC50 = 0.6 to 8.0 μM) cell. Cliotides T2, T4, T7, T10 and T12: cytotoxicity against A549 (IC50 = 0.21 to 7.59 μM) and A549/paclitaxel (IC50 = 0.45 to 7.92 μM) cells. |
|
|
psyle E (100) |
Psychotria leptothyrsa var. longicarpa |
Cytotoxicity against U937-GTB IC50 = 0.76 μM) cell. |
[153] |
|
Vigno 5 (101) |
Viola ignobilis |
Pro-apoptotic activity on HeLa cell. |
|
|
Cycloviolacin O2 (CyO2) (102) |
Viola odorata L. |
Cytotoxicity against U937 GTB (IC50 = 0.75 μM), CCRF-CEM, NCI-H69 and HT29 cells. |
|
|
Viphi A-G (103–109) |
Viola philippica |
Cytotoxicity against MM96L, HeLa, BGC-823, HFF-1 cells, but viphi D and E showed no activity against BGC-823 cell. (IC50 = 1.03 to 7.92 μM) |
[161] |
|
Vaby A (110) and D (111) |
Viola abyssinica |
Cytotoxicity against U-937 (IC50 = 2.6 and 7.6 μM, respectively) cell. |
[162] |
|
Vibi G (112) and H (113) |
Viola biflora |
Cytotoxicity against U-937 GTB (IC50 = 0.96 and 1.6 μM, respectively) cell. |
[163] |
|
Vitri A (114) |
Viola tricolor |
Cytotoxicity against U-937 GTB (IC50 = 0.6 µM) and RPMI-8226/s (IC50 = 1 µM) cells. Cytotoxicity against U251, MDA-MB-231, A549, DU145 and BEL7402 cells (IC50 = 3.07 to 6.03 μM). |
|
|
Varv A (115) and E (116) |
Viola tricolor |
Cytotoxicity against U-937 GTB (IC50 = 6 and 4 µM, respectively) and RPMI-8226/s (IC50 = 3 and 4 µM, respectively) cells. |
[164] |
|
Vitri F (117) |
Viola tricolor |
Cytotoxicity against U251, MDA-MB-231, A549, DU145 and BEL7402 cel ls (IC50 = 2.74 to 6.31 μM) |
[165] |
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-tieulent, J.; Jemal, A. Global Cancer Statistics, 2012, CA a Cancer. J. Clin. 2015, 65, 87–108.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- American Cancer Society. Global Cancer Facts & Figures 4th Edition. Atlanta: American Cancer Society. 2018. Available online: (accessed on 12 April 2021).
- Appold, K. Cancer Treatment—Top 4 Developments to Watch. 2017. Available online: (accessed on 12 April 2021).
- Kang, T.H.; Mao, C.P.; He, L.; Tsai, Y.C.; Liu, K.; La, V.; Wu, T.C.; Hung, C.F. Tumor-targeted delivery of IL-2 by NKG2D leads to accumulation of antigen-specific CD8+ T cells in the tumor loci and enhanced anti-tumor effects. PLoS ONE 2012, 7, e35141.
- Amit, D.; Tamir, S.; Hochberg, A. Development of targeted therapy for a broad spectrum of solid tumors mediated by a double promoter plasmid expressing diphtheria toxin under the control of IGF2-P4 and IGF2-P3 regulatory sequences. Int. J. Clin. Exp. Med. 2013, 6, 110–118.
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. NanoTechnol. 2007, 2, 751–760.
- Pawar, S.V.; Ho, J.C.H.; Yadav, G.D.; Yadav, V.G. The impending renaissance in discovery & development of natural products. Curr. Top. Med. Chem. 2017, 251–267.
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153.
- Joo, S.H. Cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther. 2012, 20, 19–26.
- Wipf, P. Synthetic studies of biologically active marine cyclopeptides. Chem. Rev. 1995, 95, 2115–2134.
- Pomilio, A.; Battista, M.; Vitale, A. Naturally-occurring cyclopeptides: Structures and bioactivity. Curr. Org. Chem. 2006, 10, 2075–2121.
- Tran, D.; Selsted, M.E.; Dorrestein, P.C.; Pavel, A. Cycloquest: Identification of cyclopeptides via database search of their mass spectra against genome databases. NIH Public Access. 2012, 10, 4505–4512.
- Tan, N.H.; Zhou, J. Plant cyclopeptides. Chem. Rev. 2006, 106, 840–895.
- Marahiel, M.A.; Stachelhaus, T.; Mootz, H.D. Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem. Rev. 1997, 97, 2651–2674.
- Hur, G.H.; Vickery, C.R.; Burkart, M.D. Explorations of catalytic domains in non-ribosomal peptide synthetase enzymology. Nat. Prod. Rep. 2012, 29, 1074–1098.
- Salomon, R.A.; Farias, R.N. Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J. Bacteriol. 1992.
- Luckett, S.; Garcia, R.S.; Barker, J.J.; Konarev, A.V.; Shewry, P.R.; Clarke, A.R.; Brady, R.L. High-resolution structure of a potent, cyclic proteinase inhibitor from sunflower seeds. J. Mol. Biol. 1999, 290, 525–533.
- Tang, Y.Q.; Yuan, J.; Ösapay, G.; Ösapay, K.; Tran, D.; Miller, C.J.; Ouellette, A.J.; Selsted, M.E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated α-defensins. Science 1999, 286, 498–502.
- Closse, A.; Huguenin, R. Isolierung und Strukturaufklärung von Chlamydocin. Helv. Chim. Acta 1974, 57, 533–545.
- Mollica, A.; Costante, R.; Stefanucci, A.; Novellino, E. Cyclotides: A natural combinatorial peptide library or a bioactive sequence player? J. Enzym. Inhib. Med. Chem. 2015, 30, 575–580.
- Edman, P. Chemistry of amino acids and peptides. Annu. Rev. Biochem. 1959, 28, 69–96.
- Trabi, M.; Craik, D.J. Circular proteins-No end in sight. Trends Biochem. Sci. 2002, 27, 132–138.
- Rezai, T.; Yu, B.; Millhauser, G.L.; Jacobson, M.P.; Lokey, R.S. Testing the conformational hypothesis of passive membrane permeability using synthetic cyclic peptide diastereomers. J. Am. Chem. Soc. 2006, 128, 2510–2511.
- Áron, R. Towards Targeted Photodynamic Therapy: Synthesis and Characterization of Aziridine Aldehyde-Cyclized Cancer-Targeting Peptides and Bacteriochlorin Photosensitizers. Ph.D. Thesis, University of Toronto, Toronto, ON, Canada, 2014.
- Malaker, A.; Ahmad, S.A.I. Therapeutic potency of anticancer peptides derived from marine organism. Int. J. Eng. Appl. Sci. 2013, 2, 53–65.
- Liu, J.; Gu, B.; Yang, L.; Yang, F.; Lin, H.; Yang, F. New anti-inflammatory cyclopeptides from a sponge-derived fungus Aspergillus violaceofuscus. Front. Chem. 2018, 6, 1–8.
- Desriac, F.; Jé, C.; Balnois, E.; Brillet, B.; le Chevalier, P. Antimicrobial peptides from marine proteobacteria. Mar. Drugs 2013, 11, 3632–3660.
- Wu, W.; Zhen, Z.; Niu, T.; Zhu, X.; Gao, Y.; Yan, J.; Chen, Y.; Yan, X.; Chen, H. κ-Carrageenan enhances lipopolysaccharide-induced interleukin-8 secretion by stimulating the Bcl10-NF-κB pathway in HT-29 cells and aggravates C. freundii-Induced inflammation in mice. Mediat. Inflamm. 2017, 2017, 8634865.
- Randazzo, A.; Bifulco, G.; Giannini, C.; Bucci, M.; Cirino, G.; Gomez-paloma, L.; Ponte, V.; Salerno, F.; Ii, F.; Montesano, V.D.; et al. Halipeptins A and B: Two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J. Am. Chem. Soc. 2001, 123, 10870–10876.
- Xing, H.; Tong, M.; Jiang, N.; Zhang, X.; Hu, H.; Pan, H.; Li, D. Antitumour bioactive peptides isolated from marine organisms. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1077–1082.
- Laport, M.; Santos, O.; Muricy, G. Marine sponges: Potential sources of new antimicrobial drugs. Curr. Pharm. BioTechnol. 2009, 10, 86–105.
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine Sponge derived natural products between 2001 and 2010: Trends and opportunities for discovery of bioactives. Mar. Drugs 2014, 12, 4539–4577.
- Kang, H.K.; Choi, M.C.; Seo, C.H.; Park, Y. Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int. J. Mol. Sci. 2018, 19, 919.
- Nakao, Y.; Yoshida, S.; Matsunaga, S.; Shindoh, N.; Terada, Y.; Nagai, K.; Yamashita, J.K.; Ganesan, A.; van Soest, R.W.M.; Fusetani, N. Azumamides A-E: Histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge Mycale izuensis. Angew. Chem. Int. Ed. 2006, 45, 7553–7557.
- Sriraksa, R.; Limpaiboon, T. Histone deacetylases and their inhibitors as potential therapeutic drugs for cholangiocarcinoma-cell line findings. Asian Pac. J. Cancer Prev. 2013, 14, 2503–2508.
- Abdalla, M.A. Medicinal significance of naturally occurring cyclotetrapeptides. J. Nat. Med. 2016, 70, 708–720.
- Maulucci, N.; Chini, M.G.; di Micco, S.; Izzo, I.; Cafaro, E.; Russo, A.; Gallinari, P.; Paolini, C.; Nardi, M.C.; Casapullo, A.; et al. Molecular insights into azumamide E histone deacetylases inhibitory activity. J. Am. Chem. Soc. 2007, 129, 3007–3012.
- Wen, S.; Carey, K.L.; Nakao, Y.; Fusetani, N.; Packham, G.; Ganesan, A. Total synthesis of azumamide A and azumamide E, evaluation as histone deacetylase inhibitors, and design of a more potent analogue. Org. Lett. 2007, 9, 1105–1108.
- Zhang, X.; Jacob, M.R.; Rao, R.R.; Wang, Y.H.; Agarwal, A.K.; Newman, D.J.; Khan, I.A.; Clark, A.M.; Li, X.C. Antifungal cyclic peptides from the marine sponge Microscleroderma herdmani [Corrigendum]. Res. Rep. Med. Chem. 2013, 3, 9–10.
- Bewley, C.A.; Detritus, C.; Faulkner, D.J. Microsclerodermins A and B. Antifungal cyclic peptides from the lithistid sponge Microscleroderma sp. J. Am. Chem. Soc. 1994, 116, 7631–7636.
- Guzmán, E.A.; Maers, K.; Roberts, J.; Kemami-Wangun, H.V.; Harmody, D.; Wright, A.E. The marine natural product microsclerodermin A is a novel inhibitor of the nuclear factor kappa B and induces apoptosis in pancreatic cancer cells. Investig. New Drugs. 2015, 33, 86–94.
- Kehraus, S.; Ko, G.M.; Wright, A.D.; Bonn, D.; Woerheide, G.; Reef, B. Leucamide A: A new cytotoxic heptapeptide from the Australian sponge Leucetta microraphis leucamide. J. Org. Chem. 2002, 67, 4989–4992.
- Wang, W.; Nan, F. First total synthesis of leucamide A. J. Org. Chem. 2003, 68, 1636–1639.
- Afifi, A.H.; El-Desoky, A.H.; Kato, H.; Mangindaan, R.E.P.; de Voogd, N.J.; Ammar, N.M.; Hifnawy, M.S.; Tsukamoto, S. Carteritins A and B, cyclic heptapeptides from the marine sponge Stylissa carteri. Tetrahedron Lett. 2016, 57, 1285–1288.
- Sun, J.; Cheng, W.; de Voogd, N.J.; Proksch, P.; Lin, W. Stylissatins B–D, cycloheptapeptides from the marine sponge Stylissa massa. Tetrahedron Lett. 2016, 57, 4288–4292.
- Bonnington, L.S.; Tanaka, J.; Higa, T.; Kimura, J.; Yoshimura, Y.; Nakao, Y.; Yoshida, W.Y.; Scheuer, P.J. Cupolamide A: A cytotoxic cyclic heptapeptide from two samples of the sponge Theonella cupola. J. Org. Chem. 2002, 62, 7765–7767.
- Rangel, M.; Konno, K.; Brunaldi, K.; Procopio, J.; de Freitas, J.C. Neurotoxic activity induced by a haemolytic substance in the extract of the marine sponge Geodia corticostylifera. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 207–215.
- Rangel, M.; Prado, M.P.; Konno, K.; Naoki, H.; Freitas, J.C.; Machado-Santelli, G.M. Cytoskeleton alterations induced by Geodia corticostylifera depsipeptides in breast cancer cells. Peptides 2006, 27, 2047–2057.
- Freitas, V.M.; Rangel, M.; Bisson, L.F.; Jaeger, R.G.; Machado-Santelli, G.M. The geodiamolide H, derived from Brazilian sponge Geodia corticostylifera, regulates actin cytoskeleton, migration and invasion of breast cancer cells cultured in three-dimensional environment. J. Cell. Physiol. 2008, 216, 583–594.
- Ratnayake, A.S.; Bugni, T.S.; Feng, X.; Harper, M.K.; Skalicky, J.J.; Mohammed, K.A.; Andjelic, C.D.; Barrows, L.R.; Ireland, C.M. Theopapuamide, a cyclic depsipeptide from a Papua New Guinea lithistid sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 1582–1586.
- Zampella, A.; Sepe, V.; Bellotta, F.; Luciano, P.; D’Auria, M.V.; Cresteil, T.; Debitus, C.; Petek, S.; Poupat, C.; Ahond, A. Homophymines B-E and A1-E1, a family of bioactive cyclodepsipeptides from the sponge Homophymia sp. Org. Biomol. Chem. 2009, 7, 4037–4044.
- Zampella, A.; Sepe, V.; Luciano, P.; Bellotta, F.; Monti, M.C.; D’Auria, M.V.; Jepsen, T.; Petek, S.; Adeline, M.T.; Laprévôte, O.; et al. Homophymine A, an anti-HIV cyclodepsipeptide from the sponge Homophymia sp. J. Org. Chem. 2008, 73, 5319–5327.
- Sorres, J.; Martin, M.T.; Petek, S.; Levaique, H.; Cresteil, T.; Ramos, S.; Thoison, O.; Debitus, C.; Al-Mourabit, A. Pipestelides A–C: Cyclodepsipeptides from the pacific marine sponge Pipestela candelabra. J. Nat. Prod. 2012, 75, 759–763.
- Tran, T.D.; Pham, N.B.; Fechner, G.; Zencak, D.; Vu, H.T.; Hooper, J.N.A.; Quinn, R.J. Cytotoxic cyclic depsipeptides from the Australian marine sponge Neamphius huxleyi. J. Nat. Prod. 2012, 75, 2200–2208.
- Yamano, Y.; Arai, M.; Kobayashi, M. Neamphamide B, new cyclic depsipeptide, as an anti-dormant mycobacterial substance from a Japanese marine sponge of Neamphius sp. Bioorganic Med. Chem. Lett. 2012, 22, 4877–4881.
- Stierhof, M.; Hansen, K.Ø.; Sharma, M.; Feussner, K.; Subko, K.; Díaz-Rullo, F.F.; Isaksson, J.; Pérez-Victoria, I.; Clarke, D.; Hansen, E.; et al. New cytotoxic callipeltins from the Solomon Island marine sponge Asteropus sp. Tetrahedron 2016, 72, 6929–6934.
- Coello, L.; Reyes, F.; Martín, M.J.; Cuevas, C.; Fernández, R. Isolation and structures of pipecolidepsins A and B, cytotoxic cyclic depsipeptides from the madagascan sponge Homophymia lamellosa. J. Nat. Prod. 2014, 77, 298–303.
- Gimeno, M.P. Synthesis, Structural Elucidation and Biological Evaluation of Pipecolidepsin A and Synthesis, Structural Elucidation and Biological Evaluation of Pipecolidepsin A and Phakellistatin 19. Ph.D. Thesis, University of Barcelona, Barcelona, Spain, 2013.
- Molina-Guijarro, J.M.; Moneo, V.; Martinez-Leal, J.F.; Cuevas, C.; Garcia-Fernandez, L.F.; Galmarini, C.M. Pipecolidepsin A, stellatolide A and irvalec: New cyclodepsipeptides of marine origin with antitumor activity. Eur. J. Cancer 2014, 50, 24.
- Pelay-Gimeno, M.; García-Ramos, Y.; Martin, M.J.; Spengler, J.; Molina-Guijarro, J.M.; Munt, S.; Francesch, A.M.; Cuevas, C.; Tulla-Puche, J.; Albericio, F. The first total synthesis of the cyclodepsipeptide pipecolidepsin A. Nat. Commun. 2013, 4, 1–10.
- Nicolaou, K.C.; Schlawe, D.; Kim, D.W.; Longbottom, D.A.; de Noronha, R.G.; Lizos, D.E.; Manam, R.R.; Faulkner, D.J. Total synthesis of halipeptins: Isolation of halipeptin D and synthesis of oxazoline halipeptin analogues. Chem. A Eur. J. 2005, 11, 6198–6211.
- Nicolaou, K.C.; Lizos, D.E.; Kim, D.W.; Schlawe, D.; de Noronha, R.G.; Longbottom, D.A.; Rodriquez, M.; Bucci, M.; Cirino, G. Total synthesis and biological evaluation of halipeptins A and D and analogues. J. Am. Chem. Soc. 2006, 128, 4460–4470.
- Zhan, K.; Jiao, W.; Yang, F.; Li, J.; Wang, S.; Li, Y.; Han, B.; Lin, H. Reniochalistatins A−E, cyclic peptides from the marine sponge Reniochalina stalagmitis. J. Nat. Prod. 2014, 77, 2678–2684.
- Fatino, A.; Baca, G.; Weeramange, C.; Rafferty, R.J. Total synthesis of reniochalistatin E. J. Nat. Prod. 2017, 80, 3234–3240.
- Ibrahim, S.R.M.; Edrada-Ebel, R.A.; Mohamed, G.A.; Youssef, D.T.A.; Wray, V.; Proksch, P. Callyaerin G, a new cytotoxic cyclic peptide from the marine sponge Callyspongia aerizusa. Arkivoc 2008, 12, 164–171.
- Ibrahim, S.R.M.; Min, C.C.; Teuscher, F.; Ebel, R.; Kakoschke, C.; Lin, W.; Wray, V.; Edrada-Ebel, R.; Proksch, P. Callyaerins A-F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorganic Med. Chem. 2010, 18, 4947–4956.
- Schmidt, E.W.; Raventos-Suarez, C.; Bifano, M.; Menendez, A.T.; Fairchild, C.R.; Faulkner, D.J. Scleritodermin A, a cytotoxic cyclic peptide from the Lithistid sponge Scleritoderma nodosum. J. Nat. Prod. 2004, 67, 475–478.
- Sellanes, D.; Manta, E.; Serra, G. Toward the total synthesis of Scleritodermin A: Preparation of the C1-N15 fragment. Tetrahedron Lett. 2007, 48, 1827–1830.
- Liu, S.; Cui, Y.M.; Nan, F.J. Total synthesis of the originally proposed and revised structures of scleritodermin A. Org. Lett. 2008, 10, 3765–3768.
- Kimura, M.; Wakimoto, T.; Egami, Y.; Tan, K.C.; Ise, Y.; Abe, I. Calyxamides A and B, cytotoxic cyclic peptides from the marine sponge Discodermia calyx. J. Nat. Prod. 2012, 75, 290–294.
- Kobayashi, J.; Itagaki, F.; Shigemori, I.; Takao, T.; Shimonishi, Y. Keramamides E, G, H, and J, new cyclic peptides containing an oxazole or a thiazole ring from a Theonella sponge. Tetrahedron 1995, 51, 2525–2532.
- Uemoto, H.; Yahiro, Y.; Shigemori, H.; Tsuda, M.; Takao, T.; Shimonishi, Y.; Kobayashi, J. Keramamides K and L, new cyclic peptides containing unusual tryptophan residue from Theonella sponge. Tetrahedron 1998, 54, 6719–6724.
- Tsuda, M.; Ishiyama, H.; Masuko, K.; Takao, T.; Shimonishi, Y.; Kobayashi, J. Keramamides M and N, two new cyclic peptides with a sulfate ester from Theonella sponge. Tetrahedron 1999, 55, 12543–12548.
- Junk, L.; Kazmaier, U. Total Synthesis of Keramamides A and L from a common precursor by late-stage indole synthesis and configurational revision. Angew. Chem. Int. Ed. 2018, 57, 11432–11435.
- Randazzo, A.; Dal, F.; Orru, S.; Gomez-paloma, L. Axinellins A and B: New proline-containing antiproliferative cyclopeptides from the Vanuatu sponge Axinella carteri. Eur. J. Org. Chem. 1998, 3, 2659–2665.
- Fairweather, K.A.; Sayyadi, N.; Roussakis, C.; Jolliffe, K.A. Synthesis of the cyclic heptapeptide axinellin A. Tetrahedron 2010, 66, 935–939.
- Brennan, M.R.; Costello, C.E.; Maleknia, S.D.; Pettit, G.R.; Erickson, K.L. Stylopeptide 2, a proline-rich cyclodecapeptide from the sponge Stylotella sp. J. Nat. Prod. 2008, 71, 453–456.
- Arai, M.; Yamano, Y.; Fujita, M.; Setiawan, A.; Kobayashi, M. Stylissamide X, a new proline-rich cyclic octapeptide as an inhibitor of cell migration, from an Indonesian marine sponge of Stylissa sp. Bioorganic Med. Chem. Lett. 2012, 22, 1818–1821.
- Huang, T.; Zou, Y.; Wu, M.C.; Zhao, Q.J.; Hu, H.G. Total synthesis of proline-rich cyclic octapeptide stylissamide X. Chem. Nat. Compd. 2015, 51, 523–526.
- Bewley, C.A.; He, H.; Williams, D.H.; Faulkner, D.J. Aciculitins A–C: Cytotoxic and antifungal cyclic peptides from the lithistid sponge Aciculites orientalis. J. Am. Chem. Soc. 1996, 118, 4314–4321.
- Fukuhara, K.; Takada, K.; Okada, S.; Matsunaga, S. Nazumazoles A-C, cyclic pentapeptides dimerized through a disulfide bond from the marine sponge Theonella swinhoei. Org. Lett. 2015, 17, 2646–2648.
- Youssef, D.T.A.; Shaala, L.A.; Mohamed, G.A.; Badr, J.M.; Bamanie, F.H.; Ibrahim, S.R.M. Theonellamide G, a potent antifungal and cytotoxic bicyclic glycopeptide from the red sea marine sponge Theonella swinhoei. Mar. Drugs 2014, 12, 1911–1923.
- Araki, T.; Matsunaga, S.; Nakao, Y.; Furihata, K.; West, L.; Faulkner, D.J.; Fusetani, N. Koshikamide B, a cytotoxic peptide lactone from a marine sponge Theonella sp. J. Org. Chem. 2008, 73, 7889–7894.
- Shenkar, N.; Swalla, B.J. Global diversity of Ascidiacea. PLoS ONE 2011, 6, e20657.
- Marino, A.; Rajendran, N.M. Natural products diversity of marine ascidians (Tunicates; Ascidiacea) and successful drugs in clinical development. Nat. Prod. Bioprospect 2017, 7, 1–111.
- Hockless, D.C.R.; Skelton, B.W.; White, A.H. Studies of australian ascidians. IV. mollamide, a cytotoxic cyclic heptapeptide from the compound ascidian. Aust. J. Chem. 1994, 47, 61–69.
- McKeever, B.; Pattenden, G. Total synthesis of the cytotoxic cyclopeptide mollamide, isolated from the sea squirt Didemnum molle. Tetrahedron 2003, 59, 2701–2712.
- Donia, M.S.; Wang, B.; Dunbar, D.C.; Desai, P.V.; Patny, A.; Avery, M.; Hamann, M.T. Mollamides B and C, cyclic hexapeptides from the indonesian tunicate Didemnum molle. J. Nat. Prod. 2008, 71, 941–945.
- McKeever, B.; Pattenden, G. Total synthesis of trunkamide A, a novel thiazoline-based prenylated cyclopeptide metabolite from Lissoclinum sp. Tetrahedron 2003, 59, 2713–2727.
- Bowden, B.F.; Garcia, G.D. A Cyclic Hepta-Peptide Derivative from Colonial Ascidians, Lissoclinum sp. European Patent EP0894092B1, 3 April 2002.
- Fu, X.; Su, J.; Zeng, L. Prepatellamide A, a new cyclic peptide from the ascidian Lissoclinum patella. Sci. China Ser. B Chem. 2008, 43, 643–648.
- Fernandez, A.M. Isolation and Characterization of Vitilevuamide from the Ascidians Didemnum cuculliferum and Polysyncraton lithostrotum. Ph.D. Thesis, The University of Utah, Salt Lake City, UT, USA, 1996.
- Edler, M.C.; Fernandez, A.M.; Lassota, P.; Ireland, C.M.; Barrows, L.R. Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the vinca alkaloids, and dolastatin 10. Biochem. Pharmacol. 2002, 63, 707–715.
- Pangestuti, R.; Kim, S.K. Bioactive peptide of marine origin for the prevention and treatment of non-communicable diseases. Mar. Drugs 2017, 15, 67.
- Vervoort, H.; Fenical, W.; Epifanio, R.D.A. Tamandarins A and B: New cytotoxic depsipeptides from a Brazilian ascidian of the family Didemnidae. J. Org. Chem. 2000, 65, 782–792.
- Liang, B.; Richard, D.J.; Portonovo, P.S.; Joullie, M.M. Total Syntheses and biological investigations of tamandarins A and B and tamandarin A analogs. J. Am. Chem. Soc. 2001, 123, 4469–4474.
- Rudi, A.; Aknin, M.; Gaydou, E.M.; Kashman, Y. Four new cytotoxic cyclic hexa- and heptapeptides from the marine ascidian Didemnum molle. Tetrahedron 1998, 54, 13203–13210.
- Carroll, A.; Coll, J.; Bourne, D.; Macleod, J.; Zabriskie, T.; Ireland, C.; Bowden, B. Patellins 1–6 and trunkamide A: Novel cyclic hexa-, hepta- and octa-peptides from colonial ascidians, Lissoclinum sp. Aust. J. Chem. 1996, 49, 659–667.
- Tan, K.C.; Wakimoto, T.; Takada, K.; Ohtsuki, T.; Uchiyama, N.; Goda, Y.; Abe, I. Cycloforskamide, a cytotoxic macrocyclic peptide from the sea slug Pleurobranchus forskalii. J. Nat. Prod. 2013, 76, 1388–1391.
- Rosenberg, G. A New critical estimate of named species-level diversity of the recent Mollusca. Am. Malacol. Bull. 2014, 32, 308–322.
- Chakraborty, S.; Ghosh, U. Oceans: A store house of drugs-a review. J. Pharm. Res. 2010, 3, 1293–1296.
- Wesson, K.J.; Hamann, M.T. Keenamide A, a bioactive cyclic peptide from the marine mollusk Pleurobranchus forskalii. J. Nat. Prod. 1996, 59, 629–631.
- Suenaga, K.; Mutou, T.; Shibata, T.; Itoh, T.; Fujita, T.; Takada, N.; Hayamizu, K.; Takagi, M.; Irifune, T. Aurilide, a cytotoxic depsipeptide from the sea hare Dolabella auricularia: Isolation, structure determination, synthesis, and biological activity. Tetrahedron 2004, 60, 8509–8527.
- Sato, S.I.; Murata, A.; Orihara, T.; Shirakawa, T.; Suenaga, K.; Kigoshi, H.; Uesugi, M. Marine natural product aurilide activates the opa1-mediated apoptosis by binding to prohibitin. Chem. Biol. 2011, 18, 131–139.
- Pettit, G.R.; Xu, J.P.; Hogan, F.; Williams, M.D.; Doubek, D.L.; Schmidt, J.M.; Cerny, R.L.; Boyd, M.R. Isolation and structure of the human cancer cell growth inhibitory cyclodepsipeptide dolastatin 16. J. Nat. Prod. 1997, 60, 752–754.
- Ishiwata, H.; Nemoto, T.; Ojika, M.; Yamada, K. Isolation and stereostructure of doliculide, a cytotoxic cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 1994, 59, 4710–4711.
- Ishiwata, H.; Sone, H.; Kigoshi, H.; Yamada, K. Total synthesis of doliculide, a potent cytotoxic cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 1994, 59, 4712–4713.
- Matcha, K.; Madduri, A.V.R.; Roy, S.; Ziegler, S.; Waldmann, H.; Hirsch, A.K.H.; Minnaard, A.J. Total synthesis of (-)-doliculide, structure-activity relationship studies and its binding to F-actin. ChemBioChem 2012, 13, 2537–2548.
- Foerster, F.; Braig, S.; Chen, T.; Altmann, K.H.; Vollmar, A.M. Pharmacological characterization of actin-binding (-)-doliculide. Bioorganic Med. Chem. 2015, 22, 5117–5122.
- Kimura, J.; Takada, Y.; Inayoshi, T.; Nakao, Y.; Goetz, G.; Yoshida, W.Y.; Scheuer, P.J. Kulokekahilide-1, a cytotoxic depsipeptide from the cephalaspidean mollusk Philinopsis speciosa. J. Org. Chem. 2002, 67, 1760–1767.
- Nakao, Y.; Yoshida, W.Y.; Takada, Y.; Kimura, J.; Yang, L.; Mooberry, S.L.; Scheuer, P.J. Kulokekahilide-2, a cytotoxic depsipeptide from a cephalaspidean mollusk Philinopsis speciosa. J. Nat. Prod. 2004, 67, 1332–1340.
- Umehara, M.; Negishi, T.; Tashiro, T.; Nakao, Y.; Kimura, J. Structure-related cytotoxic activity of derivatives from kulokekahilide-2, a cyclodepsipeptide in Hawaiian marine mollusk. Bioorganic Med. Chem. Lett. 2012, 22, 7422–7425.
- Umehara, M.; Negishi, T.; Maehara, Y.; Nakao, Y.; Kimura, J. Stereochemical analysis and cytotoxicity of kulokekahilide-2 and its analogues. Tetrahedron 2013, 69, 3045–3053.
- Von Schwarzenberg, K.; Vollmar, A.M. Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 2013, 332, 295–303.
- Martín-Algarra, S.; Espinosa, E.; Rubió, J.; López, J.J.L.; Manzano, J.L.; Carrión, L.A.; Plazaola, A.; Tanovic, A.; Paz-Ares, L. Phase II study of weekly kahalalide F in patients with advanced malignant melanoma. Eur. J. Cancer 2009, 45, 732–735.
- Gao, J.; Hamann, M.T. Chemistry and biology of lipids. Chem. Rev. 2009, 53, 35–43.
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25.
- Xu, W.J.; Liao, X.J.; Xu, S.H.; Diao, J.Z.; Du, B.; Zhou, X.L.; Pan, S.S. Isolation, structure determination, and synthesis of galaxamide, a rare cytotoxic cyclic pentapeptide from a marine algae Galaxaura filamentosa. Org. Lett. 2008, 10, 4569–4572.
- Lunagariya, J.; Liao, X.; Long, W.; Zhong, S.; Bhadja, P.; Li, H.; Zhao, B.; Xu, S. Cytotoxicity study of cyclopentapeptide analogues of marine natural product galaxamide towards human breast cancer cells. Oxid. Med. Cell. Longev. 2017, 2017.
- Allkin, B. Useful Plants–Medicines: At Least 28,187 Plant Species are Currently Recorded as Being of Medicinal Use. In State of the World’s Plants 201; Royal Botanic Gardens, Kew: London, UK, 2017.
- Wu, D.; Gao, Y.; Qi, Y.; Chen, L.; Ma, Y.; Li, Y. Peptide-based cancer therapy: Opportunity and challenge. Cancer Lett. 2014, 351, 13–22.
- Wélé, A.; Zhang, Y.; Ndoye, I.; Brouard, J.P.; Pousset, J.L.; Bodo, B. A cytotoxic cyclic heptapeptide from the seeds of Annona cherimola. J. Nat. Prod. 2004, 67, 1577–1579.
- Wélé, A.; Ndoye, I.; Zhang, Y.; Brouard, J.P.; Bodo, B. Cherimolacyclopeptide D, a novel cycloheptapeptide from the seeds of Annona cherimola. Phytochemistry 2005, 66, 693–696.
- Wélé, A.; Zhang, Y.; Brouard, J.P.; Pousset, J.L.; Bodo, B. Two cyclopeptides from the seeds of Annona cherimola. Phytochemistry 2005, 66, 2376–2380.
- Idrissa, N.; Adama, D.; Mamadou, B.; Rokhaya, S.G.; Yoro, T. Novel cytotoxic cycloheptapeptide from the latex of Jatropha integerrima. J. Chem. Pharm. Res. 2016, 8, 135–139.
- Mongkolvisut, W.; Sutthivaiyakit, S.; Leutbecher, H.; Mika, S.; Klaiber, I.; Möller, W.; Rösner, H.; Beifuss, U.; Conrad, J. Integerrimides A and B, cyclic heptapeptides from the latex of Jatropha integerrima. J. Nat. Prod. 2006, 69, 1435–1441.
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653.
- Okinyo-Owiti, D.P.; Dong, Q.; Ling, B.; Jadhav, P.D.; Bauer, R.; Maley, J.M.; Reaney, M.J.T.; Yang, J.; Sammynaiken, R. Evaluating the cytotoxicity of flaxseed orbitides for potential cancer treatment. Toxicol. Rep. 2015, 2, 1014–1018.
- Lomchoey, N.; Panseeta, P.; Boonsri, P.; Apiratikul, N.; Prabpai, S.; Kongsaeree, P.; Suksamrarn, S. New bioactive cyclopeptide alkaloids with rare terminal unit from the root bark of: Ziziphus cambodiana. RSC Adv. 2018, 8, 18204–18215.
- Hsieh, P.-W.; Chang, F.-R.; Wu, C.-C.; Li, C.-M.; Wu, K.-Y.; Chen, S.-L.; Yen, H.-F.; Wu, Y.-C. Longicalycinin A, a New cytotoxic cyclic peptide from Dianthus superbus var. longicalycinus (MAXIM.) WILL. Chem. Pharm. Bull. 2005, 53, 336–338.
- Ahmad, W. Solid-phase total synthesis of cyclic pentapeptide longicalycinin A, by using 2-chlorotrityl chloride resin. J. Cancer Res. Exp. Oncol. 2014, 5, 8–19.
- HoushdarTehrani, M.H.; Bamoniri, A.; Mirjalili, B.B.F.; Gholibeikian, M. Synthesis of linear and cyclic disulfide heptapeptides of longicalycinin a and evaluation of toxicity on cancerous cells HepG2 and HT-29. Iran. J. Pharm. Res. 2018, 17, 956–963.
- Hsieh, P.W.; Chang, F.R.; Wu, C.C.; Wu, K.Y.; Li, C.M.; Chen, S.L.; Wu, Y.C. New cytotoxic cyclic peptides and dianthramide from Dianthus superbus. J. Nat. Prod. 2004, 67, 1522–1527.
- Shim, Y.Y.; Young, L.W.; Arnison, P.G.; Gilding, E.; Reaney, M.J.T. Proposed systematic nomenclature for orbitides. J. Nat. Prod. 2015, 78, 645–652.
- Beirigo, P.J.S.D.; Torquato, H.F.V.; Santos, C.H.C.D.; de Carvalho, M.G.; Castro, R.N.; Paredes-Gamero, E.J.; de Sousa, P.T.; Jacinto, M.J.; da Silva, V.C. [1-8-NαlC]-Zanriorb A1, a proapoptotic orbitide from leaves of Zanthoxylum riedelianum. J. Nat. Prod. 2016, 79, 1454–1458.
- Lv, J.P.; Yang, S.; Dong, J.X.; Jin, H. New cyclopeptide alkaloids from the whole plant of Justicia procumbens L. Nat. Prod. Res. 2020, 1–9.
- Liu, Z.; Fu, J.; Xiao, S.; Wang, D. Structural characterization of ginseng cyclopeptides and detection of capability to induce apoptosis in gastrointestinal cancer cells. RSC Adv. 2019, 9, 29847–29855.
- Craik, D.J.; Daly, N.L.; Bond, T.; Waine, C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 1999, 294, 1327–1336.
- Craik, D.J.; Cemazar, M.; Wang, C.K.L.; Daly, N.L. The cyclotide family of circular miniproteins: Nature’s combinatorial peptide teplate. Biopolymer 2006, 84, 250–266.
- Zhao, S.M.; Kuang, B.; Fan, J.T.; Yan, H.; Xu, W.Y.; Tan, N.H. Antitumor cyclic hexapeptides from Rubia plants: History, chemistry, and mechanism (2005–2011). Chim. Int. J. Chem. 2011, 65, 952–956.
- Figueiredo, P.O.; Matos, M.D.F.C.; Perdomo, R.T.; Kato, W.H.; Barros, M.V.G.O.; Garcez, F.R.; Garcez, W.S. Rubiaceae-type cyclopeptides from Galianthe thalictroides. J. Nat. Prod. 2016, 79, 1165–1169.
- Wang, Z.; Zhao, S.M.; Zhao, L.M.; Chen, X.Q.; Zeng, G.Z.; Tan, N.H. Rubipodanin A, the first natural ndesmonomethyl rubiaceae-type cyclopeptide from rubia podantha, indicating an important role of the n9- methyl group in the conformation and bioactivity. PLoS ONE 2015, 10, e0144950.
- Hu, Y.Y.; Feng, L.; Wang, J.; Zhang, X.J.; Wang, Z.; Tan, N.H. Rubipodanin B, a new cytotoxic cyclopeptide from Rubia podantha. Chem. Biodivers. 2019, 16, 16–21.
- Wang, Y.; Guo, D.; He, J.; Song, L.; Chen, H.; Zhang, Z.; Tan, N. Inhibition of fatty acid synthesis arrests colorectal neoplasm growth and metastasis: Anti-cancer therapeutical effects of natural cyclopeptide RA-XII. Biochem. Biophys. Res. Commun. 2019, 512, 819–824.
- Leung, H.W.; Zhao, S.M.; Yue, G.G.L.; Lee, J.K.M.; Fung, K.P.; Leung, P.C.; Tan, N.H.; Lau, C.B.S. RA-XII inhibits tumour growth and metastasis in breast tumour-bearing mice via reducing cell adhesion and invasion and promoting matrix degradation. Sci. Rep. 2015, 5, 1–17.
- Song, L.; Wang, Z.; Wang, Y.; Guo, D.; Yang, J.; Chen, L.; Tan, N. Natural cyclopeptide RA-XII, a new autophagy inhibitor, suppresses protective autophagy for enhancing apoptosis through AMPK/mTOR/P70S6K pathways in HepG2 Cells. Molecules 2017, 22, 1934.
- Wang, J.; Wang, J.; Li, L.; Feng, L.; Wang, Y.R.; Wang, Z.; Tan, N.H. RA-XII, a bicyclic hexapeptidic glucoside isolated from Rubia yunnanensis Diels, exerts antitumor activity by inhibiting protective autophagy and activating Akt-mTOR pathway in colorectal cancer cells. J. Ethnopharmacol. 2021, 266, 113438.
- Hitotsuyanagi, Y.; Hirai, M.; Odagiri, M.; Komine, M.; Hasuda, T.; Fukaya, H.; Takeya, K. RA-XXV and RA-XXVI, Bicyclic hexapeptides from Rubia cordifolia L.: Structure, synthesis, and conformation. Chem. Asian J. 2019, 14, 205–215.
- Ding, X.; Bai, D.; Qian, J. Novel cyclotides from Hedyotis biflora inhibit proliferation and migration of pancreatic cancer cell in vitro and in vivo. Med. Chem. Res. 2014, 23, 1406–1413.
- Nguyen, G.K.T.; Zhang, S.; Nguyen, N.T.K.; Nguyen, P.Q.T.; Chiu, M.S.; Hardjojo, A.; Tam, J.P. Discovery and characterization of novel cyclotides originated from chimeric precursors consisting of albumin-1 chain a and cyclotide domains in the Fabaceae family. J. Biol. Chem. 2011, 286, 24275–24287.
- Zhang, S.; Xiao, K.Z.; Jin, J.; Zhang, Y.; Zhou, W. Chemosensitizing activities of cyclotides from clitoria ternatea in paclitaxel-resistant lung cancer cells. Oncol. Lett. 2013, 5, 641–644.
- Gerlach, U.; Burman, S.L.; Debasis Monda, R.; Goransson, D. Characterization and bioactivity of cyclotides from Psychotria leptothyrsa (Rubiaceae). J. Nat. Prod. 2010, 73, 1207–1213.
- Hashempour, H.; Koehbach, J.; Daly, N.L.; Ghassempour, A.; Gruber, C.W. Characterizing circular peptides in mixtures: Sequence fragment assembly of cyclotides from a violet plant by MALDI-TOF/TOF mass spectrometry. Amino Acids. 2013, 44, 581–595.
- Esmaeili, M.A.; Abagheri-Mahabadi, N.; Hashempour, H.; Farhadpour, M.; Gruber, C.W.; Ghassempour, A. Viola plant cyclotide vigno 5 induces mitochondria-mediated apoptosis via cytochrome C release and caspases activation in cervical cancer cells. Fitoterapia 2016, 109, 162–168.
- Herrmann, A.; Svangård, E.; Claeson, P.; Gullbo, J.; Bohlin, L.; Göransson, U. Key role of glutamic acid for the cytotoxic activity of the cyclotide cycloviolacin O2. Cell. Mol. Life Sci. 2006, 63, 235–245.
- Burman, R.; Svedlund, E.; Felth, J.; Hassan, S.; Herrmann, A.; Clark, R.J.; Craik, D.J.; Bohlin, L.; Claeson, P.; Göransson, U.; et al. Evaluation of toxicity and antitumor activity of cycloviolacin O2 in mice. Biopolymers 2010, 94, 626–634.
- Svangård, E.; Burman, R.; Gunasekera, S.; Lövborg, H.; Gullbo, J.; Göransson, U. Mechanism of action of cytotoxic cyclotides: Cycloviolacin O2 disrupts lipid membranes. J. Nat. Prod. 2007, 70, 643–647.
- Gerlach, S.L.; Rathinakumar, R.; Chakravarty, G.; Göransson, U.; Wimley, W.C.; Darwin, S.P.; Mondal, D. Anticancer and chemosensitizing abilities of cycloviolacin 02 from Viola odorata and psyle cyclotides from Psychotria leptothyrsa. Biopolymers 2010, 94, 617–625.
- Gerlach, S.L.; Yeshak, M.; Göransson, U.; Roy, U.; Izadpanah, R.; Mondal, D. Cycloviolacin O2 (CyO2) suppresses productive infection and augments the antiviral efficacy of nelfinavir in HIV-1 infected monocytic cells. Biopolymers 2013, 100, 471–479.
- He, W.; Yue, L.; Zeng, G.; Daly, N.L.; Craik, D.J.; Tan, N. Peptides Isolation and characterization of cytotoxic cyclotides from Viola philippica. Peptides 2011, 32, 1719–1723.
- Yeshak, M.Y.; Burman, R.; Asres, K.; Göransson, U. Cyclotides from an extreme habitat: Characterization of cyclic peptides from Viola abyssinica of the Ethiopian highlands. J. Nat. Prod. 2011, 74, 727–731.
- Herrmann, A.; Burman, R.; Mylne, J.S.; Karlsson, G.; Gullbo, J.; Craik, D.J.; Clark, R.J.; Göransson, U. The alpine violet, Viola biflora, is a rich source of cyclotides with potent cytotoxicity. Phytochemistry 2008, 69, 939–952.
- Svangård, E.; Göransson, U.; Hocaoglu, Z.; Gullbo, J.; Larsson, R.; Claeson, P.; Bohlin, L. Cytotoxic cyclotides from Viola tricolor. J. Nat. Prod. 2004, 67, 144–147.
- Jun, T.; Wang, C.K.; Pan, X.; Yan, H.; Zeng, G.; Xu, W.; He, W.; Chan, L.Y.; Zeng, G.; Daly, N.L.; et al. Isolation and characterization of cytotoxic cyclotides from Viola tricolor. Peptides 2010, 32, 1719–1723.




