
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | carole Ayoub Moubareck | + 8914 word(s) | 8914 | 2021-04-08 09:53:59 | | | |
| 2 | Bruce Ren | -21 word(s) | 8893 | 2021-04-12 04:16:26 | | | | |
| 3 | Bruce Ren | + 68 word(s) | 8982 | 2021-04-12 04:16:58 | | |
Video Upload Options
Human milk represents a cornerstone for growth and development of infants, with extensive array of benefits. In addition to exceptionally nutritive and bioactive components, human milk encompasses a complex community of signature bacteria that helps establish infant gut microbiota, contributes to maturation of infant immune system, and competitively interferes with pathogens. Among bioactive constituents of milk, human milk oligosaccharides (HMOs) are particularly significant.
1. Introduction to Human Milk and Importance of Breastfeeding
Over the past decades, human breast milk has been widely agreed upon as being the normal and optimal dietary start for infants, with unparalleled biological effects driven by the combined action of its nutritional and bioactive components [1]. The positive effects of breastfeeding on infants from the nutritional, physiological, and developmental viewpoints have been confirmed [2]. Worldwide, health authorities have recognized the value and benefits of breastfeeding [3]. The World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) recommend the initiation of breastfeeding within the first hour of birth and advise on exclusive breastfeeding for the first six months of life, without any other food or liquids, including water. Although the introduction of complementary food is safe starting at six months, the WHO and UNICEF recommend to pursue breastfeeding for up to two years [4]. In the breastfeeding week 2020 message, under the theme “support breastfeeding for a healthier planet”, the WHO called for protection, promotion, and support of women’s access to skilled breastfeeding, in line with the multifaceted benefits of this sustainable, biological food system [5]. Likewise, the American Academy of Pediatrics (AAP) continuously reinforces breastfeeding and mother’s milk as the normal standards for infant feeding and nutrition. It recommends exclusive breastfeeding for six months, followed by continuation of breastfeeding as complementary foods are introduced, with possible extension of breastfeeding for one year of age or beyond, as desired [6].
Human milk holds a plethora of health benefits, both on the short- and the long-term. The relationship between breastfeeding and infant’s health is based on its nutritional and non-nutritional components that have diverse roles [7]. Human milk contains the precise mix of nutrients, including carbohydrates, proteins, fats, vitamins, minerals as well as water, that together secure a dynamic composition, well developed to fulfil needs of the infant growth [8]. The main nutrient constituents of milk are shown in Table 1 [8][9][10][11]. Further to nutrients, human milk contains many bioactive constituents like immunoglobulins, growth factors, microRNAs, and human milk oligosaccharides (HMOs). These form a complex system linking the mother’s lifestyle, such as diet and gut microbiota, with outcomes on infant growth, gut microbiota, immunity, and other developmental features [12]. In the infantile gut, a human milk-directed microbiota develops, forming initial doses for seeding the mature gut microbiome. Additionally, the high concentration and structural diversity of HMOs that reach the infant colon, initiate a series of health effects [13]. To this end, this review article presents up-to-date information about human milk benefits, human milk microbiota, and HMOs, and revises possible associations between microbial milk profiles and both maternal diet and HMOs.
Table 1. Main nutrient constituents of human milk and respective concentrations [8][9][10][11].
| Macronutrients | |
| Protein | 0.9 g/dL |
| Fat | 3.5 g/dL |
| Carbohydrates (mainly glucose) | 6.7 g/dL |
| Minerals | |
| Calcium | 200–250 mg/L |
| Magnesium | 30–35 mg/L |
| Phosphorus | 120–140 mg/L |
| Sodium | 120–250 mg/L |
| Potassium | 400–550 mg/L |
| Iron | 0.3–0.9 mg/L |
| Fluoride | 4–15 µg/L |
| Fat-Soluble Vitamins | |
| Vitamin A | 0.3–0.6 mg/L |
| Vitamin D | 15 IU/day (in exclusively breastfed infants) or 0.33 µg/L |
| Vitamin E | 3–8 mg/L |
| Vitamin K | 2–3 µg/L |
| Water-Soluble Vitamins | |
| Ascorbic acid | 100 mg/L |
| Thiamine (vitamin B1) | 200 µg/L |
| Riboflavin (vitamin B2) | 0.35–0.39 mg/L |
| Niacin | 1.8–6 mg/L |
| Vitamin B6 | 0.09–0.31 mg/L |
| Folate | 80–140 µg/L |
| Vitamin B12 | 0.5–1 µg/L |
2. Human Milk Benefits
A priceless natural regimen presented by a mother to her newborn, human milk offers an extensive array of health benefits [14]. Milk from mothers whose diet is sufficient and well-balanced supplies all the necessary nutrients suitable for ideal growth (except vitamin D, whose intake should be 400 IU/day for all breastfed infants) [15]. Any amount of breastfeeding is better than none, and superior benefits accumulate with increased duration [3]. In addition to its supreme nutritive value, current scientific evidence about breastfeeding supports many beneficial effects, not only for infants who are breastfed on both short- and long-term, but also for mothers who breastfeed [16].
2.1. Short-Term Benefits for Infants
Breastfeeding possesses obvious short-term benefits for infant health, mostly in decreasing mortality and morbidity resulting from infectious diseases, such as gastrointestinal and respiratory tract infections, as well as otitis media, with a level of evidence described as convincing [15].
The protection conferred by breastfeeding against diarrhea is certainly one of the most consistent findings in the epidemiological literature [3][15][17][18][19]. A systematic review published by the WHO in 2013 suggests that breastfeeding substantially protects against morbidity/mortality from diarrhea, with a reduction of 50% in morbidity and 80–90% in mortality and hospital admissions, compared to infants with less or no breastfeeding. Such protection was higher among infants who were exclusively breastfed in the first six months of life and was provided by more intense breastfeeding. These robust results were observed across high and low-income settings [17]. An integrative review by Santos and Colleagues [20] in 2015 also replicated similar results, confirming the importance of breast feeding in the prevention of diarrhea in children under six months, especially among those who were exclusively breastfed. Such studies reinforce the recommendations for exclusive breastfeeding during the first six months of life as a key intervention for infant survival. The protective effect of breastfeeding against gastrointestinal infections has been attributed to the positive role of the microbial and immunological milk constituents and to the lack of contamination from baby bottles [19].
In addition to prevention of diarrhea, it is well documented that breastfeeding is associated with a significantly lower risk for lower respiratory tract infections and otitis media, compared with non-breastfed children [21]. Breast milk is associated with around 30% reduction in morbidity, 50% in hospital admissions, and 60% in mortality from these infections, suggesting that breastfeeding affects not only the incidence of these infections, but also their severity [17]. A systematic literature review and meta-analysis showed that suboptimal breastfeeding increased the risk of morbidity and mortality from pneumonia across different infant age groups. In particular, pneumonia mortality was higher among non-breastfed compared to exclusively breastfed infants 0–5 months of age, and among non-breastfed compared to breastfed infants and young children 6–23 months of age [22]. In a study from UK, higher risk of respiratory infection was found among infants exclusively breastfed for 4–6 months, but who stopped breastfeeding by six months, adding to the evidence of continued milk benefits after six months [23]. Furthermore, evidence shows that breastfeeding protects against acute otitis media until two years of age, but protection is greater for exclusive breastfeeding and breastfeeding of longer duration [24]. In a recent cohort analysis from Denmark in 2020, involving 815 mother-infant pairs, a strong association between breastfeeding and hospitalizations due to lower respiratory tract infection was found particularly in the first year of life [25]. In an interventional study to promote successful breastfeeding, Zivich and Colleagues showed that breastfeeding reduced incidence of mild as well as severe episodes of both respiratory infection and diarrhea in the first six months of life [26].
Recently, with the upsurge of Coronavirus Disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), recommendations about breastfeeding in infected mothers were endorsed [27]. Breastfeeding in postpartum women with SARS-CoV-2 is recommended by the WHO [28] and by the US Centers for Disease Control and Prevention (CDC) [29] for the newborn as long as health of the mother and newborn allow it. This belongs to the same context of the protective effect of mother’s milk against infections and is built on the general evidence that benefits of breastfeeding substantially outweigh the potential risks for SARS-CoV-2 transmission. Appropriate respiratory hygiene measures for infected mothers always have to be considered with direct breastfeeding [30].
Apart from infection, the American Academy of Family Physicians reports reduced incidence of atopic dermatitis (eczema) in breastfed infants [3]. Although there is evidence that exclusive breastfeeding for 3 to 4 months reduces incidence of atopic dermatitis in the first two years of life, long-term advantages for exclusive breastfeeding for prevention of atopic skin disease are not clear. Furthermore, there are no definite conclusions regarding the role of breastfeeding in preventing nor postponing the onset of specific types of food allergies in newborns [31]. In a nationwide cohort studied in Japan in 2020, breastfeeding had prophylactic effects on food allergy only among high-risk children (defined as having positive family history and eczema presentation prior to one year) with infantile eczema, whereas prolonged breastfeeding may increase the risk of food allergy [32].
Another possible benefit of human milk is being a protective factor against sudden infant death syndrome, which is the leading death cause in the United States in infants of ages 1 month to 1 year [33]. Several literature reviews have documented that sudden infant death syndrome risk decreases with breastfeeding [34][35][36], and the effect is stronger when breastfeeding is prolonged [37]. Such positive effect of breastfeeding has been deliberated in physiologic sleep studies, which have revealed that breastfed infants have lower arousal thresholds than their formula-fed counterparts, probably providing a survival mechanism for protection against this condition [38][39].
2.2. Long-Term Benefits for Infants
In addition to growth and infection prevention on the short-term, research into human milk also indicates that it may influence noncommunicable diseases or conditions later in life, perhaps offering a lifetime of benefits [40]. For instance, the relationship between breastfeeding and obesity prevention has long been sought by researchers. According to meta-analysis, breastfeeding appears to a significantly protect against obesity in childhood, although evidence on this role is still controversial, and the underlying mechanisms are still unclear [41][42]. Recently, in a prospective six-year study from Spain that followed a cohort of infants from birth up to six years of age, full breastfeeding was associated with a significant decrease in obesity. The delay in introduction of bottle feeding showed a probable protective effect against obesity at 6 years of age [43]. In trying to explain the role of breastfeeding in prevention of obesity, epidemiological data illustrate that breastfed children have healthier dietary patterns compared to formula-fed children, probably reducing their risk of obesity. These healthier patterns are, in part, caused by early opportunities for flavor learning afforded by breastfeeding. In particular, the flavors of the mother’s diet are transmitted to the infant via breastmilk, providing an additional reinforcement and flavor learning upon repeated exposure to a wide variety of flavors. The flavors experienced by the infant modulate later food preferences, acceptance of solid food, and culture onto which the infant is weaned [44]. Plus, breastfeeding favors the establishment of diverse microbial communities in infant’s gut that play a crucial role in preventing obesity via their various mechanisms of harvesting energy from food, affecting patterns of fatty acid oxidation, modulating bile acid metabolism, and influencing satiety and lipogenesis. It is still debatable to define which microbial category is responsible for obesity, and studies in this regard show discrepancies [45].
Studies linking human milk to protection against gastrointestinal tract diseases are also numerous. For the past two decades, human milk have been associated with reduced incidence in necrotizing enterocolitis in preterm infants [46][47]. Such infants’ susceptibility to enterocolitis stems from their gastrointestinal and immune system immaturity. It is thought that an exclusive human milk diet, consisting of mother’s own milk and/or donor human milk alone or fortified with a human milk-based fortifier, compensates for these immature systems by many pathways like stimulating intestinal motility, composed from lowering gastric pH, decreasing epithelial penetrability, and altering the composition of the gut microbiota [48]. In a multicenter study in a cohort of over 1000 patients with Crohn’s disease, a history of breastfeeding was inversely associated with complicated pediatric symptoms [49]. The evidence of the link between Crohn’s disease prevention and breastfeeding, however, is described as insufficient, and more reliable data are needed [15]. Similarly, limited evidence exists for the role of breastfeeding in reducing incidence of celiac disease, and further investigation is needed to indicate whether such protection only delays the onset of the disease or if it can provide permanent protection [50].
Metabolic disorders and tumors also had their share in research trying to investigate late benefits of breastfeeding. In a meta-analysis, Horta and Colleagues [40] found that breastfeeding decreased the odds of type 2 diabetes mellitus and based on high-quality studies, but no associations were found for total cholesterol or blood pressure. Breast milk has long-chain polyunsaturated fatty acids (LCPUFAs), whose supplementation is associated with a reduction in blood pressure. In addition, LCPUFAs would induce early changes in skeletal muscle which can be protective against insulin resistance and type 2 diabetes [51]. As far as cancer is concerned, and with leukemia, which accounts for 30% of childhood cancers, a meta-analysis showed that breastfeeding for six months or longer may help reduce the incidence of childhood leukemia by up to 19% [52]. Furthermore, a pediatric oncology team assessed relation between breastfeeding and cancer in a group of 300 children with cancer (leukemia, lymphoma, and solid organ cancers including brain, bone, liver, kidney, and others), and an approximately equal number of age- and sex-matched controls. Breastfeeding duration of the control group was found to be significantly longer than the patient group [53].
Perhaps the possible influence of breastfeeding on cognitive development is a subject that has not ceased to provoke considerable scientific debate. Despite substantial disparity of reported results, a positive correlation between breastfeeding and the levels of intelligence and cognitive development in children exists, although with a number of confounders like maternal age, education, socioeconomic status, and IQ [15][21]. In general, children who are breastfed for longer than six months are known to show better cognitive outcomes and a lower risk to develop attention-deficit/hyperactivity disorder [54]. Researchers from McGill University, and based on the largest randomized, interventional trial ever conducted in the field of human milk benefits, provide robust evidence that prolonged, exclusive breastfeeding enhances children’s cognitive development up to age of 6.5 years [55]. However, in a study from UK analyzing children till age of nine years breastfeeding was not associated with IQ advantage nor difference in neurological soft signs. The higher IQ associated with breastfeeding was probably accounted for by some confounding maternal and socio-economic factors [56]. The actual mechanisms linking improved cognitive development with breastfeeding remain unknown; however, it is suggested that human milk LCPUFAs are incorporated in relatively large amounts during early growth of the brain and the retina [57], and more specifically, the white matter of the brain [21]. Some of these acids, such docosahexaenoic acid (DHA) and arachidonic acid (ARA), present in human milk, may play a role in growth and development of eye, brain and nerve; these acids also exhibit potent biological activity that modulates various cellular and tissue processes [58][59]. Interestingly, and also in the field of neurologic development, a recent meta-analysis in 2020 concluded that breastfeeding for 12–24 months was associated with a significant decrease in autism spectrum disorder risk [60]. Furthermore, studies show that breastfed infants, as compared to bottle-fed infants, show higher vigor, including social approach, activity and the intensity of reaction [61]. The duration of breastfeeding was shown to correlate negatively with parent-reported antisocial conduct and aggressive behavior in children aged 4 to 11 years [62]. These and other findings continue to verify that human milk is not simply a meal at the breast, but rather a biological fluid with significant and far-reaching effects into child’s health, psychology, and social behavior.
It is worth noting that the public health significance of breastfeeding originates from a big pool of studies that are mostly observational. Large interventional or randomized controlled trials on breastfeeding are usually hindered by ethical issues. Therefore, the evidence for short-term outcomes mentioned above, such as reduced rates of infection, can be classified as strong. However, the longer term effects are probably less firm, due to difficulty in completely accounting for confounding factors [16].
2.3. Maternal Benefits
While the nutritional and physical health benefits conveyed by human milk are well established for infants, accumulating research reveals that breastfeeding mothers benefit as well. As an evidence of such profits, it was reported that mothers with premature weaning have health risks of breast and ovarian cancers, hypertension, hyperlipidemia, diabetes mellitus, myocardial infarction, and obesity, with these risks being highest in those who do not breastfeed at all [63].
Breastfeeding reduces overall risk of breast cancer [64]. In a meta-analysis, exclusive breastfeeding among parous women was found to reduce the risk of breast cancer compared with parous women who do not exclusively breastfeed [65]. Furthermore, mothers positive for the BRCA1 mutation and breastfeeding for at least one year have 37% lower breast cancer risk compared to carriers of the mutation who do not breastfeed [66]. The reduced risk of breast cancer is most evident in postmenopausal women and is directly proportional to the duration of lactation. The protective effect of breastfeeding is attributed to differentiation of breast cells, decreased number of ovulatory cycles, and estrogen excretion through milk, reducing overall exposure to this hormone [67]. In addition to breast cancer, evidence suggests that breastfeeding reduces risk of ovarian cancer [63]. Modugno and Colleagues [68] concluded that breastfeeding for as few as three months was associated with reduced risk of ovarian cancer, and the association was long-lived. The probable mechanism of breastfeeding in ovarian cancer protection may be due to absence of ovulation. Monthly ovulation might increase the odds of genetic mutations, and ovarian overstimulation by elevated gonadotropins may trigger hyperproliferation, with possible malignant transformation [69].
Prolonged breastfeeding has beneficial effects in reducing the long-term risk of coronary artery disease. Parous women who breastfeed for five or more months in at least one pregnancy seem to be at decreased risk of coronary artery disease, whereas parous women who either did not breastfeed at all or discontinued breastfeeding early seem to be at elevated risk [70]. The European Society of Cardiology concluded that breastfeeding women had a lower risk of coronary artery disease later in life compared to those who do not breastfeed [71]. Data from studies in both animals and humans suggest that lactation may alter maternal sugar and lipid homeostasis and may exert effects on blood pressure regulation. This might have significant effects on lipid homeostasis, with lower triglyceride and higher HDL cholesterol levels, possibly reducing risks of heart diseases [72]. Breastfeeding also affects maternal risks of hypertension and diabetes mellitus [63][64][73]. Park and Choi [74] studied a population of over 3000 nonsmoking postmenopausal women aged 50 years or above, and showed that more children breastfed and longer breastfeeding duration were associated with lower risk of hypertension. A systematic review and meta-analysis determined that variable breastfeeding durations have different protective effects against maternal hypertension, especially if continued beyond 12 months [75]. Longer duration of breastfeeding, whether full or partial, is associated with lower maternal risk of hypertension and heart disease irrespective of pre-pregnancy body mass index and abdominal adiposity seven years after delivery [76]. Further, mothers who breastfeed have less visceral obesity and smaller waist circumference, probably accounting for a lower maternal risk of type 2 diabetes mellitus [77], and this was recently proved in a prospective cohort of women followed for 14.2 years [78]. Few common mechanisms of relationships between breastfeeding and maternal risks of hypertension and diabetes were suggested. For example, maternal metabolism (e.g., fat accumulation and insulin resistance) may be reversed by breastfeeding, which decreases both diseases risk. During pregnancy, visceral fat accumulates, insulin resistance rises, and lipid and triglyceride levels increase. These changes appear to retune more rapidly, and more entirely, with lactation—the so called “reset hypothesis” [79]. Another theory is that oxytocin, needed in glucose homeostasis and the release of which is stimulated by breastfeeding, may be associated with decreased risk of type 2 diabetes mellitus [80]. It is worth mentioning that milk production, which consumes about 500 kcal per day for an infant who is exclusively breastfed, reduces maternal obesity later in life [81].
Apart from cardiovascular health, breastfeeding affects psychological well-being. Breastfeeding mothers report less anxiety, less negative mood, and less stress, as well as increased sleep duration and reduced sleep disturbances when compared to formula-feeding mothers [82][83]. On another note, studies on post-partum depression demonstrate that breastfeeding may protect mothers from this disorder, and researchers have strived to explain the biological processes that explain this protection. For example, lactation attenuates neuro-endocrine responses to stress, and this may be related to fewer post-partum depressive symptoms [84][85]. Moreover, early breastfeeding cessation was linked to higher risk of post-partum depression [86]. It is the psychological pressure to exclusively breastfeed that contributes to postpartum depression symptoms in mothers unable to achieve their breastfeeding intentions [87][88]. In a prospective follow-up for eight weeks postpartum, mothers with breastfeeding problems (including mastitis, nipple pain, need for frequent expressing of milk, or over-supply or under-supply of milk) showed poor mental health [89].
With all the aforementioned benefits of human milk on infant and maternal wellness, breastfeeding continues to form a cornerstone for prevention of many short- and long-term health risks. It is a real investment in health, rather than a mere lifestyle decision. The promotion of human milk as a highly active biological milieu is substantially imperative, and further research into its numerous potential benefits is always appealing.
Figure 1 summarizes data from Section 2.1, Section 2.2 and Section 2.3 of this review pertaining to benefits of human milk and breastfeeding.
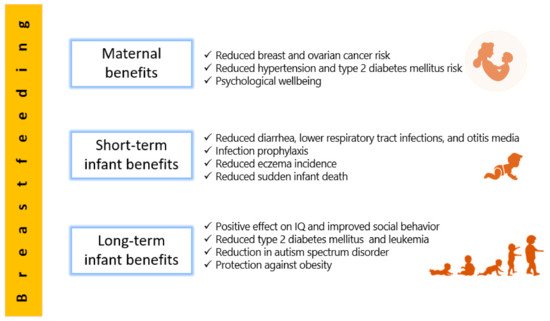
Figure 1. A summarized description of the benefits of breastfeeding and effects of human milk on the early and long-term infant health, as well as benefits for the mother.
3. A Focused Insight into Human Milk Microbiota
In addition to its diverse nutritional and health benefits, human milk is characterized by a rich microbiota which constitutes a source for the infantile gut microbiota [90]. Except for initial observations of occurrence of microbes in milk in the 1950s, while studying the possibility of transmission of infections to infants via breastfeeding [91], human milk was thought of as a sterile fluid [92]. Around the beginning of the twenty-first century, a study in Spain by Martin and Colleagues [93] demonstrated, for the first time, the presence of the lactic acid bacterium, Lactobacillus gasseri, from milk and stools of mother–infant pairs, indicating that breast milk formed a potential source for infant microbiota. The non-sterility of breast milk has, ever since this initial observation, started to become extensively researched, and regarded as a protective factor arising from the wide range of microbes, in addition to the universally acknowledged nutritional role [94].
3.1. The Origin of Human Milk Microbiota
The origin of microbial populations in human milk is not completely understood and remains debatable. However, milk microbial populations have been proposed to originate endogenously, from the maternal digestive system through a complex pathway involving immune cells, or from the mother’s skin or infant’s mouth [95], or from the breast tissue itself [92]. It is plausible that more than one pathway contribute to the bacterial contents of milk [96].
3.1.1. The Entero-Mammary Pathway
The discovery of anaerobic species, such as bifidobacteria, in human milk suggested the transfer of microbiota from the mother’s gut to milk, especially since these anaerobes are not commonly cultivated from breast skin swabs [90]. A physiological translocation mechanism is thought to carry maternal intestinal microbiota to the lactating mammary gland. This pathway is supported by the presence of bacterial communities in colostrum, collected even before first infant suckling occurs [97]. The unique process of translocation is aided by physiological and hormonal variations during late pregnancy and increased permeability of the intestinal epithelial lining [98], and is also immunologically mediated. Such active migration of microbes involves mononuclear cells, dendritic cells, and CD18 cells, delivering nonpathogenic bacteria from the intestine to the lactating mammary gland [99]. Dendritic cells are capable of penetrating the gut epithelium by loosening the tight junctions between intestinal epithelial cells and taking up bacteria from the gut lumen. Bacteria are then transported by macrophages to mesenteric lymph nodes and finally to the mammary gland [98][100].
3.1.2. Retrograde Origin
Ultrasound imaging studies of nursing mothers have proved retrograde back flow of milk from the infant oral cavity due to infant suckling, suggesting a backward movement that transfers bacteria from infant’s mouth into the mother’s mammary gland [101]. This theory of milk microbiota origin is supported by similarities between the infant oral microbiota and human milk microbiota. For instance, Streptococcus, an abundant bacterial genus of breast milk, also dominates the salivary bacteria. Nevertheless, whether both bacterial communities share the same species and strains of Streptococcus remains to be determined [92][102].
3.1.3. Transfer from Maternal Skin
3.1.4. Mammary Tissue Origin
In 2014, Urbaniak and Colleagues [105] showed, using culture and 16S rRNA gene sequencing, the presence of various genera of bacteria in breast tissue of Canadian and Irish women aged 18–90, with or without history of lactation, and without signs or symptoms of infection. Other investigations also showed that the breast tissue itself has an established microbiota, which is quite different from that of breast skin tissue and breast skin swabs [106]. Investigations on nipple aspirate fluid collected aseptically by application of negative pressure on the nipple, showed presence of bacterial DNA, probably from breast ducts [107]. It is probable that the existence of six to eight ductal openings on the surface of the human nipple permits microbes environmental microbes to access the ductal system of the breast [108]. It is also likely that such microbiota may influence the one present in human milk [92].
In summary, regardless of the relative contribution of the above four sources to microbes in milk, it has become generally acceptable that milk microbiota is not a secondary contamination, but rather a discrete microbiota, which is distinct from others in both the mother and the infant.
3.2. Types of Microbes in Human Milk
Both the early, culture-based methods, as well as the new, genomic-based and sophisticated techniques, such as next-generation sequencing, have been applied to analyze human milk microbiota and embrace its diversity [109]. Breast milk is a niche to several hundreds of bacterial species, and harbors about 1000 colony-forming units of bacteria/mL [110]. Following initial exposure to microbes upon birth, breast milk is the next immediate important source of various microbes to seed the infant’s gut [103]. As an evidence of this vertical transfer from milk microbiota to infant intestine, it was found that infants who primarily breastfeed during the first month of life, share 28% of their stool microbes with microbes of their mother’s milk. The number of shared microbes rises with the amount of daily breast milk intake in a dose-related manner, and microbes in the infant gut most resemble those from their own mother. The types and proportions of different microbes in human milk exhibit interindividual variation [103][111]. The major types are presented below.
3.2.1. Bacteria in Human Milk
A very recent systematic review in 2019 determined the bacterial repertoire of human milk, showing the presence of about 820 species, mainly belonging to Gram-positive Firmicutes and Gram-negative Proteobacteria [112]. Firmicutes include members of the genera Staphylococcus, Streptococcus, Lactobacillus, Bifidobacterium, Enterococcus, Veillonella, Gemella, Clostridium, and others. On the other hand, Proteobacteria include Escherichia, Pseudomonas, Enterobacter, Serratia, Ralstonia, Sphingomonas, Bradyrhizobium, and others. Additionally, milk contains Actinobacteria, like Actinomyces, Corynebacterium, and Propionibacterium, Fusobacteria such as Leptotrichia, as well as Bacteroidetes, such as Prevotella [113]. Since 2011, scientific consensus has been molded towards the existence of a “core” bacteriome in human milk, consisting of the following nine genera: Staphylococcus, Streptococcus, Serratia, Pseudomonas, Corynebacterium, Ralstonia, Propionibacterium, Sphingomonas, and Bradyrhizobium. These represent about half of the microbial milk community, although their abundance might diverge among milk [87][102]. In terms of relative proportions, Streptococcus and Staphylococcus species are the most common, and this is universally true, irrespective of variability in geographic location or analytical methods of milk analysis, whether culture or molecular based. They were followed by Bifidobacterium, Lactobacillus, Propionibacterium, Enterococcus, and members of the Enterobacteriaceae family [114]. During breastfeeding course, the overall bacterial diversity decreases compared with colostrum and transition milk. Moreover, the composition of the bacteria shifts from skin and enteric organisms such as Staphylococcus and Streptococcus, to infant mouth and skin organisms, such as Veillonella, Leptotrichia, and Prevotella, which increase as lactation progresses and mature milk is secreted [115].
3.2.2. Fungi in Human Milk
Most studies of human milk microbiome have overlooked fungi, and they have not been thoroughly assessed. In a cohort of Australian women, Candida was detected in breast milk in both culture and PCR-based methods [116]. Furthermore, a metagenomic analysis of milk samples showed not only fungal-related reads of DNA, but reads related to Archea and protozoa as well [117]. In 2020, a cohort of 271 samples of human milk revealed, through DNA sequencing, the presence of fungi in over 20% of samples, dominated by the genera Candida, Alternaria, and Rhodotorula [118]. In a study comparing transient and mature human milk samples, the most abundant fungal species in transient milk were Saccharomyces cerevisiae and Aspergillus glaucus. While A. glaucus was the second most common species in mature milk, S. cerevisiae disappeared, and Penicillium rubens appeared as the most abundant species, suggesting variable composition of milk mycobiome with maturity [119]. Further research is needed to establish the prevalence and role of fungi in human milk.
3.2.3. Human Milk Virome
Recent evidence is emerging to show that the neonatal virome is modulated by breastfeeding [120], and human milk contains viruses transmitted from the mother to the infant to colonize the infant gastrointestinal tract [121]. These viruses include eukaryotic viruses, bacteriophages, and other viral particles [122]. Specifically, bacteriophages form a majority, and they have the ability to kill bacteria or provide them with potentially beneficial genes [109].
3.3. Milk Microbiota Diversity and Associated Factors
A myriad of environmental, genetic, and immune factors personalizes a mother’s milk microbiome. Among different women, and in the same woman experiencing diverse physiological, hormonal, and pathological conditions, an inconsistency is expected among communities of milk microbiota [92].
Primarily, maternal factors contribute to differences in milk microbiota composition. Khodayar-Pardo and Colleagues [123] showed that Cesarean section births were associated with total bacterial concentrations in milk higher than vaginal delivery, and significantly higher levels of Streptococcus, but significantly lower levels of Bifidobacterium. This was associated in some studies with risk of immune-mediated and inflammatory disorders in infants born by Cesarean delivery [124]. Possibly, physiological stress or hormonal signals of delivery could influence the microbial transmission process to milk [115]. The difference in composition of milk microbiota among vaginal deliveries and Cesarean section, however, was not significant in other investigations [125], warranting further analysis. A fail-safe mechanism has been proposed, whereby the mother passes along her bacterial imprint irrespective of how the baby is born, eliminating major differences in milk microbiome composition with mode of delivery [125]. The differences in milk microbiota among cesarean and vaginal deliveries are, however, thought to be only short lived when reflected on infant gut microbiome. By one month, vaginal and cesarean section infants cannot be separated on the basis of composition of their microbiota [126]. Based on available evidence, it is difficult to draw clear-cut conclusions regarding milk microbiota composition and both mode of delivery and milk maturity.
Maternal body weight also influences milk microbiota. Milk from obese mothers was found to include a bacterial community of lower diversity compared with milk from normal-weight mothers, according to findings of a randomized controlled trial, but was associated with higher numbers of Staphylococcus and lower numbers of Bifidobacterium [115]. The link between gut microbiota and obesity may possibly be extended to milk, substantiating the conception that obesity influences the milk microbiota via its influence on the gut microbiota [115][127]. Indeed, maternal diet influences milk microbiota, and this is elaborated in Section 3.4.
In 2015, and also relating to maternal factors on milk microbiota composition, Olivares and Colleagues [128] showed that levels of bifidobacteria were reduced in milk of mothers with celiac disease compared to healthy mothers, and this could, theoretically, diminish the protective effects of breastfeeding on the child’s risk of developing celiac disease. Further, breast milk favored Clostridium leptum, Bifidobacterium longum, and Bifidobacterium breve gut colonization, and these were associated with the HLA type of low genetic risk for celiac disease [129]. In an analysis of intrapartum antibiotic administration on human milk microbiota, it was found that the species Bifidobacterium was uniquely found in breast milk samples of mothers who did not receive antibiotics. Such observation may be significant, since reduced fecal bifidobacteria in early infancy may be associated with higher risk of non-communicable diseases in childhood, like atopy and overweight [130].
On another note, maternal postnatal psychosocial distress may alter maternal gut microbiota, which, in turn, may affect bacteria present in milk. Browne and Colleagues [131] found no significant differences in the relative proportions of major bacterial genera between women with high and low levels of psychosocial distress. However, progressive and distinct decrease in Firmicutes, Proteobacteria, and Bacteroidetes at the phylum level, and increase in Acinetobacter, Flavobacterium, and Lactobacillus at the genus level, were evident in milk samples of women with low psychosocial distress. High maternal psychosocial distress was also related to significantly lower bacterial diversity in milk three months after delivery. These findings suggest a likely relationship between maternal psychosocial distress and milk microbiota, indicating that post-partum psychological symptoms may impact infantile development and health, through their influence on milk.
Milk bacterial profiles do not appear to significantly differ in relation to maternal age, infant gender, or race/ethnicity in a given geographical region [109], but do differ across geographic locations of Europe, Africa, and Asia. Across these continents, Kumar and Colleagues [132] analyzed milk samples of 80 women from 4 different countries (China, Finland, South Africa, and Spain). Spanish women had highest abundance of Bacteroidetes, whereas Chinese women had highest abundance of Actinobacteria. Women who had a cesarean section had higher amount of Proteobacteria as observed in the milk of the Spanish and South African women. Interestingly, and in the emerging field of human milk mycobiome, a core of four genera was shared across milk samples from the above four countries, consisting of Malassezia, Davidiella, Sistotrema, and Penicillium, which are also present in the infant gut, supporting potential role of breast milk in the initial seeding of fungal species in the infant gut [133]. In a very recent investigation from Dubai [134], the genus Hydrogenophaga (of the beta-Proteobacteria), previously reported in breast cancer tissue [106], was significantly higher in the breast milk of local women compared to expatriates living in Dubai. This finding may shed a light on possible influence of race and lifestyle on human milk microbiota, but the importance of such data needs to be further explored.
Other determining factors of human milk microbiota have been investigated. In one survey, of milk samples collected at different stages of lactation, Bifidobacterium spp. concentration was significantly higher in milk samples from term gestations compared to preterm ones, indicating that gestational age plays a role in structuring milk microbiota [123]. This was not reproducible in another study on Canadian women, whereby comparison of bacterial profiles between preterm and term births showed no statistically significant differences [125]. The number of lactobacilli- or bifidobacteria-positive samples was significantly lower in women treated with antibiotics during pregnancy or lactation [135]. This emphasizes that consideration needs to be given to the impact of drugs administered to the breastfeeding mother, not only on potential consequences for the infant health, but also in diverting the normal milk microbiota [109].
To summarize the above, a review about the multitude of factors influencing human milk composition was very nicely and recently elaborated by Zimmermann and Curtis [136] in 2020. This review of 44 studies recognized some evidence that gestational age, infant sex, delivery mode, parity, lactation stage, diet, body mass index, composition of breast milk, geographic location, HIV infection, and method of collection affect composition of the breast milk microbiota. However, many studies were small and findings possibly conflicting, indicating need for further research setting some benchmarks on the internal and external players affecting the nature of human milk microbiota.
3.4. The Effect of Maternal Diet on Human Milk Microbiota and on Intestinal Microbiota of Infants
In addition to the numerous physiological, medical and environmental factors described above, it was shown that the assembly of microbes in human milk is also affected by maternal diet [137]. The associations between numerous macro- and micronutrients in maternal diet and the milk microbiota have been investigated.
In fact, maternal diet affects the concentration of certain substances, like fatty acids, in milk. In other words, maternal nutrient intake may indirectly help shape the bacterial community membership in human milk simply because of its impact on milk nutrient composition. For example, Kumar and Colleagues [132] documented multiple associations between human milk fatty acid profiles and variations in milk microbiota. Monounsaturated fatty acids of milk were negatively associated with Proteobacteria, but positively associated with Lactobacillus genus.
Additionally, maternal nutrient intake affects gastrointestinal bacterial communities, which in turn, may become part of the milk microbiota via the entero-mammary pathway (Section 3.1.1). For example, Carrothers and Colleagues [138] provided initial evidence for associations between maternal nutrition and maternal fecal microbial community structure during lactation. Increased intake of pantothenic acid, riboflavin, vitamin B6, and vitamin B12 were related to increased relative abundance of Prevotella and decreased relative abundance of Bacteroides in the maternal gut. Intake of copper, magnesium, manganese, and molybdenum were positively associated with Firmicutes and negatively associated with Bacteroidetes. Overall, findings steadily suggest that high consumption of a more nutrient-rich and calorie-loaded diet was positively associated with relative abundance of Firmicutes. It was suggested that such maternal dietary factors that directly influence the maternal gastrointestinal bacterial community, might also indirectly affect the milk microbiota [137].
Correlations between maternal dietary intake and milk microbiota composition have been validated by several studies. In one investigation from Brazil, postpartum women went through a validated food frequency questionnaire that covered the whole pregnancy period, and correlations were obtained between their diet and their milk microbiota. A global, significant association with milk microbiota diversity was observed for the intake of ascorbic acid during pregnancy [139]. In light of ascorbic acid importance, higher maternal consumption of citrus fruits, as well as of vegetables and β-carotene during pregnancy were protective against eczema in the offspring, in line with the positive effects of ascorbic acid on the human immune system [140]. Furthermore, positive correlations were found between Bifidobacterium in the milk and intake of polyunsaturated and linoleic fatty acids during lactation [139]. This is consistent with the conversion of polyunsaturated fatty acids to conjugated linoleic acid and conjugated linolenic acid, known to favor Bifidobacterium growth [141].
In a study of 21 healthy breastfeeding women at Washington State University and University of Idaho, diet records, collected over 9 dietary assessments over six months postpartum, were correlated with milk microbiota. The results showed that relative abundances of several bacterial groups were associated with changes in maternal dietary intake. For instance, intake of saturated fatty acids and monounsaturated fatty acids were inversely associated with the relative abundance of Corynebacterium; total carbohydrates, disaccharides, and lactose were negatively associated with Firmicutes; and protein consumption was positively correlated with the increase in Gemella, a genus belonging to the Streptococcaceae family [137].
In an interesting animal model of diet followed for 31 months, it was demonstrated that alone, diet may modulate mammary gland microbiota. Mediterranean diet resulted in approximately 10-fold increase in mammary gland Lactobacillus abundance compared with mammary tissue from Western diet-fed animals [142]. Lactobacillus is often thought of as commensal organism and is commonly included in probiotic formulations, where it directly contributes to beneficial nutritional, physiological, microbiological, and immunological effects in the host [143]. Again, with Lactobacillus transferred from maternal gut to human milk, and then from milk to intestinal tract of infants, a dynamic pathway exists in which maternal diet will play a major role in determining the profile of infant gut microbiota [144].
Another recent study reinforcing the hypothesis that maternal diet affects milk microbiota found, at one-month postpartum, significant negative correlation between Streptococcus in maternal milk and the intake of unsaturated fatty acids and the abundance of oleic acid. This was explained by the fact that streptococci may be sensitive to the antibacterial effects of fatty acids, causing a direct inhibition [145]. The same group reported that Bifidobacterium, pivotal in development of the infant gut microbiome, was detected, at extremely low abundances, in milk samples [145]. This observation is in favor of the current view that maternal milk provides a microbial source for colonization of the infant intestine. However, the main effect of milk on the baby’s gut composition does not necessarily come from the milk bacteria only, but rather from other milk components, as well, that help to enrich specific bacterial groups. This could explain why bacterial breast milk composition and fecal microbiota composition are not exactly the same, but correlations do exist [146]. In light of benefits of Bifidobacterium, a trial investigated maternal supplementation of this bacterium as well as Lactobacillus to maternal diet four weeks prior to the due date and for three months after delivery. The intervention did not significantly affect overall composition of breast milk microbiota transferred to the infant during breastfeeding, analyzed by 16S rRNA sequencing, thus questioning the substantial effect of probiotic supplementation to maternal diet on microbiota composition of breast milk [147].
The currently available reports on maternal diet and microbiota of human milk are still few and reports on the role of nutrients in the metabolism of bacteria in human milk are much needed. Furthermore, the somehow incomplete knowledge regarding the availability of macro- and micronutrients in milk, which are directly useful for bacteria, further complicate data interpretation. The relationship between maternal diet and milk microbiota has much yet to be investigated, and in-depth studies of various nutritive components and their concentrations on microbiota of milk are warranted.
3.5. Beneficial Effects of Milk Microbiota on Infant Health
The commensal and beneficial microbes in human milk play a pioneer role in shaping infant health, and perhaps represent an example of how intimate contact with the microbial world is necessary for normal development in early life [148]. Human milk microbiota affects the establishment of the largest human microbial reservoir in the gastrointestinal tract; also, the reciprocal relation between milk microbiota and gut microbiota is reflected on the development of immunity and protection against pathogens.
After birth, milk microbiota forms the most important determinant of infant gut colonization, which occurs in a stepwise fashion. With initial exposure to microbes transferred to the infant gut from mother’s milk, colonization of the intestine of infants starts, and may be essential for the maturation of the gut-associated lymphoid tissue, homeostasis of the intestinal epithelium, and development of intestinal physiology [149]. In the first few days after birth, the infant intestine is characterized by a heterogeneous population of microbes characterized by facultative anaerobes that belong to Enterobacteriaceae, Streptococcus, Enterococcus, and Staphylococcus that thrive on oxygen availability in the newborn gut. Escherichia coli, Enterococcus faecalis, and Enterococcus faecium are the most characterized species among first colonizers. Gradual oxygen consumption by such facultative anaerobes creates a reduced oxygen environment that allows expansion of obligate anaerobes such as Bifidobacterium, Bacteroides, and Clostridium. Intestinal colonization undergoes further changes upon introduction of solid food. The genus Bifidobacterium is detected in the first few months, predominates by 12 months, then declines towards the second year, at the end of which the infant microbiota becomes more diverse [150]. It is essential here to note the distinction between gut microbiota of breastfed and formula-fed infants: It is well known today that Bifidobacterium is the predominant intestinal genus in both feeding modes [151], with species variations. The species Bifidobacterium longum, Bifidobacterium infantis, Bifidobacterium breve, and Bifidobacterium bifidum are commonly detected in breastfed babies, while Bifidobacterium adolescentis and Bifidobacterium pseudocatenulatum, commonly seen among the intestinal adult microbiota, predominate in formula-fed babies [152]. Collectively, formula-fed infants in general have relatively stable and diverse microbial intestinal communities with higher levels of facultative anaerobes and strict anaerobes when compared to breast-fed infants. Fecal samples from breast-fed infants are less complex, with higher numbers of aerobic organisms, and with more critical changes in microbial composition up to the first year. Once weaning, or the introduction of solid foods into the feeding pattern, begins, differences in microbial communities between breastfed and formula-fed infants diminish, and the microbial profile shifts towards the adult intestinal microbiome [153].
The establishment and development of bifidobacterial and lactic acid bacteria in infant’s gut from viable inocula in human milk was demonstrated by several studies. For example, a study of Spanish full-term breastfed infants evaluated similarity between fecal bacteria and those from corresponding milk samples. It was shown that in one-day-old newborns, Enterococcus and Streptococcus were the microorganisms most frequently isolated, while from 10 days until 3 months of age, bifidobacteria became predominant. In corresponding breast-milk, Streptococcus genus was most frequently isolated, and Lactobacillus and Bifidobacterium were also obtained [154]. With an approximate viable bacterial density of 2–4 log colony-forming units/mL of human milk, resulting in a projected daily ingestion of 5–7 log cells with regular breastfeeding, it is expected that neonatal gut microbiota echoes the bacterial composition of breast milk [155]. A trend consistent with breastfeeding is that breast milk selects for a highly adapted intestinal microbiota, dominated by bifidobacteria and termed a “milk-oriented microbiota” [150]. When a disbalance occurs in such orientation health changes may result, for example, preterm infants with altered microbiota are susceptible to necrotizing enterocolitis due to the immaturity of their gastrointestinal and immune systems [48]. In 2020, a meta-analysis of necrotizing enterocolitis in premature infants showed that a mixture of Bifidobacterium and Lactobacillus could reduce morbidity, illustrating role of human milk microbiota in health and disease [156].
The human milk microbiota has a putative role in prevention of infections in newborns, and this occurs through its contribution to the competitive exclusion of pathogens and its involvement in the maturation of the immune system [155]. One possible example of the competitive exclusion of pathogens is the genus Staphylococcus. Several Staphylococcus species, especially S. epidermidis, colonize human milk and the intestine of breastfed infants. In an analysis of milk and stool samples from mother–infant pairs, Jiménez and Colleagues [157] found that S. epidermidis was the predominant species in the milk and stool of breast-fed infants, while it was less prevalent in those of formula fed-infants. The presence of adhesion-related genes in S. epidermidis was very high, while the biofilm-related operon icaD and the gene mecA were only detected only rarely the S. epidermidis strains. Hence, the bacterial attachment factors were present but less commonly the virulence or antibiotic resistance determinants. The commensal staphylococcal strains provided by breast milk to the infant gut may successfully compete with potentially harmful strains. In another, more recent investigation on intestinal Enterococcus from breastfed infants, the commensal enterococci harbored antibiotic resistance and virulence genes. However, the patterns of these genes were not consistent with those described for antibiotic-resistant hospital-associated enterococci, and none were resistant to vancomycin. The frequency of virulence determinants like hemolysin and gelatinase was also low, while some genes linked to colonization were abundant. Taken together, these findings suggest a possible benefit of enterococci in preparing the infant gut for effective opposition against pathogens [158]. It is possible that the pre-colonization of infants with maternal commensal strains will help later in preventing acquisition of infection by more virulent ones [155]. Interestingly, and in the field of HIV infection, lactobacilli cultured from human milk were able to inhibit HIV-1 infection in vitro by blockade of CCR5 co-receptor, and to a lesser extent CXCR4 or both coreceptors, suggesting a probable role for lactobacilli in mucosal protection against HIV-1 [159].
In summary, an association between human milk microbiota, and consequently gut microbiota, and infant health and disease is an important contributor to infant hemostasis. In addition to the relationships described above, ongoing studies are continuing to investigate influence of microbiota on infant irritable bowel syndrome, inflammatory bowel disorder, and type 1 diabetes mellitus [160]. The functions of human milk microbiota and HMOs are summarized in Figure 2 below.
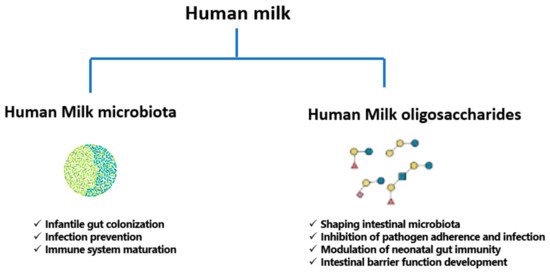
Figure 2. Functions of human milk microbiota and human milk oligosaccharides (HMOs).
3.6. Probiotics of Human Milk
The term “Probiotic” means “for life” and it is currently used to name bacteria associated with beneficial effects for humans and animals. In 2001, the Food and Agriculture Organization of the United Nations (FAO) and the WHO defined probiotics as “live microorganism which, when administered in adequate amounts confer a health benefit on the host” [161]. This definition was revised in 2014 by the International Scientific Association for Probiotics and Prebiotics, including for probiotics “microorganism for which there are scientific evidence of safety and efficacy [162]. Probiotics that have been principally studied in humans include species from Lactobacillus and Bifidobacterium genera, both of which have been used safely for quite a long time [90]. Probiotic administration early in life may be effective in prevention and treatment of some disorders, leading to a correct microbial colonization while the gut microbiota is still being established [163]. Increasingly administered to infants, probiotics are intended to decrease the risk of certain ailments like necrotizing enterocolitis and late-onset sepsis in preterm infants, colic and antibiotic-associated diarrhea in term infants, in addition to some chronic diseases of childhood such as asthma and atopic disease [164]. The mechanisms responsible for probiotic action still require full elucidation; however, they include modification of the gut microbiota and normalizing its perturbation, competitive adherence to the mucosa and epithelium for pathogen exclusion, strengthening of the gut epithelial barrier, improving the digestion process by complementing the functions of absent digestive enzymes, and modulating the immune system to offer an advantage to the host [162][165]. There are agreed-upon criteria that a probiotic must harbor to be considered efficacious, such as capacity to survive in the GI tract, resistance to stomach acidity, lack of mobile antibiotic resistance genes, and demonstration of health benefits through efficacy testing followed by clinical trials [90][166].
Human milk-derived bacterial strains can be considered as potential probiotics; isolation of strains from milk for subsequent use in infant health and nutrition markets is used [90]. For example, human milk strains of Lactobacillus reuteri, a well-studied probiotic found in the gut and breast milk, can reduce the production of pro-inflammatory cytokines, promote T cell development, and decrease the microbial translocation across the intestinal epithelium from the gut lumen to tissues, thereby reducing inflammation. Notably, the decrease in L. reuteri in humans in the past decades was correlated with an increase in prevalence of inflammatory disorders. Direct supplementation of L. reuteri may be an attractive preventive and/or therapeutic model against inflammatory diseases [167]. In a murine model of asthma developed in 2020, Li and Colleagues [168] showed that oral administration of L. reuteri was more effective in asthma prevention than five other Lactobacillus species, where it reduced the risk of asthma by modulating specific gut microbiota to improve the immune environment of the lungs. L. reuteri supplementation, may, therefore, be a candidate against asthma and other allergic diseases. The same organism from human milk attenuated weight gain, fat accumulation, hypertriglyceridemia, and hypercholesterolemia in mice, thus opening a new horizon for the development of relevant foods to prevent metabolic disorders [169]. In a randomized controlled trial, L. reuteri administration was preventive in reducing pediatric consultations, parental discomfort, and the use of pain relievers for infant colic [170].
Apart from L. reuteri, Lactobacillus fermentum strains from human milk have been studied as probiotic candidates, and proved to be safe, well tolerated and useful for the prophylaxis against community-acquired infections [171]. They also showed cholesterol-lowering effects in simulated models of liver and gastrointestinal tract [172]. Both L. fermentum, and another milk-derived species, Lactobacillus salivarius, enhanced both natural and acquired immune responses, via activation of natural killer and T cell subsets and induction of a broad array of cytokines in peripheral blood mononuclear cells in vitro [173]. An interesting investigation of milk-derived Lactobacillus casei and Lactobacillus paracasei strains, demonstrated anticancer and antioxidant effect in HeLa cell lines of cervical cancer [174]. Likewise, anticancer potential was demonstrated by Rajoka and Colleagues [175] for Lactobacillus rhamnosus isolated from human milk, through induction of apoptosis and down-regulation of the bcl-2 protooncogene, suggesting potential anticancer capability. A review of available studies on the use of probiotics in infantile acute gastroenteritis and antibiotic-associated diarrhea found that L. rhamnosus had the highest compelling evidence of efficacy in reducing duration of gastroenteritis by one day, and was the most effective probiotic in prevention of diarrhea [176].
As for bifidobacteria of human milk, studies are also available to support their use as probiotics. Maldonado and Colleagues [177] conducted a randomized controlled trial on the addition of a strain of Bifidobacterium breve, originally isolated from human milk, to infant formula. They concluded that this probiotic reduced crying rates, is safe, and induces beneficial effects on health. Bifidobacterium longum, isolated from human breast milk, had anti-oncogenic and tumor suppressor potential in murine colorectal cancer [178]. Park and Colleagues [179] showed that a probiotics formula containing both B. longum and Lactobacillus acidophilus alleviated fever, vomiting, and diarrhea with no adverse events in hospitalized infants with rotavirus infection.
Although Bifidobacterium and Lactobacillus strains from human milk persist as the most commonly sought bacteria for probiotic use, the health endorsing effects of other bacteria from milk is also under investigation. Enterococcus faecium, a probable probiotic isolated from breast milk was evaluated both in vitro and in vivo for its safety. The strain was non-hemolytic, sensitive to majority of antibiotics, and showed no alteration of normal growth and development in male rats. No adverse effects on general status nor behavior; additionally, no significant changes were noted in the hematological results, blood biochemistry, organ weights, and histopathology, and none of the vital organs of treated animals displayed signs of bacteremia nor infectivity. These findings indicated the candidature of E. faecium as a potential safe probiotic [180]. In another analysis of E. faecium and E. faecalis strains isolated from breast milk, these bacteria inhibited the growth of Escherichia coli, Listeria monocytogenes, Salmonella typhi, Staphylococcus aureus, Shigella dysenteriae, and Streptococcus agalactiae. However, phenotypic and genotypic virulence analysis indicated hyaluronidase enzyme production and vancomycin resistance in E. faecalis, calling for careful monitoring of probiotic strains for safety parameters [181]. Moreover, a study of cells and cytoplasmic fractions of E. faecalis and Staphylococcus hominis isolated from breast milk against MCF-7 breast cancer cell line revealed significant decrease in cellular proliferation in concentration- and time-dependent style. Morphological signals of apoptosis such as cell shrinkage, cell death, and membrane blebbing were observed in over one-third of the tumor cells [182].
In short, the available data on possible probiotics isolated from human milk is abundant. Further, focused evaluation of safety and efficacy of this rich milieu of microbes is needed to determine which members are good alternative nutraceuticals with health-promoting profiles or promising therapeutic indices.
References
- Mosca, F.; Giannì, M.L. Human Milk: Composition and Health Benefits. Pediatr. Med. Chir. 2017, 39, 155.
- Johnson, A.; Kirk, R.; Rosenblum, K.L.; Muzik, M. Enhancing Breastfeeding Rates among African American Women: A Systematic Review of Current Psychosocial Interventions. Breastfeed Med. 2015, 10, 45–62.
- Westerfield, K.L.; Koenig, K.; Oh, R. Breastfeeding: Common Questions and Answers. Am. Fam. Phys. 2018, 98, 368–373.
- Bagci Bosi, A.T.; Eriksen, K.G.; Sobko, T.; Wijnhoven, T.M.A.; Breda, J. Breastfeeding Practices and Policies in WHO European Region Member States. Public Health Nutr. 2016, 19, 753–764.
- World Breastfeeding Week 2020 Message. Available online: (accessed on 19 September 2020).
- Section on Breastfeeding. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841.
- Ojo-Okunola, A.; Cacciatore, S.; Nicol, M.P.; du Toit, E. The Determinants of the Human Milk Metabolome and Its Role in Infant Health. Metabolites 2020, 10, 77.
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. 2013, 60, 49–74.
- Gidrewicz, D.A.; Fenton, T.R. A Systematic Review and Meta-Analysis of the Nutrient Content of Preterm and Term Breast Milk. BMC Pediatr. 2014, 14, 216.
- Dror, D.K.; Allen, L.H. Overview of Nutrients in Human Milk. Adv. Nutr. 2018, 9, 278S–294S.
- Picciano, M.F. Representative Values for Constituents of Human Milk. Pediatr. Clin. N. Am. 2001, 48, 263–264.
- Gay, M.C.L.; Koleva, P.T.; Slupsky, C.M.; Toit, E.D.; Eggesbo, M.; Johnson, C.C.; Wegienka, G.; Shimojo, N.; Campbell, D.E.; Prescott, S.L.; et al. Worldwide Variation in Human Milk Metabolome: Indicators of Breast Physiology and Maternal Lifestyle? Nutrients 2018, 10, 1151.
- Musilova, S.; Rada, V.; Vlkova, E.; Bunesova, V. Beneficial Effects of Human Milk Oligosaccharides on Gut Microbiota. Benef. Microbes 2014, 5, 273–283.
- Bhandari, N.; Prajapati, R. Prevalence of Exclusive Breast Feeding and Its Associated Factors among Mothers. Kathmandu Univ. Med. J. KUMJ 2018, 16, 166–170.
- Gertosio, C.; Meazza, C.; Pagani, S.; Bozzola, M. Breastfeeding and Its Gamut of Benefits. Minerva. Pediatr. 2016, 68, 201–212.
- Binns, C.; Lee, M.; Low, W.Y. The Long-Term Public Health Benefits of Breastfeeding. Asia Pac. J. Public Health 2016, 28, 7–14.
- Horta, B.L.; Victora, C.G. Short-Term Effects of Breastfeeding. A Systematic Review on the Benefits of Breastfeeding on Diarrhoea and Pneumonia Mortality; World Health Organization: Geneva, Switzerland, 2013.
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal Breastfeeding Practices and Infant and Child Mortality: A Systematic Review and Meta-Analysis. Acta Paediatr. 2015, 104, 3–13.
- Lamberti, L.M.; Fischer Walker, C.L.; Noiman, A.; Victora, C.; Black, R.E. Breastfeeding and the Risk for Diarrhea Morbidity and Mortality. BMC Public Health 2011, 11 (Suppl. 3), S15.
- Santos, F.S.; Santos, F.C.S.; dos Santos, L.H.; Leite, A.M.; de Mello, D.F. Breastfeeding and Protection against Diarrhea: An Integrative Review of Literature. Einstein Sao Paulo 2015, 13, 435–440.
- Rouw, E.; von Gartzen, A.; Weißenborn, A. The importance of breastfeeding for the infant. Bundesgesundheitsblatt Gesundh. Gesundh. 2018, 61, 945–951.
- Lamberti, L.M.; Zakarija-Grković, I.; Fischer Walker, C.L.; Theodoratou, E.; Nair, H.; Campbell, H.; Black, R.E. Breastfeeding for Reducing the Risk of Pneumonia Morbidity and Mortality in Children under Two: A Systematic Literature Review and Meta-Analysis. BMC Public Health 2013, 13 (Suppl. 3), S18.
- Quigley, M.A.; Carson, C.; Sacker, A.; Kelly, Y. Exclusive Breastfeeding Duration and Infant Infection. Eur. J. Clin. Nutr. 2016, 70, 1420–1427.
- Bowatte, G.; Tham, R.; Allen, K.J.; Tan, D.J.; Lau, M.; Dai, X.; Lodge, C.J. Breastfeeding and Childhood Acute Otitis Media: A Systematic Review and Meta-Analysis. Acta Paediatr. 2015, 104, 85–95.
- Christensen, N.; Bruun, S.; Søndergaard, J.; Christesen, H.T.; Fisker, N.; Zachariassen, G.; Sangild, P.T.; Husby, S. Breastfeeding and Infections in Early Childhood: A Cohort Study. Pediatrics 2020, 146.
- Zivich, P.; Lapika, B.; Behets, F.; Yotebieng, M. Implementation of Steps 1–9 to Successful Breastfeeding Reduces the Frequency of Mild and Severe Episodes of Diarrhea and Respiratory Tract Infection Among 0–6 Month Infants in Democratic Republic of Congo. Matern. Child Health J. 2018, 22, 762–771.
- Davanzo, R.; Moro, G.; Sandri, F.; Agosti, M.; Moretti, C.; Mosca, F. Breastfeeding and Coronavirus Disease-2019: Ad Interim Indications of the Italian Society of Neonatology Endorsed by the Union of European Neonatal & Perinatal Societies. Matern. Child Nutr. 2020, 16, e13010.
- World Health Organization Breastfeeding and COVID-19. Available online: (accessed on 30 January 2021).
- CDC Coronavirus Disease (COVID-19) and Breastfeeding. Available online: (accessed on 30 January 2021).
- Fernández-Carrasco, F.J.; Vázquez-Lara, J.M.; González-Mey, U.; Gómez-Salgado, J.; Parrón-Carreño, T.; Rodríguez-Díaz, L. Coronavirus Covid-19 Infection and Breastfeeding: An Exploratory Review. Available online: (accessed on 20 September 2020).
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition; Section on Allergy and Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143.
- Matsumoto, N.; Yorifuji, T.; Nakamura, K.; Ikeda, M.; Tsukahara, H.; Doi, H. Breastfeeding and Risk of Food Allergy: A Nationwide Birth Cohort in Japan. Allergol. Int. 2020, 69, 91–97.
- Carlin, R.F.; Moon, R.Y. Risk Factors, Protective Factors, and Current Recommendations to Reduce Sudden Infant Death Syndrome: A Review. JAMA Pediatr. 2017, 171, 175–180.
- Alm, B.; Wennergren, G.; Möllborg, P.; Lagercrantz, H. Breastfeeding and Dummy Use Have a Protective Effect on Sudden Infant Death Syndrome. Acta Paediatr. 2016, 105, 31–38.
- Horne, R.S.C. Sudden Infant Death Syndrome: Current Perspectives. Intern. Med. J. 2019, 49, 433–438.
- Adams, S.M.; Ward, C.E.; Garcia, K.L. Sudden Infant Death Syndrome. Am. Fam. Phys. 2015, 91, 778–783.
- Hauck, F.R.; Thompson, J.M.D.; Tanabe, K.O.; Moon, R.Y.; Vennemann, M.M. Breastfeeding and Reduced Risk of Sudden Infant Death Syndrome: A Meta-Analysis. Pediatrics 2011, 128, 103–110.
- Franco, P.; Scaillet, S.; Wermenbol, V.; Valente, F.; Groswasser, J.; Kahn, A. The Influence of a Pacifier on Infants’ Arousals from Sleep. J. Pediatr. 2000, 136, 775–779.
- Horne, R.S.C.; Parslow, P.M.; Ferens, D.; Watts, A.-M.; Adamson, T.M. Comparison of Evoked Arousability in Breast and Formula Fed Infants. Arch. Dis. Child. 2004, 89, 22–25.
- Horta, B.L.; Loret de Mola, C.; Victora, C.G. Long-Term Consequences of Breastfeeding on Cholesterol, Obesity, Systolic Blood Pressure and Type 2 Diabetes: A Systematic Review and Meta-Analysis. Acta Paediatr. 2015, 104, 30–37.
- Marseglia, L.; Manti, S.; D’Angelo, G.; Cuppari, C.; Salpietro, V.; Filippelli, M.; Trovato, A.; Gitto, E.; Salpietro, C.; Arrigo, T. Obesity and Breastfeeding: The Strength of Association. Women Birth 2015, 28, 81–86.
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The Association between Breastfeeding and Childhood Obesity: A Meta-Analysis. BMC Public Health 2014, 14, 1267.
- Ortega-García, J.A.; Kloosterman, N.; Alvarez, L.; Tobarra-Sánchez, E.; Cárceles-Álvarez, A.; Pastor-Valero, R.; López-Hernández, F.A.; Sánchez-Solis, M.; Claudio, L. Full Breastfeeding and Obesity in Children: A Prospective Study from Birth to 6 Years. Child Obes. 2018, 14, 327–337.
- Ak, V. Does Breastfeeding Shape Food Preferences? Links to Obesity. Available online: (accessed on 20 September 2020).
- Dahiya, D.K.; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut Microbiota Modulation and Its Relationship with Obesity Using Prebiotic Fibers and Probiotics: A Review. Front. Microbiol. 2017, 8, 563.
- Patel, A.L.; Kim, J.H. Human Milk and Necrotizing Enterocolitis. Semin. Pediatr. Surg. 2018, 27, 34–38.
- Cotten, C.M. Modifiable Risk Factors in Necrotizing Enterocolitis. Clin. Perinatol. 2019, 46, 129–143.
- Maffei, D.; Schanler, R.J. Human Milk Is the Feeding Strategy to Prevent Necrotizing Enterocolitis! Semin. Perinatol. 2017, 41, 36–40.
- Lindoso, L.; Mondal, K.; Venkateswaran, S.; Somineni, H.K.; Ballengee, C.; Walters, T.D.; Griffiths, A.; Noe, J.D.; Crandall, W.; Snapper, S.; et al. The Effect of Early-Life Environmental Exposures on Disease Phenotype and Clinical Course of Crohn’s Disease in Children. Am. J. Gastroenterol. 2018, 113, 1524–1529.
- Silano, M.; Agostoni, C.; Sanz, Y.; Guandalini, S. Infant Feeding and Risk of Developing Celiac Disease: A Systematic Review. BMJ Open 2016, 6, e009163.
- Murff, H.J.; Edwards, T.L. Endogenous Production of Long-Chain Polyunsaturated Fatty Acids and Metabolic Disease Risk. Curr. Cardiovasc. Risk Rep. 2014, 8.
- Amitay, E.L.; Keinan-Boker, L. Breastfeeding and Childhood Leukemia Incidence: A Meta-Analysis and Systematic Review. JAMA Pediatr. 2015, 169, e151025.
- Küçükçongar, A.; Oğuz, A.; Pınarlı, F.G.; Karadeniz, C.; Okur, A.; Kaya, Z.; Çelik, B. Breastfeeding and Childhood Cancer: Is Breastfeeding Preventative to Childhood Cancer? Pediatric Hematol. Oncol. 2015, 32, 374–381.
- Bar, S.; Milanaik, R.; Adesman, A. Long-Term Neurodevelopmental Benefits of Breastfeeding. Curr. Opin. Pediatr. 2016, 28, 559–566.
- Kramer, M.S.; Aboud, F.; Mironova, E.; Vanilovich, I.; Platt, R.W.; Matush, L.; Igumnov, S.; Fombonne, E.; Bogdanovich, N.; Ducruet, T.; et al. Breastfeeding and Child Cognitive Development: New Evidence from a Large Randomized Trial. Arch. Gen. Psychiatry 2008, 65, 578–584.
- Holme, A.; MacArthur, C.; Lancashire, R. The Effects of Breastfeeding on Cognitive and Neurological Development of Children at 9 Years. Child Care Health Dev. 2010, 36, 583–590.
- Koletzko, B.; Rodriguez-Palmero, M. Polyunsaturated Fatty Acids in Human Milk and Their Role in Early Infant Development. J. Mammary Gland Biol. Neoplasia 1999, 4, 269–284.
- Bernard, J.Y.; Armand, M.; Peyre, H.; Garcia, C.; Forhan, A.; De Agostini, M.; Charles, M.-A.; Heude, B.; EDEN Mother-Child Cohort Study Group (Etude des Déterminants pré- et postnatals précoces du développement et de la santé de l’Enfant). Breastfeeding, Polyunsaturated Fatty Acid Levels in Colostrum and Child Intelligence Quotient at Age 5–6 Years. J. Pediatr. 2017, 183, 43–50.e3.
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216.
- Ghozy, S.; Tran, L.; Naveed, S.; Quynh, T.T.H.; Helmy Zayan, A.; Waqas, A.; Sayed, A.K.H.; Karimzadeh, S.; Hirayama, K.; Huy, N.T. Association of Breastfeeding Status with Risk of Autism Spectrum Disorder: A Systematic Review, Dose-Response Analysis and Meta-Analysis. Asian J. Psychiatr. 2020, 48, 101916.
- Kielbratowska, B.; Kazmierczak, M.; Michalek, J.; Preis, K. Temperament and the Mother-Infant Dyad: Associations with Breastfeeding and Formula Feeding with a Bottle. Infant Ment. Health J. 2015, 36, 243–250.
- Shelton, K.H.; Collishaw, S.; Rice, F.J.; Harold, G.T.; Thapar, A. Using a Genetically Informative Design to Examine the Relationship between Breastfeeding and Childhood Conduct Problems. Eur. Child Adolesc. Psychiatry 2011, 20, 571–579.
- Schwarz, E.B.; Nothnagle, M. The Maternal Health Benefits of Breastfeeding. Am. Fam. Phys. 2015, 91, 603–604.
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st Century: Epidemiology, Mechanisms, and Lifelong Effect. Lancet 2016, 387, 475–490.
- Unar-Munguía, M.; Torres-Mejía, G.; Colchero, M.A.; González de Cosío, T. Breastfeeding Mode and Risk of Breast Cancer: A Dose-Response Meta-Analysis. J. Hum. Lact. 2017, 33, 422–434.
- Pan, H.; He, Z.; Ling, L.; Ding, Q.; Chen, L.; Zha, X.; Zhou, W.; Liu, X.; Wang, S. Reproductive Factors and Breast Cancer Risk among BRCA1 or BRCA2 Mutation Carriers: Results from Ten Studies. Cancer Epidemiol. 2014, 38, 1–8.
- Manrique Tejedor, J.; Figuerol Calderó, M.I.; Cuéllar De Frutos, A. Breastfeeding as a method of breast cancer prevention. Rev. Enferm. 2015, 38, 32–38.
- Modugno, F.; Goughnour, S.L.; Wallack, D.; Edwards, R.P.; Odunsi, K.; Kelley, J.L.; Moysich, K.; Ness, R.B.; Brooks, M.M. Breastfeeding Factors and Risk of Epithelial Ovarian Cancer. Gynecol. Oncol. 2019, 153, 116–122.
- Sung, H.K.; Ma, S.H.; Choi, J.-Y.; Hwang, Y.; Ahn, C.; Kim, B.-G.; Kim, Y.-M.; Kim, J.W.; Kang, S.; Kim, J.; et al. The Effect of Breastfeeding Duration and Parity on the Risk of Epithelial Ovarian Cancer: A Systematic Review and Meta-Analysis. J. Prev. Med. Public Health 2016, 49, 349–366.
- Rajaei, S.; Rigdon, J.; Crowe, S.; Tremmel, J.; Tsai, S.; Assimes, T.L. Breastfeeding Duration and the Risk of Coronary Artery Disease. J. Womens Health Larchmt 2019, 28, 30–36.
- Peters, S.A.; van der Schouw, Y.T.; Wood, A.M.; Sweeting, M.J.; Moons, K.G.; Weiderpass, E.; Arriola, L.; Benetou, V.; Boeing, H.; Bonnet, F.; et al. Parity, Breastfeeding and Risk of Coronary Heart Disease: A Pan-European Case-Cohort Study. Eur. J. Prev. Cardiol. 2016, 23, 1755–1765.
- Stuebe, A.M.; Michels, K.B.; Willett, W.C.; Manson, J.E.; Rexrode, K.; Rich-Edwards, J.W. Duration of Lactation and Incidence of Myocardial Infarction in Middle-to-Late Adulthood. Am. J. Obstet. Gynecol. 2009, 200, 138.e1–138.e8.
- Poudel, R.R.; Shrestha, D. Breastfeeding for Diabetes Prevention. J. Pak. Med. Assoc. 2016, 66, S88–S90.
- Park, S.; Choi, N.-K. Breastfeeding and Maternal Hypertension. Am. J. Hypertens. 2018, 31, 615–621.
- Qu, G.; Wang, L.; Tang, X.; Wu, W.; Sun, Y. Association Between Duration of Breastfeeding and Maternal Hypertension: A Systematic Review and Meta-Analysis. Breastfeed Med. 2018, 13, 318–326.
- Kirkegaard, H.; Bliddal, M.; Støvring, H.; Rasmussen, K.M.; Gunderson, E.P.; Køber, L.; Sørensen, T.I.A.; Nohr, E.A. Breastfeeding and Later Maternal Risk of Hypertension and Cardiovascular Disease—The Role of Overall and Abdominal Obesity. Prev. Med. 2018, 114, 140–148.
- Jäger, S.; Jacobs, S.; Kröger, J.; Fritsche, A.; Schienkiewitz, A.; Rubin, D.; Boeing, H.; Schulze, M.B. Breast-Feeding and Maternal Risk of Type 2 Diabetes: A Prospective Study and Meta-Analysis. Diabetologia 2014, 57, 1355–1365.
- Luo, J.; Hendryx, M.; LeBlanc, E.S.; Shadyab, A.H.; Qi, L.; Sealy-Jefferson, S.; Manson, J.E. Associations Between Parity, Breastfeeding, and Risk of Maternal Type 2 Diabetes Among Postmenopausal Women. Obstet. Gynecol. 2019, 134, 591–599.
- Stuebe, A.M.; Rich-Edwards, J.W. The Reset Hypothesis: Lactation and Maternal Metabolism. Am. J. Perinatol. 2009, 26, 81–88.
- Wang, T.; Xu, Y.; Xu, M.; Ning, G.; Lu, J.; Dai, M.; Xu, B.; Sun, J.; Sun, W.; Lai, S.; et al. Circulating Prolactin and Risk of Type 2 Diabetes: A Prospective Study. Am. J. Epidemiol. 2016, 184, 295–301.
- Bobrow, K.L.; Quigley, M.A.; Green, J.; Reeves, G.K.; Beral, V.; Million Women Study Collaborators. Persistent Effects of Women’s Parity and Breastfeeding Patterns on Their Body Mass Index: Results from the Million Women Study. Int. J. Obes. 2013, 37, 712–717.
- Groër, M.W. Differences between Exclusive Breastfeeders, Formula-Feeders, and Controls: A Study of Stress, Mood, and Endocrine Variables. Biol. Res. Nurs. 2005, 7, 106–117.
- Doan, T.; Gardiner, A.; Gay, C.L.; Lee, K.A. Breast-Feeding Increases Sleep Duration of New Parents. J. Perinat. Neonatal. Nurs. 2007, 21, 200–206.
- Figueiredo, B.; Dias, C.C.; Brandão, S.; Canário, C.; Nunes-Costa, R. Breastfeeding and Postpartum Depression: State of the Art Review. J. Pediatr. Rio J. 2013, 89, 332–338.
- Da Silva Tanganhito, D.; Bick, D.; Chang, Y.-S. Breastfeeding Experiences and Perspectives among Women with Postnatal Depression: A Qualitative Evidence Synthesis. Women Birth 2020, 33, 231–239.
- Lara-Cinisomo, S.; McKenney, K.; Di Florio, A.; Meltzer-Brody, S. Associations Between Postpartum Depression, Breastfeeding, and Oxytocin Levels in Latina Mothers. Breastfeed Med. 2017, 12, 436–442.
- Diez-Sampedro, A.; Flowers, M.; Olenick, M.; Maltseva, T.; Valdes, G. Women’s Choice Regarding Breastfeeding and Its Effect on Well-Being. Nurs. Womens Health 2019, 23, 383–389.
- Silva, C.S.; Lima, M.C.; Sequeira-de-Andrade, L.A.S.; Oliveira, J.S.; Monteiro, J.S.; Lima, N.M.S.; Santos, R.M.A.B.; Lira, P.I.C. Association between Postpartum Depression and the Practice of Exclusive Breastfeeding in the First Three Months of Life. J. Pediatr. Rio J. 2017, 93, 356–364.
- Cooklin, A.R.; Amir, L.H.; Nguyen, C.D.; Buck, M.L.; Cullinane, M.; Fisher, J.R.W.; Donath, S.M.; CASTLE Study Team. Physical Health, Breastfeeding Problems and Maternal Mood in the Early Postpartum: A Prospective Cohort Study. Arch. Womens Ment. Health 2018, 21, 365–374.
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 39.
- Rantasalo, I.; Kauppinen, M.A. The Occurrence of Staphylococcus Aureus in Mother’s Milk. Ann. Chir. Gynaecol. Fenn. 1959, 48, 246–258.
- Ojo-Okunola, A.; Nicol, M.; du Toit, E. Human Breast Milk Bacteriome in Health and Disease. Nutrients 2018, 10, 1643.
- Martín, R.; Langa, S.; Reviriego, C.; Jimínez, E.; Marín, M.L.; Xaus, J.; Fernández, L.; Rodríguez, J.M. Human Milk Is a Source of Lactic Acid Bacteria for the Infant Gut. J. Pediatr. 2003, 143, 754–758.
- Civardi, E.; Garofoli, F.; Tzialla, C.; Paolillo, P.; Bollani, L.; Stronati, M. Microorganisms in Human Milk: Lights and Shadows. J. Matern. Fetal. Neonatal. Med. 2013, 26 (Suppl. 2), 30–34.
- Ruiz, L.; García-Carral, C.; Rodriguez, J.M. Unfolding the Human Milk Microbiome Landscape in the Omics Era. Front. Microbiol. 2019, 10, 1378.
- Moossavi, S.; Sepehri, S.; Robertson, B.; Bode, L.; Goruk, S.; Field, C.J.; Lix, L.M.; de Souza, R.J.; Becker, A.B.; Mandhane, P.J.; et al. Composition and Variation of the Human Milk Microbiota Are Influenced by Maternal and Early-Life Factors. Cell Host Microbe 2019, 25, 324–335.e4.
- Damaceno, Q.S.; Souza, J.P.; Nicoli, J.R.; Paula, R.L.; Assis, G.B.; Figueiredo, H.C.; Azevedo, V.; Martins, F.S. Evaluation of Potential Probiotics Isolated from Human Milk and Colostrum. Probiotics Antimicrob. Proteins 2017, 9, 371–379.
- Rodríguez, J.M. The Origin of Human Milk Bacteria: Is There a Bacterial Entero-Mammary Pathway during Late Pregnancy and Lactation? Adv. Nutr. 2014, 5, 779–784.
- Perez, P.F.; Doré, J.; Leclerc, M.; Levenez, F.; Benyacoub, J.; Serrant, P.; Segura-Roggero, I.; Schiffrin, E.J.; Donnet-Hughes, A. Bacterial Imprinting of the Neonatal Immune System: Lessons from Maternal Cells? Pediatrics 2007, 119, e724–e732.
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.P.; Ricciardi-Castagnoli, P. Dendritic Cells Express Tight Junction Proteins and Penetrate Gut Epithelial Monolayers to Sample Bacteria. Nat. Immunol. 2001, 2, 361–367.
- Geddes, D.T. The Use of Ultrasound to Identify Milk Ejection in Women—Tips and Pitfalls. Int. Breastfeed J. 2009, 4, 5.
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.E.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the Diversity and Temporal Stability of Bacterial Communities in Human Milk. PLoS ONE 2011, 6, e21313.
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K.; et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017, 171, 647–654.
- Demmelmair, H.; Jiménez, E.; Collado, M.C.; Salminen, S.; McGuire, M.K. Maternal and Perinatal Factors Associated with the Human Milk Microbiome. Curr. Dev. Nutr. 2020, 4, nzaa027.
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of Human Breast Tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014.
- Hieken, T.J.; Chen, J.; Hoskin, T.L.; Walther-Antonio, M.; Johnson, S.; Ramaker, S.; Xiao, J.; Radisky, D.C.; Knutson, K.L.; Kalari, K.R.; et al. The Microbiome of Aseptically Collected Human Breast Tissue in Benign and Malignant Disease. Sci. Rep. 2016, 6, 30751.
- Chan, A.A.; Bashir, M.; Rivas, M.N.; Duvall, K.; Sieling, P.A.; Pieber, T.R.; Vaishampayan, P.A.; Love, S.M.; Lee, D.J. Characterization of the Microbiome of Nipple Aspirate Fluid of Breast Cancer Survivors. Sci. Rep. 2016, 6, 28061.
- Going, J.J.; Moffat, D.F. Escaping from Flatland: Clinical and Biological Aspects of Human Mammary Duct Anatomy in Three Dimensions. J. Pathol. 2004, 203, 538–544.
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361.
- Jeurink, P.V.; van Bergenhenegouwen, J.; Jiménez, E.; Knippels, L.M.J.; Fernández, L.; Garssen, J.; Knol, J.; Rodríguez, J.M.; Martín, R. Human Milk: A Source of More Life than We Imagine. Benef. Microbes 2013, 4, 17–30.
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical Mother-Neonate Transfer of Maternal Gut Bacteria via Breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904.
- Togo, A.; Dufour, J.-C.; Lagier, J.-C.; Dubourg, G.; Raoult, D.; Million, M. Repertoire of Human Breast and Milk Microbiota: A Systematic Review. Future Microbiol. 2019, 14, 623–641.
- Latuga, M.S.; Stuebe, A.; Seed, P.C. A Review of the Source and Function of Microbiota in Breast Milk. Semin. Reprod. Med. 2014, 32, 68–73.
- Fitzstevens, J.L.; Smith, K.C.; Hagadorn, J.I.; Caimano, M.J.; Matson, A.P.; Brownell, E.A. Systematic Review of the Human Milk Microbiota. Nutr. Clin. Pract. 2017, 32, 354–364.
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The Human Milk Microbiome Changes over Lactation and Is Shaped by Maternal Weight and Mode of Delivery. Am. J. Clin. Nutr. 2012, 96, 544–551.
- Amir, L.H.; Donath, S.M.; Garland, S.M.; Tabrizi, S.N.; Bennett, C.M.; Cullinane, M.; Payne, M.S. Does Candida and/or Staphylococcus Play a Role in Nipple and Breast Pain in Lactation? A Cohort Study in Melbourne, Australia. BMJ Open 2013, 3.
- Jiménez, E.; de Andrés, J.; Manrique, M.; Pareja-Tobes, P.; Tobes, R.; Martínez-Blanch, J.F.; Codoñer, F.M.; Ramón, D.; Fernández, L.; Rodríguez, J.M. Metagenomic Analysis of Milk of Healthy and Mastitis-Suffering Women. J. Hum. Lact. 2015, 31, 406–415.
- Moossavi, S.; Fehr, K.; Derakhshani, H.; Sbihi, H.; Robertson, B.; Bode, L.; Brook, J.; Turvey, S.E.; Moraes, T.J.; Becker, A.B.; et al. Human Milk Fungi: Environmental Determinants and Inter-Kingdom Associations with Milk Bacteria in the CHILD Cohort Study. BMC Microbiol. 2020, 20, 146.
- Dinleyici, M.; Pérez-Brocal, V.; Arslanoglu, S.; Aydemir, O.; Ozumut, S.S.; Tekin, N.; Vandenplas, Y.; Moya, A.; Dinleyici, E.C. Human Milk Mycobiota Composition: Relationship with Gestational Age, Delivery Mode, and Birth Weight. Benef. Microbes 2020, 11, 151–162.
- Liang, G.; Zhao, C.; Zhang, H.; Mattei, L.; Sherrill-Mix, S.; Bittinger, K.; Kessler, L.R.; Wu, G.D.; Baldassano, R.N.; DeRusso, P.; et al. The Stepwise Assembly of the Neonatal Virome Is Modulated by Breastfeeding. Nature 2020, 581, 470–474.
- Pannaraj, P.S.; Ly, M.; Cerini, C.; Saavedra, M.; Aldrovandi, G.M.; Saboory, A.A.; Johnson, K.M.; Pride, D.T. Shared and Distinct Features of Human Milk and Infant Stool Viromes. Front. Microbiol. 2018, 9, 1162.
- Mohandas, S.; Pannaraj, P.S. Beyond the Bacterial Microbiome: Virome of Human Milk and Effects on the Developing Infant. Milk Mucosal Immun. Microbiome Impact Neonate 2020, 94, 86–93.
- Khodayar-Pardo, P.; Mira-Pascual, L.; Collado, M.C.; Martínez-Costa, C. Impact of Lactation Stage, Gestational Age and Mode of Delivery on Breast Milk Microbiota. J. Perinatol. 2014, 34, 599–605.
- Decker, E.; Engelmann, G.; Findeisen, A.; Gerner, P.; Laass, M.; Ney, D.; Posovszky, C.; Hoy, L.; Hornef, M.W. Cesarean Delivery Is Associated with Celiac Disease but Not Inflammatory Bowel Disease in Children. Pediatrics 2010, 125, e1433–e1440.
- Urbaniak, C.; Angelini, M.; Gloor, G.B.; Reid, G. Human Milk Microbiota Profiles in Relation to Birthing Method, Gestation and Infant Gender. Microbiome 2016, 4, 1.
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the Infant Microbiome Community Structure and Function across Multiple Body Sites and in Relation to Mode of Delivery. Nat. Med. 2017, 23, 314–326.
- Gérard, P. Gut Microbiota and Obesity. Cell. Mol. Life Sci. 2016, 73, 147–162.
- Olivares, M.; Albrecht, S.; De Palma, G.; Ferrer, M.D.; Castillejo, G.; Schols, H.A.; Sanz, Y. Human Milk Composition Differs in Healthy Mothers and Mothers with Celiac Disease. Eur. J. Nutr. 2015, 54, 119–128.
- Valitutti, F.; Cucchiara, S.; Fasano, A. Celiac Disease and the Microbiome. Nutrients 2019, 11, 2403.
- Hermansson, H.; Kumar, H.; Collado, M.C.; Salminen, S.; Isolauri, E.; Rautava, S. Breast Milk Microbiota Is Shaped by Mode of Delivery and Intrapartum Antibiotic Exposure. Front. Nutr. 2019, 6, 4.
- Browne, P.D.; Aparicio, M.; Alba, C.; Hechler, C.; Beijers, R.; Rodríguez, J.M.; Fernández, L.; de Weerth, C. Human Milk Microbiome and Maternal Postnatal Psychosocial Distress. Front. Microbiol. 2019, 10, 2333.
- Kumar, H.; du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct Patterns in Human Milk Microbiota and Fatty Acid Profiles Across Specific Geographic Locations. Front. Microbiol. 2016, 7, 1619.
- Boix-Amorós, A.; Puente-Sánchez, F.; du Toit, E.; Linderborg, K.M.; Zhang, Y.; Yang, B.; Salminen, S.; Isolauri, E.; Tamames, J.; Mira, A.; et al. Mycobiome Profiles in Breast Milk from Healthy Women Depend on Mode of Delivery, Geographic Location, and Interaction with Bacteria. Appl. Environ. Microbiol. 2019, 85.
- Ayoub Moubareck, C.; Lootah, M.; Tahlak, M.; Venema, K. Profiles of Human Milk Oligosaccharides and Their Relations to the Milk Microbiota of Breastfeeding Mothers in Dubai. Nutrients 2020, 12, 1727.
- Soto, A.; Martín, V.; Jiménez, E.; Mader, I.; Rodríguez, J.M.; Fernández, L. Lactobacilli and Bifidobacteria in Human Breast Milk: Influence of Antibiotherapy and Other Host and Clinical Factors. J. Pediatric Gastroenterol. Nutr. 2014, 59, 78–88.
- Zimmermann, P.; Curtis, N. Breast Milk Microbiota: A Review of the Factors That Influence Composition. J. Infect. 2020, 81, 17–47.
- Williams, J.E.; Carrothers, J.M.; Lackey, K.A.; Beatty, N.F.; York, M.A.; Brooker, S.L.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; et al. Human Milk Microbial Community Structure Is Relatively Stable and Related to Variations in Macronutrient and Micronutrient Intakes in Healthy Lactating Women. J. Nutr. 2017, 147, 1739–1748.
- Carrothers, J.M.; York, M.A.; Brooker, S.L.; Lackey, K.A.; Williams, J.E.; Shafii, B.; Price, W.J.; Settles, M.L.; McGuire, M.A.; McGuire, M.K. Fecal Microbial Community Structure Is Stable over Time and Related to Variation in Macronutrient and Micronutrient Intakes in Lactating Women123. J. Nutr. 2015, 145, 2379–2388.
- Padilha, M.; Danneskiold-Samsøe, N.B.; Brejnrod, A.; Hoffmann, C.; Cabral, V.P.; Iaucci, J.d.M.; Sales, C.H.; Fisberg, R.M.; Cortez, R.V.; Brix, S.; et al. The Human Milk Microbiota Is Modulated by Maternal Diet. Microorganisms 2019, 7, 502.
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Consumption of Vegetables, Fruit, and Antioxidants during Pregnancy and Wheeze and Eczema in Infants. Allergy 2010, 65, 758–765.
- Coakley, M.; Ross, R.P.; Nordgren, M.; Fitzgerald, G.; Devery, R.; Stanton, C. Conjugated Linoleic Acid Biosynthesis by Human-Derived Bifidobacterium Species. J. Appl. Microbiol. 2003, 94, 138–145.
- Shively, C.A.; Register, T.C.; Appt, S.E.; Clarkson, T.B.; Uberseder, B.; Clear, K.Y.J.; Wilson, A.S.; Chiba, A.; Tooze, J.A.; Cook, K.L. Consumption of Mediterranean versus Western Diet Leads to Distinct Mammary Gland Microbiome Populations. Cell Rep. 2018, 25, 47–56.e3.
- Zhang, Z.; Lv, J.; Pan, L.; Zhang, Y. Roles and Applications of Probiotic Lactobacillus Strains. Appl. Microbiol. Biotechnol. 2018, 102, 8135–8143.
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703.
- Babakobi, M.D.; Reshef, L.; Gihaz, S.; Belgorodsky, B.; Fishman, A.; Bujanover, Y.; Gophna, U. Effect of Maternal Diet and Milk Lipid Composition on the Infant Gut and Maternal Milk Microbiomes. Nutrients 2020, 12, 2539.
- Turroni, F.; Milani, C.; Duranti, S.; Mancabelli, L.; Mangifesta, M.; Viappiani, A.; Lugli, G.A.; Ferrario, C.; Gioiosa, L.; Ferrarini, A.; et al. Deciphering Bifidobacterial-Mediated Metabolic Interactions and Their Impact on Gut Microbiota by a Multi-Omics Approach. ISME J. 2016, 10, 1656–1668.
- Simpson, M.R.; Avershina, E.; Storrø, O.; Johnsen, R.; Rudi, K.; Øien, T. Breastfeeding-Associated Microbiota in Human Milk Following Supplementation with Lactobacillus Rhamnosus GG, Lactobacillus Acidophilus La-5, and Bifidobacterium Animalis Ssp. Lactis Bb-12. J. Dairy Sci. 2018, 101, 889–899.
- Rautava, S. Early Microbial Contact, the Breast Milk Microbiome and Child Health. J. Dev. Orig. Health Dis. 2016, 7, 5–14.
- Gueimonde, M.; Laitinen, K.; Salminen, S.; Isolauri, E. Breast Milk: A Source of Bifidobacteria for Infant Gut Development and Maturation? Neonatology 2007, 92, 64–66.
- Pacheco, A.R.; Barile, D.; Underwood, M.A.; Mills, D.A. The Impact of the Milk Glycobiome on the Neonate Gut Microbiota. Annu. Rev. Anim. Biosci. 2015, 3, 419–445.
- Turroni, F.; Peano, C.; Pass, D.A.; Foroni, E.; Severgnini, M.; Claesson, M.J.; Kerr, C.; Hourihane, J.; Murray, D.; Fuligni, F.; et al. Diversity of Bifidobacteria within the Infant Gut Microbiota. PLoS ONE 2012, 7, e36957.
- Turroni, F.; Foroni, E.; Pizzetti, P.; Giubellini, V.; Ribbera, A.; Merusi, P.; Cagnasso, P.; Bizzarri, B.; de’Angelis, G.L.; Shanahan, F.; et al. Exploring the Diversity of the Bifidobacterial Population in the Human Intestinal Tract. Appl. Environ. Microbiol. 2009, 75, 1534–1545.
- Chong, C.Y.L.; Bloomfield, F.H.; O’Sullivan, J.M. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 2018, 10, 274.
- Solís, G.; de Los Reyes-Gavilan, C.G.; Fernández, N.; Margolles, A.; Gueimonde, M. Establishment and Development of Lactic Acid Bacteria and Bifidobacteria Microbiota in Breast-Milk and the Infant Gut. Anaerobe 2010, 16, 307–310.
- Bergmann, H.; Rodríguez, J.M.; Salminen, S.; Szajewska, H. Probiotics in Human Milk and Probiotic Supplementation in Infant Nutrition: A Workshop Report. Br. J. Nutr. 2014, 112, 1119–1128.
- Jiao, X.; Fu, M.-D.; Wang, Y.-Y.; Xue, J.; Zhang, Y. Bifidobacterium and Lactobacillus for Preventing Necrotizing Enterocolitis in Very-Low-Birth-Weight Preterm Infants: A Systematic Review and Meta-Analysis. World J. Pediatr. 2020, 16, 135–142.
- Jiménez, E.; Delgado, S.; Maldonado, A.; Arroyo, R.; Albújar, M.; García, N.; Jariod, M.; Fernández, L.; Gómez, A.; Rodríguez, J.M. Staphylococcus Epidermidis: A Differential Trait of the Fecal Microbiota of Breast-Fed Infants. BMC Microbiol. 2008, 8, 143.
- Landete, J.M.; Peirotén, Á.; Medina, M.; Arqués, J.L.; Rodríguez-Mínguez, E. Virulence and Antibiotic Resistance of Enterococci Isolated from Healthy Breastfed Infants. Microb. Drug Resist. 2018, 24, 63–69.
- Martín, V.; Maldonado, A.; Fernández, L.; Rodríguez, J.M.; Connor, R.I. Inhibition of Human Immunodeficiency Virus Type 1 by Lactic Acid Bacteria from Human Breastmilk. Breastfeed Med. 2010, 5, 153–158.
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81.
- FAO/WHO. Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. In Proceedings of the Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, Cordoba, Argentina, 1–4 October 2001.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Bozzi Cionci, N.; Baffoni, L.; Gaggìa, F.; Di Gioia, D. Therapeutic Microbiology: The Role of Bifidobacterium Breve as Food Supplement for the Prevention/Treatment of Paediatric Diseases. Nutrients 2018, 10, 1723.
- Halloran, K.; Underwood, M.A. Probiotic Mechanisms of Action. Early Hum. Dev. 2019, 135, 58–65.
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174.
- Montalban-Arques, A.; De Schryver, P.; Bossier, P.; Gorkiewicz, G.; Mulero, V.; Gatlin, D.M.; Galindo-Villegas, J. Selective Manipulation of the Gut Microbiota Improves Immune Status in Vertebrates. Front. Immunol. 2015, 6, 512.
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus Reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757.
- Li, L.; Fang, Z.; Liu, X.; Hu, W.; Lu, W.; Lee, Y.-K.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus Reuteri Attenuated Allergic Inflammation Induced by HDM in the Mouse and Modulated Gut Microbes. PLoS ONE 2020, 15, e0231865.
- Li, S.; Qi, C.; Zhu, H.; Yu, R.; Xie, C.; Peng, Y.; Yin, S.-W.; Fan, J.; Zhao, S.; Sun, J. Lactobacillus Reuteri Improves Gut Barrier Function and Affects Diurnal Variation of the Gut Microbiota in Mice Fed a High-Fat Diet. Food Funct. 2019, 10, 4705–4715.
- Savino, F.; Ceratto, S.; Poggi, E.; Cartosio, M.E.; Cordero di Montezemolo, L.; Giannattasio, A. Preventive Effects of Oral Probiotic on Infantile Colic: A Prospective, Randomised, Blinded, Controlled Trial Using Lactobacillus Reuteri DSM 17938. Benef. Microbes 2015, 6, 245–251.
- López-Huertas, E. Safety and Efficacy of Human Breast Milk Lactobacillus Fermentum CECT 5716. A Mini-Review of Studies with Infant Formulae. Benef. Microbes 2015, 6, 219–224.
- Asan-Ozusaglam, M.; Gunyakti, A. Lactobacillus Fermentum Strains from Human Breast Milk with Probiotic Properties and Cholesterol-Lowering Effects. Food Sci. Biotechnol. 2019, 28, 501–509.
- Pérez-Cano, F.J.; Dong, H.; Yaqoob, P. In Vitro Immunomodulatory Activity of Lactobacillus Fermentum CECT5716 and Lactobacillus Salivarius CECT5713: Two Probiotic Strains Isolated from Human Breast Milk. Immunobiology 2010, 215, 996–1004.
- Riaz Rajoka, M.S.; Zhao, H.; Lu, Y.; Lian, Z.; Li, N.; Hussain, N.; Shao, D.; Jin, M.; Li, Q.; Shi, J. Anticancer Potential against Cervix Cancer (HeLa) Cell Line of Probiotic Lactobacillus Casei and Lactobacillus Paracasei Strains Isolated from Human Breast Milk. Food Funct. 2018, 9, 2705–2715.
- Riaz Rajoka, M.S.; Zhao, H.; Mehwish, H.M.; Li, N.; Lu, Y.; Lian, Z.; Shao, D.; Jin, M.; Li, Q.; Zhao, L.; et al. Anti-Tumor Potential of Cell Free Culture Supernatant of Lactobacillus Rhamnosus Strains Isolated from Human Breast Milk. Food Res. Int. 2019, 123, 286–297.
- Guarino, A.; Guandalini, S.; Lo Vecchio, A. Probiotics for Prevention and Treatment of Diarrhea. J. Clin. Gastroenterol. 2015, 49 (Suppl. 1), S37–S45.
- Maldonado, J.; Gil-Campos, M.; Maldonado-Lobón, J.A.; Benavides, M.R.; Flores-Rojas, K.; Jaldo, R.; Jiménez Del Barco, I.; Bolívar, V.; Valero, A.D.; Prados, E.; et al. Evaluation of the Safety, Tolerance and Efficacy of 1-Year Consumption of Infant Formula Supplemented with Lactobacillus Fermentum CECT5716 Lc40 or Bifidobacterium Breve CECT7263: A Randomized Controlled Trial. BMC Pediatr. 2019, 19, 361.
- Fahmy, C.A.; Gamal-Eldeen, A.M.; El-Hussieny, E.A.; Raafat, B.M.; Mehanna, N.S.; Talaat, R.M.; Shaaban, M.T. Bifidobacterium Longum Suppresses Murine Colorectal Cancer through the Modulation of OncomiRs and Tumor Suppressor MiRNAs. Nutr. Cancer 2019, 71, 688–700.
- Park, M.S.; Kwon, B.; Ku, S.; Ji, G.E. The Efficacy of Bifidobacterium Longum BORI and Lactobacillus Acidophilus AD031 Probiotic Treatment in Infants with Rotavirus Infection. Nutrients 2017, 9, 887.
- Khalkhali, S.; Mojgani, N. In Vitro and in Vivo Safety Analysis of Enterococcus Faecium 2C Isolated from Human Breast Milk. Microb. Pathog. 2018, 116, 73–77.
- Khalkhali, S.; Mojgani, N. Bacteriocinogenic Potential and Virulence Traits of Enterococcus Faecium and E. Faecalis Isolated from Human Milk. Iran. J. Microbiol. 2017, 9, 224–233.
- Hassan, Z.; Mustafa, S.; Rahim, R.A.; Isa, N.M. Anti-Breast Cancer Effects of Live, Heat-Killed and Cytoplasmic Fractions of Enterococcus Faecalis and Staphylococcus Hominis Isolated from Human Breast Milk. Cell. Dev. Biol. Anim. 2016, 52, 337–348.




