
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paweł Halik | + 2851 word(s) | 2851 | 2021-04-08 04:27:09 | | | |
| 2 | Karina Chen | -66 word(s) | 2785 | 2021-04-19 02:31:40 | | |
Video Upload Options
It is now known that vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptors (VEGFRs) play a pivotal role in angiogenesis process. Nowadays, the use of inhibitors of angiogenesis promoting factors is a powerful tool in anticancer combination therapeutic strategies, especially in cancer anti-angiogenic therapy (AAT).
1. VEGF Glycoproteins
The first reports on VEGF appeared in 1980s, when it was recognised as vascular permeability factor [1], vasculotropin [2] and, as currently known, vascular endothelial growth factor [3], an endogenous effector of prominent pro-angiogenic action through direct activation of vascular endothelial cells. VEGF belongs to the mammalian peptide family consisting of constituents originating from different genes: VEGF-A, VEGF-B, VEGF-C, VEGF-D and PlGF (placenta growth factor), but also viral homolog VEGF-E [4] and VEGF-F of snake venom origin [5]. The common feature of these glycoproteins is the creation of dimeric forms through specific sequence of cysteines forming disulphide bridges between two monomers [6]. Each VEGF family protein occurs as a glycosylated peptide monomer; however, it has to homodimerise or heterodimerise to activate its biological function.
VEGF-A (commonly called VEGF), is the most researched representative of the family and occurs in multiple isoforms (e.g., VEGF-A121, VEGF-A145, VEGF-A165, VEGF-A183, VEGF-A189 and VEGF-A206) due to an alternative splicing of mRNA obtained in the transcription process of the human gene VEGFA [7][8]. The VEGFA gene consists of eight exons that are highly conserved between species. In the first five constitutive exons are encoded the fundamental signal sequence, dimerisation cysteine fragment, specific VEGF receptors recognition domain, fragment employed in glycosylation and plasmin cleavage site, respectively. Furthermore, exons 6 and 7 encode an alternative heparine binding sequence and neuropilin binding domain, while last exon 8 encodes the unique VEGF domain. Alternative splicing results in variability of the primarily structure between isoforms, which affects their bioavailability and biological potency, mainly due to the isoform affinity to heparin sulphate and proteoglycan present on the extracellular surface competing with VEGF receptors [9]. Therefore, VEGF-A121 is freely diffusible and highly active isoform because it binds to neither neuropilins nor heparin sulphate, while VEGF-A165 and VEGF-A189 bind to both, resulting in expansion of their retention on the cellular surface or extracellular matrix.
Althought VEGF-A is highly recognised as a critical angiogenic inductor, it shows broad pleiotropic action in mammals, namely,
(I) significant mitogenic effect on vascular endothelial cells [10], as well as anti-apoptotic impact on these cells [11];
(II) increase of vascular permeability, resulting in increased serum peptides extravasation and local intra-tissue pressure [12];
(III) induction of chemotaxis and activation of monocytes and haematopoietic stem cells [13][14][15];
(IV) neurotrophic and neuroprotective action [16].
The production of VEGF-A glycoproteins occurs in the endothelium and vascular smooth muscle cells, but also in activated platelets, fibroblasts, lymphocytes and macrophages [17], where the production may be stimulated by numerous factors. This process is especially noticeable in tumour cells, that hyperexpress VEGF to stimulate the promotion of tumour growth neoangiogenesis [18]. The main initiator of the transcription of mRNA encoding VEGF-A is hypoxia state, especially noticeable in the necrotic and cancer cells [19]. This phenomenon is associated with the formation of hypoxia induced factor in these cells, which is called hypoxia inducible factor-1 (HIF-1) [20][21]. In contrast to hypoxia, HIF-1 cellular concentration is strictly regulated under physiological conditions. Other significant stimulating factors of VEGF-A cellular synthesis are cytokines (interleukin 1b, IL-1b and tumour necrosis factor alpha, TNF-α), several hormones and specific growth factors [22][23], activation of oncogenes RAS and SRC, mutation in suppressor genes p53 and von Hippel–Lindau (VHL) [23][24][25], as well as nitric oxide and oxygen radicals [26][27]. These factors are more or less known as indirect initiators of angiogenesis, acting on the synthesis of VEGF-A.
The activity of other mammalian VEGF proteins is more specific than that of VEGF-A, however effects in site of action of all VEGF glycoproteins are more or less similar. VEGF-B has a relatively limited angiogenic action only towards ischemic myocardium, which is associated with VEGF-B level decrease [28]. More recently, it has been revealed that potent metabolic and antioxidative action of VEGF-B is possibly related to pro-angiogenic effects [29][30][31]. It contributes to the homeostasis of lipids in numerous tissues and the upregulation of brown adipose tissue, resulting in reduced risks of obesity and insulin resistance induced by diet rich in fat. Moreover, there are also reports of neuroprotective activity of exogenous VEGF-B186 isoform in the distal neuropathy and Parkinson’s disease models [32][33]. This effect is assumed to be induced directly on the motor neurons, similar to VEGF-A, not through their vascularity.
Some similarities to VEGF-B action exhibits placenta growth factor. PlGF is expressed dominantly by placental trophoblasts, but also during early embryonic development and to a lesser extent in a few adult organs such as heart, lungs, thyroid or skeletal muscles [34]. Contribution of PlGF in physiological angiogenesis in adults is negligible, however under pathological conditions such as ischemia, it prominently stimulates vascular endothelium proliferation and also differentiation and activation of the monocytes into the macrophages recognised as an angiogenic feedback stimulant [35]. Moreover, PlGF increases vessel permeability and inflammation in degenerations as rheumatoid arthritis and atherosclerosis promoting neoangiogenesis [36]. In addition, several types of tumour cell lines have the ability of PlGF expression, which favours the pro-angiogenic M2-phenotype tumour-associated macrophages [37].
VEGF-C is recognised as the fundamental promotor of proliferation and migration of the lymphatic system endothelium [38]. It also stimulates the cytokine-inducted migration and permeability of the vascular endothelial cells, although to a lesser extent than VEGF-A and independently of hypoxia stimulus. Similar in structure and function to VEGF-C, VEGF-D plays a secondary role in the physiological stimulation of human endothelium of vascular and lymphatic systems. Concomitantly, the high expression of both growth factors significantly promote and correlate with the metastasis through the lymphatic vessels in a variety of cancers [39][40][41].
2. VEGF Receptors and Their Co-Receptors
The site of action of VEGF glycoproteins are their specific receptors presented on the surface of target cells. There are three such receptors: VEGFR-1 (also known as FLT1, due to the same name of its gene), VEGFR-2 (known as KDR or FLK1, encoded by KDR gene) and VEGFR-3 (FLT4, encoded by FLT4 gene).
VEGFRs are classified as members of receptor tyrosine kinase superfamily due to their autophosphorylation ability induced by recognition of specific ligands. They are present in the form of homo- or heterodimers consisting of three functional fragments defined as extracellular part with seven Ig-like subunits, lipophilic single transmembrane domain and intracellular domain with distinctive tyrosine kinase activity. Individual VEGF proteins (and their isoforms) have different affinity towards each receptor. It is well known that VEGFR-1 binds VEGF-A, VEGF-B and PlGF, while VEGFR-2 binds VEGF-A as well as post-proteolytic VEGF-C and VEGF-D. Both VEGF-C and VEGF-D have affinity mainly towards VEGFR-3 [42] (Figure 1).
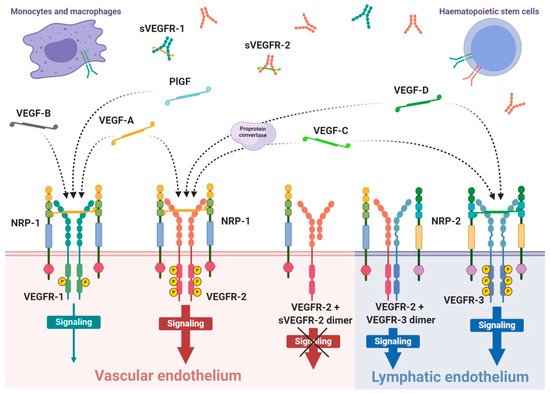
Interaction of growth factor with its receptor becomes much stronger with the participation of specific co-receptors that facilites the creation of the molecular complex ligand-receptor [43]. These co-receptors, known as neuropilins, occur as neuropilin 1 (NRP-1) that participates in VEGFR-1 or VEGFR-2 interactions with ligands and neuropilin 2 (NRP-2) mostly assigned to VEGFR-3 (Figure 1). Both types of neuropilins are expressed on endothelial cells and specific types of tumours [43][44]. NRP-1 binding differs between VEGF isoforms, so that VEGF-A165 and VEGF-A189 create stronger complexes with VEGFR-2 and NRP-1 than VEGF-A121, which is deprived of NRP-1 binding domain [43]. Nevertheless, direct interaction of VEGF-A121 with NRP-1 can regulate endothelial cell migration and sprouting independently of specific VEGF receptors [45].
The expression of VEGFR-1 occurs predominantly on endothelial cells of blood vessels, but also on monocytes and macrophages, placental trophoblasts as well as renal mesangial cells [46][47]. Similarly, VEGFR-2 occurs mostly on blood vessel endothelium, as well as platelets, haematopoietic and retinal stem cells. Both receptors are clearly expressed on cell surfaces of solid cancers and haematopoietic system neoplasms [48][49]. VEGFR-3 expression is specified only on endothelial cells of lymphatic system [50]. Therefore, a substantial share of VEGFR-1 and VEGFR-2 on vascular endothelium shows their significant contribution in angiogenesis, while VEGFR-3 and NRP-2 highly contribute in lymphangiogenesis [42][51].
For ligand binding receptors require at least the first three Ig-like domains, however, not all must participate in ligand binding. Simultaneously, if the ligand binds to neuropilin, then the third and fourth domains of the receptor will also attach to neuropilin. Moreover, besides ligand interaction, receptors also have to dimerise to be able to transduct signals intracellularly [52][53]. When both conditions are met, ligand can trigger the mutual autophosphorylation of the receptor intracellular tyrosine subunits and activation of specific signalling pathways inside the cell.
Different ligands can stimulate various biological effects through activated receptors, as well as activation of VEGFR-1 and VEGFR-2 by VEGF-A cause a different induction of intracellular signalling pathways [11][53]. Activation of VEGFR-2 leads to stimulation of the cell cycle, proliferation, migration, cell differentiation, angiogenesis, increased permeability of blood vessels but also inhibition of the apoptotic death and up-regulation of VEGF-A synthesis in endothelial cells [11][54]. On the contrary, VEGF-A can bind to VEGFR-1, activating its low-efficient tyrosine kinases, which has insignificant influence on endothelial cells [53][55]. Despite the high abundance of this receptor on endothelium, second receptor exerts even 10-fold higher density on endothelial cells [53][56]. Concomitantly, VEGF-A has about 10-fold lower affinity to VEGFR-2 compared to VEGFR-1. Hence, it is suspected that VEGFR-1 acts as concomitant decoy receptor and uptakes VEGF-A before it can bind to adjacent VEGFR-2, ergo VEGFR-1 plays an angiogenic-regulation role [35][56]. However, the same receptor interaction with PlGF promotes VEGF-A pool for endothelial angiogenic action through VEGFR-2 [35] and can regulate transphosphorylation of VEGFR-2 [57], thus amplifying angiogenesis through VEGFR-2. VEGFR-1 signalling can also regulate paracrine release in the vascular endothelial cells of other tissue endothelium growth factors inducing intestinal organogenesis and morphogenesis before vascular flow formation [58].
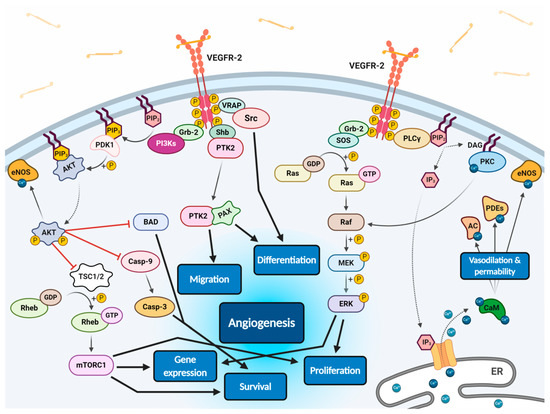
All VEGF isoforms that bind selectively to VEGFR-2 are capable to elicit receptor autophosphorylation, thus triggering the activation of numerous intracellular signalling pathways (Figure 2) [11][53][56][59][60]. Phosphorylated receptor subunits bind many adaptor molecules such as Shb (SH2 domain-containing adapter protein B), SOS (Son of sevenless proteins) or Grb-2 (Growth factor receptor-bound protein 2) that activate Ras GPTase. This last protein stimulates MAPK pathway responsible for endothelium proliferation. Simultaneously, phosphorylated intracellular VEGFR-2 domain activates phospholipase C-gamma (PLC-γ), which catalyses hydrolysis of phosphatidylinositol bisphosphate (PIP2) to inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 triggers intracellular release of Ca2+ form endoplasmic reticulum, which employs calcium modulated protein calmodulin to stimulate cAMP phosphodiesterase, adenylate cyclase and site-specific endothelial NO synthase (eNOS) and consequently increase NO-driven vasodilation and vascular permeability. However, DAG activates calcium-dependent protein kinase C (PKC), a multi-target kinase stimulating indirect cell proliferation and migration. Additionally, phosphorylated VEGFR-2 induces protein kinase B (commonly known as AKT) at the beginning of PI3K/AKT/mTOR pathway, an important signalling regulator of the cell cycle and metabolism, reducing risk of apoptosis and promoting cellular transcription, proliferation and migration [11][59]. Moreover, phosphorylated VEGFR-2 activates signalling of focal adhesion kinase (FAK) observed during cellular migration, adhesion, cytoskeleton rearrangement and tumour progression [60][61]. Nevertheless, it was observed that VEGF-A can regulate endothelial cell attachment independently of VEGFR-2 through NPR-1 [62].
VEGF receptors, in addition to transmembrane forms, can also occur in soluble forms, known as sVEGFR-1 and sVEGFR-2 (Figure 1) [61][63]. Their formation can be explained by two mechanisms, namely, a proteolysis of extracellular binding domain [64][65] and alternative splicing of primary gene transcript [61][66], both forming freely diffusible proteins consisting of only six of seven Ig-like subunits [67]. Soluble receptors are secreted by identical cells that express regular receptors, mostly by vascular endothelial cells [63]. Due to the fact that sVEGFRs exhibit comparable binding affinity on a similar basis as regular receptors, but are deprived of effector domains of tyrosine kinases, they can demonstrate only a regulatory decoy function. Both soluble receptors compete for VEGF-A with regular receptors inhibiting angiogenic and other actions of the growth factor. Simultaneously, sVEGFR-2 can uptake VEGF-C and VEGF-D reducing their overall supply intended for lymphangiogenesis stimulation through VEGFR-3 [66]. Moreover, creation of heterodimers from soluble and regular receptors precludes cellular signalling [63]; however, it is suspected that interaction of sVEGFRs with NRP-1 can mediate VEGF-A trigger of intracellular PKC pathway signalling [68].
Interestingly, several reports have shown the reverse correlation between sVEGFR expression and cancerous angiogenesis or metastasis. Such research has indicated that sVEGFR-1 permanently suppresses tumour growth and decreases metastasis promoting overall survival rate in rodents or humans with fibrosarcoma and glioblastoma [69], advanced renal cancer [70], breast cancer [71][72], acute myeloid leukaemia [73], colorectal cancer [74] and non-small cell lung cancer [75]. Similar results were presented for sVEGFR-2 [72][76][77][78][79], demonstrating significant biomarker role of these receptors in diagnosis of numerous cancers.
3. Anti-Angiogenic Therapy Strategies for Tumour Treatment
Although various angiogenesis-stimulating factors exist, VEGF-A is considered the most potent and predominant one. This also applies to sustained angiogenesis in cancers. Currently, it is known that angiogenesis, besides its crucial role in the tumour growth, stimulates the progression of invasiveness and development of vascular network in the surrounding tumour microenvironment [80][81]. The concept of angiogenesis targeting for cancer diagnosis and treatment seems promising, therefore, a wide variety of therapeutic strategies have been directed at visualisation and interfering with tumour-stimulated angiogenesis. However, since the first FDA approval of bevacizumab (BV), humanised anti-VEGF-A mAb, for the combinational chemotherapy regimen with 5-fluorouracil of metastatic colorectal cancer [82], only a few AAT strategies have been granted similar approval. It has become a challenge to evaluate these strategies almost personally for each patient, due to considerable variability of the angiogenic process in each treated entity [83]. Although the correlation between tumour progression and VEGF-A expression is well established, it does not transfer into intended anti-angiogenic therapeutic effects. This is due to the heterogeneity of the same tumour between patients, but also between different tumours in an individual patient, that occurs and changes at different stages of the lesion development. This raises the need for appropriate methods of assessing how the patient responds to the proposed therapy. In terms of AAT, this applies to clinically significant parameters as the lesion location with regard to tumour admission of therapeutic agents and expression of endogenous growth factors in tumour microenvironment affecting the saturation of target receptors involved in angiogenesis. Despite the complexity of this issue, the use of radiopharmaceuticals is increasingly proposed for independent preliminary screening, which can provide the prediction of patient clinical response [84]. Radioligands successfully targeting VEGF/VEGFR system in vivo are potentially valuable tracers for the study of angiogenic processes [85], stratification of patients to AATs [86], as well as monitoring therapy efficacy and clinical outcomes [87][88].
Basically, the aforementioned radiopharmaceuticals are based on various approaches to VEGF/VEGFR system targeting including radiolabelled derivatives of human VEGF-A ligands, anti-VEGF or anti-VEGFR antibodies, VEGFR binding peptides, small molecular inhibitors of tyrosine kinase domain of VEGF receptors and peptidomimetic ligands targeting NRP-1 co-receptor. Additionally, depending on specific radiation features of applied radionuclide, the radiopharmaceuticals are dedicated for diagnostic, therapeutic or theranostic purposes. This multitude of radiopharmaceutical solutions allows for the design of tailor-made therapeutic tool and its evaluation on a specific cancer model. The broad selection of above listed biovectors enables choice of one that provides the desired multiple molecular targets or just specific one, exhibits eligible pharmacokinetics, predicts response of certain chemotherapeutic strategy, or shows confirmed complemental contribution to the selected chemotherapy.
AAT methods have especially found a place in clinical practice applied in monotherapy. Currently, it is well known that even these methods used alone are inefficient, they advantageously support conventional chemotherapy effects [89]. Interestingly, the AAT contributes to normalisation of the tumour vasculature resulting in enhanced metabolic rate and delivery capacity of the tumour; hence, AAT can increase efficacy of the radiotherapy or activity of immune system in the close tumour surroundings.
References
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Harvey, V.S.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985.
- Plouet, J.; Schilling, J.; Gospodarowicz, D. Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT-20 cells. EMBO J. 1989, 8, 3801–3806.
- Ferrara, N.; Hanzel, W.J. Pituitary folicular cells secrete a novel heparinbinding growth factor specific for vascular endothelial cells. BioChem. Biophys. Res. Commun. 1989, 161, 851–858.
- Holmes, D.I.R.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209.
- Yamazaki, Y.; Matsunaga, Y.; Tokunaga, Y.; Obayashi, S.; Saito, M.; Morita, T. Snake Venom Vascular Endothelial Growth Factors (VEGF-Fs) Exclusively Vary Their Structures and Functions among Species. J. Biol. Chem. 2009, 284, 9885–9891.
- Pötgens, A.J.; Lubsen, N.H.; van Altena, M.C.; Vermeulen, R.; Bakker, A.; Schoenmakers, J.G.; Ruiter, D.J.; de Waal, R.M. Covalent Dimerization of Vascular Permeability Factor/Vascular Endothelial Growth Factor Is Essential for Its Biological Activity. J. Biol. Chem. 1994, 269, 32879–32885.
- Nowak, D.G.; Woolard, J.; Amin, E.M.; Konopatskaya, O.; Saleem, M.A.; Churchill, A.J.; Ladomery, M.R.; Harper, S.J.; Bates, D.O. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J. Cell Sci. 2008, 121, 3487–3495.
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264.
- Houck, K.A.; Leung, D.W.; Rowland, A.M.; Winer, J.; Ferrara, N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 1992, 267, 26031–26037.
- Ferrara, N.; Davis-Smith, T. The Biology of Vascular Endothelial Growth Factor. Endocr. Rev. 1997, 18, 4–25.
- Gerber, H.P.; McMurtrey, S.; Kowalski, J.; Yan, M.; Keyt, B.A.; Dixit, V.; Ferrara, N. Vascular Endothelial Growth Factor Regulates Endothelial Cell Survival through the Phosphatidylinositol 3′-Kinase/Akt Signal Transduction Pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998, 273, 30336–30343.
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039.
- Cluass, M.; Gerlach, M.; Gerlach, H.; Brett, J.; Wang, F.; Familletti, P.C.; Pan, Y.C.; Olander, J.V.; Connolly, D.T.; Stern, D. Vascular Permeability Factor: A Tumor-derived Polypeptide that Induces Endothelial Cell and Monocyte Procoagulant Activity, and Promotes Monocyte Migration. J. Exp. Med. 1990, 172, 1535–1545.
- Broxmeyer, H.E.; Cooper, S.; Li, Z.H.; Lu, L.; Song, H.Y.; Kwon, B.S.; Warren, R.E.; Donner, D.B. Myeloid progenitor cell regulatory effects of vascular endothelial cell growth factor. Int. J. Hematol. 1995, 62, 203–215.
- Hattori, K.; Dias, S.; Heissig, B.; Hackett, N.R.; Lyden, D.; Tateno, M.; Hicklin, D.J.; Zhu, Z.; Witte, L.; Crystal, R.G.; et al. Vascular Endothelial Growth Factor and Angiopoietin-1 Stimulate Postnatal Hematopoiesis by Recruitment of Vasculogenic and Hematopoietic Stem Cells. J. Exp. Med. 2001, 193, 1005–1014.
- Storkebaum, E.; Carmeliet, P. VEGF: A critical player in neurodegeneration. J. Clin. Investig. 2004, 113, 14–18.
- Berse, B.; Brown, L.F.; Van de Water, L.; Dvorak, H.F.; Senger, D.R. Vascular Permeability Factor (Vascular Endothelial Growth Factor) Gene is Expressed Differentially in Normal Tissues, Macrophages, and Tumors. Mol. Biol. Cell 1992, 3, 211–220.
- Dvorak, H.F.; Sioussat, T.M.; Brown, L.F.; Berse, B.; Nagy, J.A.; Sotrel, A.; Manseau, E.J.; Van de Water, L.; Senger, D.R. Distribution of Vascular Permeability Factor (Vascular Endothelial Growth Factor) in Tumors: Concentration in Tumor Blood Vessels. J. Exp. Med. 1991, 174, 1275–1278.
- Semenza, G. Angiogenesis in ischemic and neoplastic disorders. Annu. Rev. Med. 2003, 54, 17–28.
- Forsythe, J.A.; Jiang, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4604–4613.
- Warren, R.S.; Yuan, H.; Matli, M.R.; Ferrara, N.; Donner, D.B. Induction of vascular endothelial growth factor by insulin-like growth factor 1 in colorectal carcinoma. J. Biol. Chem. 1996, 271, 29483–29488.
- Ben-Av, P.; Crofford, L.J.; Wilder, R.L.; Hla, T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-l: A potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995, 372, 83–87.
- Enholm, B.; Paavonen, K.; Ristimäki, A.; Kumar, V.; Gunji, Y.; Klefstrom, J.; Kivinen, L.; Laiho, M.; Olofsson, B.; Joukov, V.; et al. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 1997, 14, 2475–2483.
- Siemesiter, G.; Weindel, K.; Mohrs, K.; Barleon, B.; Martiny-Baron, G.; Marmé, D. Reversion of deregulated expression of vascular endothelial growth factor in human renal carcinoma cells by von Hippel-Lindau tumor suppressor protein. Cancer Res. 1996, 56, 2299–2301.
- Eliceiri, B.P.; Paul, R.; Schwartzberg, P.L.; Hood, J.D.; Leng, J.; Cheresh, D.A. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell 1999, 4, 915–924.
- Kimura, H.; Esumi, H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim. Pol. 2003, 50, 49–59.
- Ushio-Fukai, M.; Nakamura, Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008, 266, 37–52.
- Li, X.; Tjwa, M.; Van Hove, I.; Enholm, B.; Neven, E.; Paavonen, K.; Jeltsch, M.; Juan, T.D.; Sievers, R.E.; Chorianopoulos, E.; et al. Reevaluation of the Role of VEGF-B Suggests a Restricted Role in the Revascularization of the Ischemic Myocardium. Arter. Thromb. Vasc. Biol. 2008, 28, 1614–1620.
- Hagberg, C.E.; Falkevall, A.; Wang, X.; Larsson, E.; Huusko, J.; Nilsson, I.; van Meeteren, L.A.; Samen, E.; Lu, L.; Vanwildemeersch, M.; et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature 2010, 464, 917–921.
- Hagberg, C.E.; Mehlem, A.; Falkevall, A.; Muhl, L.; Fam, B.C.; Orstäter, H.; Scotney, P.; Nyqvist, D.; Samen, E.; Lu, L.; et al. Targeting VEGF-B as a novel treatment for insulin resistance and type 2 diabetes. Nature 2012, 490, 426–430.
- Arjunan, P.; Lin, X.; Tang, Z.; Du, Y.; Kumar, A.; Liu, L.; Yin, X.; Huang, L.; Chen, W.; Chen, Q.; et al. VEGF-B is a potent antioxidant. Proc. Natl. Acad. Sci. USA 2018, 115, 10351–10356.
- Dhondt, J.; Peeraer, E.; Verheyen, A.; Nuydens, R.; Buysschaert, I.; Poesen, K.; Van Geyte, K.; Beerens, M.; Shibuya, M.; Haigh, J.J.; et al. Neuronal FLT1 receptor and its selective ligand VEGF-B protect against retrograde degeneration of sensory neurons. FASEB J. 2011, 25, 1461–1473.
- Yue, X.; Hariri, D.J.; Caballero, B.; Zhang, S.; Bartlett, M.J.; Kaut, O.; Mount, D.W.; Wullner, U.; Sherman, S.J.; Falk, T. Comparative study of the neurotrophic effects elicited by VEGF-B and GDNF in preclinical in vivo models of Parkinson’s disease. Neuroscience 2014, 258, 385–400.
- De Falco, S. The discovery of placenta growth factor and its biological activity. Exp. Mol. Med. 2012, 44, 1–9.
- Carmeliet, P.; Moons, L.; Luttun, A.; Vincenti, V.; Compernolle, V.; De Mol, M.; Wu, Y.; Bono, F.; Devy, L.; Beck, H.; et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001, 7, 575–583.
- Oura, H.; Bertoncini, J.; Velasco, P.; Brown, L.F.; Carmeliet, P.; Detmar, M. A critical role of placental growth factor in the induction of inflammation and edema formation. Blood 2003, 101, 560–567.
- Rolny, C.; Mazzone, M.; Tugues, S.; Laoui, D.; Johansson, I.; Coulon, C.; Squadrito, M.L.; Segura, I.; Li, X.; Knevels, E.; et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through down-regulation of PlGF. Cancer Cell. 2011, 19, 31–44.
- Jussila, L.; Alitalo, K. Vascular growth factors and lymphangiogenesis. Physiol. Rev. 2002, 82, 673–700.
- Tsurusaki, T.; Kanda, S.; Sakai, H.; Kanetake, H.; Saito, Y.; Alitalo, K.; Koji, T. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br. J. Cancer 1999, 80, 309–313.
- Mandriota, S.J.; Jussila, L.; Jeltsch, M.; Compagni, A.; Baetens, D.; Prevo, R.; Banerji, S.; Huarte, J.; Montesano, R.; Jackson, D.G.; et al. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001, 20, 672–682.
- Stacker, S.A.; Caesar, C.; Baldwin, M.E.; Thornton, G.E.; Williams, R.A.; Prevo, R.; Jackson, D.G.; Nishikawa, S.; Kubo, H.; Achen, M.G. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 2001, 7, 186–191.
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560.
- Soker, S.; Fidder, H.; Neufeld, G.; Klagsbrun, M. Characterization of novel vascular endothelial growth factor (VEGF) receptors on tumor cells that bind VEGF165 via its exon 7-encoded domain. J. Biol. Chem. 1996, 271, 5761–5767.
- Soker, S.; Takashima, S.; Miao, H.Q.; Neufeld, G.; Klagsbrun, M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 1998, 92, 735–745.
- Pan, Q.; Chathery, Y.; Wu, Y.; Rathore, N.; Tong, R.K.; Peale, F.; Bagri, A.; Tessier-Lavigne, M.; Koch, A.W.; Watts, R.J. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J. Biol. Chem. 2007, 282, 24049–24056.
- Jakeman, L.B.; Armanini, M.; Philips, H.S.; Ferrara, N. Developmental expression of binding sites and mRNA for vascular endothelial growth factor suggests a role for this protein in vasculogenesis and angiogenesis. Endocrinology 1993, 133, 848–859.
- Shen, H.; Clauss, M.; Ryan, J.; Schmidt, A.M.; Tijburg, P.; Borden, L.; Connolly, D.; Stern, D.; Kao, J. Characterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytes. Blood 1993, 81, 2767–2773.
- Walter, J.W.; North, P.E.; Waner, M.; Mizeracki, A.; Blei, F.; Walker, J.W.; Reinisch, J.F.; Marchuk, D.A. Somatic mutation of vascular endothelial growth factor receptors in juvenile hemangioma. Genes Chromosomes Cancer 2002, 33, 295–303.
- Guo, S.; Colbert, L.S.; Fuller, M.; Zhang, Y.; Gonzalez-Perez, R.R. Vascular endothelial growth factor receptor-2 in breast cancer. Biochim. Biophys. Acta 2010, 1806, 108–121.
- Pajusola, K.; Aprelikova, O.; Korhonen, J.; Kaipainen, A.; Pertovaara, L.; Alitalo, R.; Alitalo, K. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992, 52, 5738–5743.
- Yuan, L.; Moyon, D.; Pardanaud, L.; Breant, C.; Karkkainen, M.J.; Alitalo, K.; Eichmann, A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002, 129, 4797–4806.
- Mac Gabhann, F.; Popel, A.S. Dimerization of VEGF receptors and implications for signal transduction: A computational study. Biophys. Chem. 2007, 128, 125–139.
- Waltenberger, J.; Claesson-Welsh, L.; Siegbahn, A.; Shibuya, M.; Heldin, C.H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 1994, 269, 26988–26995.
- Sondell, M.; Lundborg, G.; Kanje, M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J. Neuro Sci. 1999, 19, 5731–5740.
- De Vries, C.; Escobedo, J.A.; Ueno, H.; Houck, K.; Ferrara, N.; Williams, L.T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255, 989–991.
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signaling—In control of vascular function. Nat. Rev. Mol. Cell. Biol. 2006, 7, 359–371.
- Autiero, M.; Waltenberger, J.; Communi, D.; Kranz, A.; Moons, L.; Lambrechts, D.; Kroll, J.; Plaisance, S.; De Mol, M.; Bono, F.; et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat. Med. 2003, 9, 936–943.
- Matsumoto, K.; Yoshitomi, H.; Rossant, J.; Zaret, K.S. Liver organogenesis promoted by endothelial cells prior to vascular function. Science 2001, 294, 559–563.
- Byzova, T.V.; Goldman, C.K.; Pampori, N.; Thomas, K.A.; Bett, A.; Shattil, S.J.; Plow, E.F. A mechanism for modulation of cellular responses to VEGF: Activation of the integrins. Mol. Cell 2000, 6, 851–860.
- Le Boeuf, F.; Houle, F.; Huot, J. Regulation of Vascular Endothelial Growth Factor Receptor 2-mediated Phosphorylation of Focal Adhesion Kinase by Heat Shock Protein 90 and Src Kinase Activities. J. Biol. Chem. 2004, 279, 39175–39185.
- Kendall, R.L.; Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 10705–10709.
- Murga, M.; Fernandez-Capetillo, O.; Tosato, G. Neuropilin-1 regulates attachment in human endothelial cells independently of vascular endothelial growth factor receptor-2. Blood 2005, 105, 1992–1999.
- Horing, C.; Weich, H.A. Soluble VEGF receptors. Angiogenesis 1999, 3, 33–39.
- Ebos, J.M.L.; Bocci, G.; Man, S.; Thrope, P.E.; Hicklin, D.J.; Zhou, D.; Jia, X.; Kerbel, R.S. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol. Cancer Res. 2004, 2, 315–326.
- Cai, J.; Jiang, W.G.; Grant, M.B.; Boulton, M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J. Biol. Chem. 2006, 281, 3604–3613.
- Albuquerque, R.J.C.; Hayashi, T.; Cho, W.G.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030.
- Barleon, B.; Totzke, F.; Herzog, C.; Blanke, S.; Kremmer, E.; Siemeister, G.; Marmé, D.; Martiny-Baron, G. Mapping of sites for ligand binding and receptor dimerization at the extracellular domain of the vascular endothelial growth factor receptor FLT-1. J. Biol. Chem. 1997, 272, 10382–10388.
- Lorquet, S.; Berndt, S.; Blacher, S.; Gengoux, E.; Peulen, O.; Maquoi, E.; Noël, A.; Foidart, J.M.; Munaut, C.; Péqueux, C. Soluble forms of VEGF receptor-1 and -2 promote vascular maturation via mural cell recruitment. FASEB J. 2010, 24, 3782–3795.
- Goldman, C.K.; Kendall, R.L.; Cabrera, G.; Soroceanu, L.; Heike, Y.; Gillespie, G.Y.; Siegal, G.P.; Mao, X.; Bett, A.J.; Huckle, W.R.; et al. Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis, and mortality rate. Proc. Natl. Acad. Sci. USA 1998, 95, 8795–8800.
- Harris, A.L.; Reusch, P.; Barleon, B.; Hang, C.; Dobbs, N.; Marme, D. Soluble Tie2 and Flt1 extracellular domains in serum of patients with renal cancer and response to antiangiogenic therapy. Clin. Cancer Res. 2001, 7, 1992–1997.
- Toi, M.; Bando, H.; Ogawa, T.; Muta, M.; Hornig, C.; Weich, H.A. Significance of vascular endothelial growth factor (VEGF)/soluble VEGF receptor-1 relationship in breast cancer. Int. J. Cancer 2002, 98, 14–18.
- Bando, H.; Weich, H.A.; Brokelmann, M.; Horiguchi, S.; Funata, N.; Ogawa, T.; Toi, M. Association between intratumoral free and total VEGF, soluble VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br. J. Cancer 2005, 92, 553–561.
- Aref, S.; El Sherbiny, M.; Goda, T.; Fouda, M.; Al Askalany, H.; Abdalla, D. Soluble VEGF/sFLt1 ratio is an independent predictor of AML patient outcome. Hematology 2005, 10, 131–134.
- Yamaguchi, T.; Bando, H.; Mori, T.; Takahashi, K.; Matsumoto, H.; Yasutome, M.; Weich, H.; Toi, M. Overexpression of soluble vascular endothalial growth factor receptor 1 in colorectal cancer: Association with progression and prognosis. Cancer Sci. 2007, 98, 405–410.
- Kopczyńska, E.; Dancewicz, M.; Kowalewski, J.; Makarewicz, R.; Kardymowicz, H.; Kaczmarczyk, A.; Tyrakowski, T. Time-dependent changes of plasma concentrations of angiopoietins, vascular endothelial growth factor, and soluble forms of their receptors in nonsmall cell lung cancer patients following surgical resection. ISRN Oncol. 2012, 2012, 638352.
- Kou, B.; Li, Y.; Zhang, L.; Zhu, G.; Wang, X.; Li, Y.; Xia, J.; Shi, Y. In vivo inhibition of tumor angiogenesis by a soluble VEGFR-2 fragment. Exp. Mol. Pathol. 2004, 76, 129–137.
- Faderl, S.; Do, K.A.; Johnson, M.M.; Keating, M.; O’Brien, S.; Jilani, I.; Ferrajoli, A.; Ravandi-Kashani, F.; Aguilar, C.; Dey, A.; et al. Angiogenic factors may have a different prognostic role in adult acute lymphoblastic leukemia. Blood 2005, 106, 4303–4307.
- Jayasinghe, C.; Simiantonaki, N.; Michel-Schmidt, R.; Kirkpatrick, C.J. Comparative study of human colonic tumor-derives endothelial cells (HCTEC) and normal colonic microvascular endothelial cells (HCMEC): Hypoxia-induces sVEGFR-1 and sVEGFR-2 levels. Oncol. Rep. 2009, 21, 933–939.
- Kikuchi, S.; Obata, Y.; Yagyu, K.; Lin, Y.; Nakajima, T.; Kobayashi, O.; Kikuichi, M.; Ushijima, R.; Kurosawa, M.; Ueda, J. Reduced serum vascular endothelial growth factor receptor-2 (sVEGFR-2) and sVEGFR-1 levels in gastric cancer patients. Cancer Sci. 2011, 102, 866–869.
- Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990, 82, 4–6.
- Skobe, M.; Rockwell, P.; Goldstein, N.; Vosseler, S.; Fusenig, N.E. Halting angiogenesis suppresses carcinoma cell invasion. Nat. Med. 1997, 3, 1222–1227.
- Yang, J.C.; Haworth, L.; Sherry, R.M.; Hwu, P.; Schwartzentruber, D.J.; Topalian, S.L.; Steinberg, S.M.; Chen, H.X.; Rosenberg, S.A. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 2003, 349, 427–434.
- Longo, R.; Gasparini, G. Challenges for patient selection with VEGF inhibitors. Cancer Chemother. Pharmacol. 2007, 60, 151–170.
- Stephen, R.M.; Gilles, R.J. Promise and progress for functional and molecular imaging of response to targeted therapies. Pharm. Res. 2007, 24, 1172–1185.
- Stacy, M.R.; Maxfield, M.W.; Sinusas, A.J. Targeted Molecular Imaging of Angiogenesis in PET and SPECT: A review. Yale J. Biol. Med. 2012, 85, 75–86.
- Wang, H.; Cai, W.; Chen, K.; Li, Z.B.; Kashefi, A.; He, L.; Chen, X. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 2001–2010.
- Cai, W.; Chen, K.; Mohamedali, K.A.; Cao, Q.; Gambhir, S.S.; Rosenblum, M.G.; Chen, X. PET of vascular endothelial growth factor receptor expression. J. Nucl. Med. 2006, 47, 2048–2056.
- Backer, M.V.; Levashova, Z.; Patel, V.; Jehning, B.T.; Claffey, K.; Blankenberg, F.G.; Backer, J.M. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat. Med. 2007, 13, 504–509.
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011, 91, 1071–7121.





