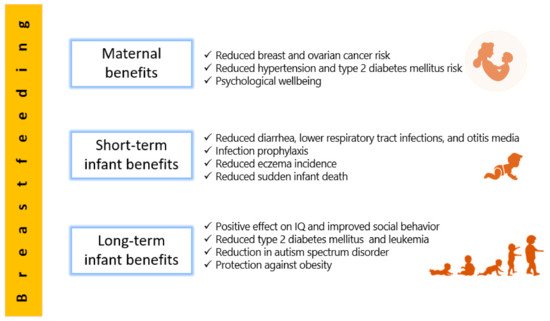
In addition to its diverse nutritional and health benefits, human milk is characterized by a rich microbiota which constitutes a source for the infantile gut microbiota [
90]. Except for initial observations of occurrence of microbes in milk in the 1950s, while studying the possibility of transmission of infections to infants via breastfeeding [
91], human milk was thought of as a sterile fluid [
92]. Around the beginning of the twenty-first century, a study in Spain by Martin and Colleagues [
93] demonstrated, for the first time, the presence of the lactic acid bacterium,
Lactobacillus gasseri, from milk and stools of mother–infant pairs, indicating that breast milk formed a potential source for infant microbiota. The non-sterility of breast milk has, ever since this initial observation, started to become extensively researched, and regarded as a protective factor arising from the wide range of microbes, in addition to the universally acknowledged nutritional role [
94].
3.3. Milk Microbiota Diversity and Associated Factors
A myriad of environmental, genetic, and immune factors personalizes a mother’s milk microbiome. Among different women, and in the same woman experiencing diverse physiological, hormonal, and pathological conditions, an inconsistency is expected among communities of milk microbiota [
92].
Primarily, maternal factors contribute to differences in milk microbiota composition. Khodayar-Pardo and Colleagues [
123] showed that Cesarean section births were associated with total bacterial concentrations in milk higher than vaginal delivery, and significantly higher levels of
Streptococcus, but significantly lower levels of
Bifidobacterium. This was associated in some studies with risk of immune-mediated and inflammatory disorders in infants born by Cesarean delivery [
124]. Possibly, physiological stress or hormonal signals of delivery could influence the microbial transmission process to milk [
115]. The difference in composition of milk microbiota among vaginal deliveries and Cesarean section, however, was not significant in other investigations [
125], warranting further analysis. A fail-safe mechanism has been proposed, whereby the mother passes along her bacterial imprint irrespective of how the baby is born, eliminating major differences in milk microbiome composition with mode of delivery [
125]. The differences in milk microbiota among cesarean and vaginal deliveries are, however, thought to be only short lived when reflected on infant gut microbiome. By one month, vaginal and cesarean section infants cannot be separated on the basis of composition of their microbiota [
126]. Based on available evidence, it is difficult to draw clear-cut conclusions regarding milk microbiota composition and both mode of delivery and milk maturity.
Maternal body weight also influences milk microbiota. Milk from obese mothers was found to include a bacterial community of lower diversity compared with milk from normal-weight mothers, according to findings of a randomized controlled trial, but was associated with higher numbers of
Staphylococcus and lower numbers of
Bifidobacterium [
115]. The link between gut microbiota and obesity may possibly be extended to milk, substantiating the conception that obesity influences the milk microbiota via its influence on the gut microbiota [
115,
127]. Indeed, maternal diet influences milk microbiota, and this is elaborated in
Section 3.4.
In 2015, and also relating to maternal factors on milk microbiota composition, Olivares and Colleagues [
128] showed that levels of bifidobacteria were reduced in milk of mothers with celiac disease compared to healthy mothers, and this could, theoretically, diminish the protective effects of breastfeeding on the child’s risk of developing celiac disease. Further, breast milk favored
Clostridium leptum,
Bifidobacterium longum, and
Bifidobacterium breve gut colonization, and these were associated with the HLA type of low genetic risk for celiac disease [
129]. In an analysis of intrapartum antibiotic administration on human milk microbiota, it was found that the species
Bifidobacterium was uniquely found in breast milk samples of mothers who did not receive antibiotics. Such observation may be significant, since reduced fecal bifidobacteria in early infancy may be associated with higher risk of non-communicable diseases in childhood, like atopy and overweight [
130].
On another note, maternal postnatal psychosocial distress may alter maternal gut microbiota, which, in turn, may affect bacteria present in milk. Browne and Colleagues [
131] found no significant differences in the relative proportions of major bacterial genera between women with high and low levels of psychosocial distress. However, progressive and distinct decrease in Firmicutes, Proteobacteria, and Bacteroidetes at the phylum level, and increase in
Acinetobacter,
Flavobacterium, and
Lactobacillus at the genus level, were evident in milk samples of women with low psychosocial distress. High maternal psychosocial distress was also related to significantly lower bacterial diversity in milk three months after delivery. These findings suggest a likely relationship between maternal psychosocial distress and milk microbiota, indicating that post-partum psychological symptoms may impact infantile development and health, through their influence on milk.
Milk bacterial profiles do not appear to significantly differ in relation to maternal age, infant gender, or race/ethnicity in a given geographical region [
109], but do differ across geographic locations of Europe, Africa, and Asia. Across these continents, Kumar and Colleagues [
132] analyzed milk samples of 80 women from 4 different countries (China, Finland, South Africa, and Spain). Spanish women had highest abundance of Bacteroidetes, whereas Chinese women had highest abundance of Actinobacteria. Women who had a cesarean section had higher amount of Proteobacteria as observed in the milk of the Spanish and South African women. Interestingly, and in the emerging field of human milk mycobiome, a core of four genera was shared across milk samples from the above four countries, consisting of
Malassezia, Davidiella, Sistotrema, and
Penicillium, which are also present in the infant gut, supporting potential role of breast milk in the initial seeding of fungal species in the infant gut [
133]. In a very recent investigation from Dubai [
134], the genus
Hydrogenophaga (of the beta-Proteobacteria), previously reported in breast cancer tissue [
106], was significantly higher in the breast milk of local women compared to expatriates living in Dubai. This finding may shed a light on possible influence of race and lifestyle on human milk microbiota, but the importance of such data needs to be further explored.
Other determining factors of human milk microbiota have been investigated. In one survey, of milk samples collected at different stages of lactation,
Bifidobacterium spp. concentration was significantly higher in milk samples from term gestations compared to preterm ones, indicating that gestational age plays a role in structuring milk microbiota [
123]. This was not reproducible in another study on Canadian women, whereby comparison of bacterial profiles between preterm and term births showed no statistically significant differences [
125]. The number of lactobacilli- or bifidobacteria-positive samples was significantly lower in women treated with antibiotics during pregnancy or lactation [
135]. This emphasizes that consideration needs to be given to the impact of drugs administered to the breastfeeding mother, not only on potential consequences for the infant health, but also in diverting the normal milk microbiota [
109].
To summarize the above, a review about the multitude of factors influencing human milk composition was very nicely and recently elaborated by Zimmermann and Curtis [
136] in 2020. This review of 44 studies recognized some evidence that gestational age, infant sex, delivery mode, parity, lactation stage, diet, body mass index, composition of breast milk, geographic location, HIV infection, and method of collection affect composition of the breast milk microbiota. However, many studies were small and findings possibly conflicting, indicating need for further research setting some benchmarks on the internal and external players affecting the nature of human milk microbiota.
3.4. The Effect of Maternal Diet on Human Milk Microbiota and on Intestinal Microbiota of Infants
In addition to the numerous physiological, medical and environmental factors described above, it was shown that the assembly of microbes in human milk is also affected by maternal diet [
137]. The associations between numerous macro- and micronutrients in maternal diet and the milk microbiota have been investigated.
In fact, maternal diet affects the concentration of certain substances, like fatty acids, in milk. In other words, maternal nutrient intake may indirectly help shape the bacterial community membership in human milk simply because of its impact on milk nutrient composition. For example, Kumar and Colleagues [
132] documented multiple associations between human milk fatty acid profiles and variations in milk microbiota. Monounsaturated fatty acids of milk were negatively associated with Proteobacteria, but positively associated with
Lactobacillus genus.
Additionally, maternal nutrient intake affects gastrointestinal bacterial communities, which in turn, may become part of the milk microbiota via the entero-mammary pathway (
Section 3.1.1). For example, Carrothers and Colleagues [
138] provided initial evidence for associations between maternal nutrition and maternal fecal microbial community structure during lactation. Increased intake of pantothenic acid, riboflavin, vitamin B6, and vitamin B12 were related to increased relative abundance of
Prevotella and decreased relative abundance of
Bacteroides in the maternal gut. Intake of copper, magnesium, manganese, and molybdenum were positively associated with Firmicutes and negatively associated with Bacteroidetes. Overall, findings steadily suggest that high consumption of a more nutrient-rich and calorie-loaded diet was positively associated with relative abundance of Firmicutes. It was suggested that such maternal dietary factors that directly influence the maternal gastrointestinal bacterial community, might also indirectly affect the milk microbiota [
137].
Correlations between maternal dietary intake and milk microbiota composition have been validated by several studies. In one investigation from Brazil, postpartum women went through a validated food frequency questionnaire that covered the whole pregnancy period, and correlations were obtained between their diet and their milk microbiota. A global, significant association with milk microbiota diversity was observed for the intake of ascorbic acid during pregnancy [
139]. In light of ascorbic acid importance, higher maternal consumption of citrus fruits, as well as of vegetables and β-carotene during pregnancy were protective against eczema in the offspring, in line with the positive effects of ascorbic acid on the human immune system [
140]. Furthermore, positive correlations were found between
Bifidobacterium in the milk and intake of polyunsaturated and linoleic fatty acids during lactation [
139]. This is consistent with the conversion of polyunsaturated fatty acids to conjugated linoleic acid and conjugated linolenic acid, known to favor
Bifidobacterium growth [
141].
In a study of 21 healthy breastfeeding women at Washington State University and University of Idaho, diet records, collected over 9 dietary assessments over six months postpartum, were correlated with milk microbiota. The results showed that relative abundances of several bacterial groups were associated with changes in maternal dietary intake. For instance, intake of saturated fatty acids and monounsaturated fatty acids were inversely associated with the relative abundance of
Corynebacterium; total carbohydrates, disaccharides, and lactose were negatively associated with Firmicutes; and protein consumption was positively correlated with the increase in
Gemella, a genus belonging to the Streptococcaceae family [
137].
In an interesting animal model of diet followed for 31 months, it was demonstrated that alone, diet may modulate mammary gland microbiota. Mediterranean diet resulted in approximately 10-fold increase in mammary gland
Lactobacillus abundance compared with mammary tissue from Western diet-fed animals [
142].
Lactobacillus is often thought of as commensal organism and is commonly included in probiotic formulations, where it directly contributes to beneficial nutritional, physiological, microbiological, and immunological effects in the host [
143]. Again, with
Lactobacillus transferred from maternal gut to human milk, and then from milk to intestinal tract of infants, a dynamic pathway exists in which maternal diet will play a major role in determining the profile of infant gut microbiota [
144].
Another recent study reinforcing the hypothesis that maternal diet affects milk microbiota found, at one-month postpartum, significant negative correlation between
Streptococcus in maternal milk and the intake of unsaturated fatty acids and the abundance of oleic acid. This was explained by the fact that streptococci may be sensitive to the antibacterial effects of fatty acids, causing a direct inhibition [
145]. The same group reported that
Bifidobacterium, pivotal in development of the infant gut microbiome, was detected, at extremely low abundances, in milk samples [
145]. This observation is in favor of the current view that maternal milk provides a microbial source for colonization of the infant intestine. However, the main effect of milk on the baby’s gut composition does not necessarily come from the milk bacteria only, but rather from other milk components, as well, that help to enrich specific bacterial groups. This could explain why bacterial breast milk composition and fecal microbiota composition are not exactly the same, but correlations do exist [
146]. In light of benefits of
Bifidobacterium, a trial investigated maternal supplementation of this bacterium as well as
Lactobacillus to maternal diet four weeks prior to the due date and for three months after delivery. The intervention did not significantly affect overall composition of breast milk microbiota transferred to the infant during breastfeeding, analyzed by 16S rRNA sequencing, thus questioning the substantial effect of probiotic supplementation to maternal diet on microbiota composition of breast milk [
147].
The currently available reports on maternal diet and microbiota of human milk are still few and reports on the role of nutrients in the metabolism of bacteria in human milk are much needed. Furthermore, the somehow incomplete knowledge regarding the availability of macro- and micronutrients in milk, which are directly useful for bacteria, further complicate data interpretation. The relationship between maternal diet and milk microbiota has much yet to be investigated, and in-depth studies of various nutritive components and their concentrations on microbiota of milk are warranted.
3.5. Beneficial Effects of Milk Microbiota on Infant Health
The commensal and beneficial microbes in human milk play a pioneer role in shaping infant health, and perhaps represent an example of how intimate contact with the microbial world is necessary for normal development in early life [
148]. Human milk microbiota affects the establishment of the largest human microbial reservoir in the gastrointestinal tract; also, the reciprocal relation between milk microbiota and gut microbiota is reflected on the development of immunity and protection against pathogens.
After birth, milk microbiota forms the most important determinant of infant gut colonization, which occurs in a stepwise fashion. With initial exposure to microbes transferred to the infant gut from mother’s milk, colonization of the intestine of infants starts, and may be essential for the maturation of the gut-associated lymphoid tissue, homeostasis of the intestinal epithelium, and development of intestinal physiology [
149]. In the first few days after birth, the infant intestine is characterized by a heterogeneous population of microbes characterized by facultative anaerobes that belong to Enterobacteriaceae,
Streptococcus,
Enterococcus, and
Staphylococcus that thrive on oxygen availability in the newborn gut.
Escherichia coli,
Enterococcus faecalis, and
Enterococcus faecium are the most characterized species among first colonizers. Gradual oxygen consumption by such facultative anaerobes creates a reduced oxygen environment that allows expansion of obligate anaerobes such as
Bifidobacterium,
Bacteroides, and
Clostridium. Intestinal colonization undergoes further changes upon introduction of solid food. The genus
Bifidobacterium is detected in the first few months, predominates by 12 months, then declines towards the second year, at the end of which the infant microbiota becomes more diverse [
150]. It is essential here to note the distinction between gut microbiota of breastfed and formula-fed infants: It is well known today that
Bifidobacterium is the predominant intestinal genus in both feeding modes [
151], with species variations. The species
Bifidobacterium longum,
Bifidobacterium infantis,
Bifidobacterium breve, and
Bifidobacterium bifidum are commonly detected in breastfed babies, while
Bifidobacterium adolescentis and
Bifidobacterium pseudocatenulatum, commonly seen among the intestinal adult microbiota, predominate in formula-fed babies [
152]. Collectively, formula-fed infants in general have relatively stable and diverse microbial intestinal communities with higher levels of facultative anaerobes and strict anaerobes when compared to breast-fed infants. Fecal samples from breast-fed infants are less complex, with higher numbers of aerobic organisms, and with more critical changes in microbial composition up to the first year. Once weaning, or the introduction of solid foods into the feeding pattern, begins, differences in microbial communities between breastfed and formula-fed infants diminish, and the microbial profile shifts towards the adult intestinal microbiome [
153].
The establishment and development of bifidobacterial and lactic acid bacteria in infant’s gut from viable inocula in human milk was demonstrated by several studies. For example, a study of Spanish full-term breastfed infants evaluated similarity between fecal bacteria and those from corresponding milk samples. It was shown that in one-day-old newborns,
Enterococcus and
Streptococcus were the microorganisms most frequently isolated, while from 10 days until 3 months of age, bifidobacteria became predominant. In corresponding breast-milk,
Streptococcus genus was most frequently isolated, and
Lactobacillus and
Bifidobacterium were also obtained [
154]. With an approximate viable bacterial density of 2–4 log colony-forming units/mL of human milk, resulting in a projected daily ingestion of 5–7 log cells with regular breastfeeding, it is expected that neonatal gut microbiota echoes the bacterial composition of breast milk [
155]. A trend consistent with breastfeeding is that breast milk selects for a highly adapted intestinal microbiota, dominated by bifidobacteria and termed a “milk-oriented microbiota” [
150]. When a disbalance occurs in such orientation health changes may result, for example, preterm infants with altered microbiota are susceptible to necrotizing enterocolitis due to the immaturity of their gastrointestinal and immune systems [
48]. In 2020, a meta-analysis of necrotizing enterocolitis in premature infants showed that a mixture of
Bifidobacterium and
Lactobacillus could reduce morbidity, illustrating role of human milk microbiota in health and disease [
156].
The human milk microbiota has a putative role in prevention of infections in newborns, and this occurs through its contribution to the competitive exclusion of pathogens and its involvement in the maturation of the immune system [
155]. One possible example of the competitive exclusion of pathogens is the genus
Staphylococcus. Several
Staphylococcus species, especially
S. epidermidis, colonize human milk and the intestine of breastfed infants. In an analysis of milk and stool samples from mother–infant pairs, Jiménez and Colleagues [
157] found that
S. epidermidis was the predominant species in the milk and stool of breast-fed infants, while it was less prevalent in those of formula fed-infants. The presence of adhesion-related genes in
S. epidermidis was very high, while the biofilm-related operon
icaD and the gene
mecA were only detected only rarely the
S. epidermidis strains. Hence, the bacterial attachment factors were present but less commonly the virulence or antibiotic resistance determinants. The commensal staphylococcal strains provided by breast milk to the infant gut may successfully compete with potentially harmful strains. In another, more recent investigation on intestinal
Enterococcus from breastfed infants, the commensal enterococci harbored antibiotic resistance and virulence genes. However, the patterns of these genes were not consistent with those described for antibiotic-resistant hospital-associated enterococci, and none were resistant to vancomycin. The frequency of virulence determinants like hemolysin and gelatinase was also low, while some genes linked to colonization were abundant. Taken together, these findings suggest a possible benefit of enterococci in preparing the infant gut for effective opposition against pathogens [
158]. It is possible that the pre-colonization of infants with maternal commensal strains will help later in preventing acquisition of infection by more virulent ones [
155]. Interestingly, and in the field of HIV infection, lactobacilli cultured from human milk were able to inhibit HIV-1 infection in vitro by blockade of CCR5 co-receptor, and to a lesser extent CXCR4 or both coreceptors, suggesting a probable role for lactobacilli in mucosal protection against HIV-1 [
159].
In summary, an association between human milk microbiota, and consequently gut microbiota, and infant health and disease is an important contributor to infant hemostasis. In addition to the relationships described above, ongoing studies are continuing to investigate influence of microbiota on infant irritable bowel syndrome, inflammatory bowel disorder, and type 1 diabetes mellitus [
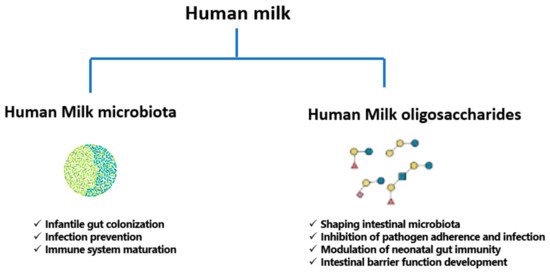
160]. The functions of human milk microbiota and HMOs are summarized in below.
Figure 2. Functions of human milk microbiota and human milk oligosaccharides (HMOs).
3.6. Probiotics of Human Milk
The term “Probiotic” means “for life” and it is currently used to name bacteria associated with beneficial effects for humans and animals. In 2001, the Food and Agriculture Organization of the United Nations (FAO) and the WHO defined probiotics as “live microorganism which, when administered in adequate amounts confer a health benefit on the host” [
161]. This definition was revised in 2014 by the International Scientific Association for Probiotics and Prebiotics, including for probiotics “microorganism for which there are scientific evidence of safety and efficacy [
162]. Probiotics that have been principally studied in humans include species from
Lactobacillus and
Bifidobacterium genera, both of which have been used safely for quite a long time [
90]. Probiotic administration early in life may be effective in prevention and treatment of some disorders, leading to a correct microbial colonization while the gut microbiota is still being established [
163]. Increasingly administered to infants, probiotics are intended to decrease the risk of certain ailments like necrotizing enterocolitis and late-onset sepsis in preterm infants, colic and antibiotic-associated diarrhea in term infants, in addition to some chronic diseases of childhood such as asthma and atopic disease [
164]. The mechanisms responsible for probiotic action still require full elucidation; however, they include modification of the gut microbiota and normalizing its perturbation, competitive adherence to the mucosa and epithelium for pathogen exclusion, strengthening of the gut epithelial barrier, improving the digestion process by complementing the functions of absent digestive enzymes, and modulating the immune system to offer an advantage to the host [
162,
165]. There are agreed-upon criteria that a probiotic must harbor to be considered efficacious, such as capacity to survive in the GI tract, resistance to stomach acidity, lack of mobile antibiotic resistance genes, and demonstration of health benefits through efficacy testing followed by clinical trials [
90,
166].
Human milk-derived bacterial strains can be considered as potential probiotics; isolation of strains from milk for subsequent use in infant health and nutrition markets is used [
90]. For example, human milk strains of
Lactobacillus reuteri, a well-studied probiotic found in the gut and breast milk, can reduce the production of pro-inflammatory cytokines, promote T cell development, and decrease the microbial translocation across the intestinal epithelium from the gut lumen to tissues, thereby reducing inflammation. Notably, the decrease in
L. reuteri in humans in the past decades was correlated with an increase in prevalence of inflammatory disorders. Direct supplementation of
L. reuteri may be an attractive preventive and/or therapeutic model against inflammatory diseases [
167]. In a murine model of asthma developed in 2020, Li and Colleagues [
168] showed that oral administration of
L. reuteri was more effective in asthma prevention than five other
Lactobacillus species, where it reduced the risk of asthma by modulating specific gut microbiota to improve the immune environment of the lungs.
L. reuteri supplementation, may, therefore, be a candidate against asthma and other allergic diseases. The same organism from human milk attenuated weight gain, fat accumulation, hypertriglyceridemia, and hypercholesterolemia in mice, thus opening a new horizon for the development of relevant foods to prevent metabolic disorders [
169]. In a randomized controlled trial,
L. reuteri administration was preventive in reducing pediatric consultations, parental discomfort, and the use of pain relievers for infant colic [
170].
Apart from
L. reuteri,
Lactobacillus fermentum strains from human milk have been studied as probiotic candidates, and proved to be safe, well tolerated and useful for the prophylaxis against community-acquired infections [
171]. They also showed cholesterol-lowering effects in simulated models of liver and gastrointestinal tract [
172]. Both
L. fermentum, and another milk-derived species,
Lactobacillus salivarius, enhanced both natural and acquired immune responses, via activation of natural killer and T cell subsets and induction of a broad array of cytokines in peripheral blood mononuclear cells in vitro [
173]. An interesting investigation of milk-derived
Lactobacillus casei and
Lactobacillus paracasei strains, demonstrated anticancer and antioxidant effect in HeLa cell lines of cervical cancer [
174]. Likewise, anticancer potential was demonstrated by Rajoka and Colleagues [
175] for
Lactobacillus rhamnosus isolated from human milk, through induction of apoptosis and down-regulation of the
bcl-2 protooncogene, suggesting potential anticancer capability. A review of available studies on the use of probiotics in infantile acute gastroenteritis and antibiotic-associated diarrhea found that
L. rhamnosus had the highest compelling evidence of efficacy in reducing duration of gastroenteritis by one day, and was the most effective probiotic in prevention of diarrhea [
176].
As for bifidobacteria of human milk, studies are also available to support their use as probiotics. Maldonado and Colleagues [
177] conducted a randomized controlled trial on the addition of a strain of
Bifidobacterium breve, originally isolated from human milk, to infant formula. They concluded that this probiotic reduced crying rates, is safe, and induces beneficial effects on health.
Bifidobacterium longum, isolated from human breast milk, had anti-oncogenic and tumor suppressor potential in murine colorectal cancer [
178]. Park and Colleagues [
179] showed that a probiotics formula containing both
B. longum and
Lactobacillus acidophilus alleviated fever, vomiting, and diarrhea with no adverse events in hospitalized infants with rotavirus infection.
Although
Bifidobacterium and
Lactobacillus strains from human milk persist as the most commonly sought bacteria for probiotic use, the health endorsing effects of other bacteria from milk is also under investigation.
Enterococcus faecium, a probable probiotic isolated from breast milk was evaluated both in vitro and in vivo for its safety. The strain was non-hemolytic, sensitive to majority of antibiotics, and showed no alteration of normal growth and development in male rats. No adverse effects on general status nor behavior; additionally, no significant changes were noted in the hematological results, blood biochemistry, organ weights, and histopathology, and none of the vital organs of treated animals displayed signs of bacteremia nor infectivity. These findings indicated the candidature of
E. faecium as a potential safe probiotic [
180]. In another analysis of
E. faecium and
E. faecalis strains isolated from breast milk, these bacteria inhibited the growth of
Escherichia coli,
Listeria monocytogenes,
Salmonella typhi,
Staphylococcus aureus,
Shigella dysenteriae, and
Streptococcus agalactiae. However, phenotypic and genotypic virulence analysis indicated hyaluronidase enzyme production and vancomycin resistance in
E. faecalis, calling for careful monitoring of probiotic strains for safety parameters [
181]. Moreover, a study of cells and cytoplasmic fractions of
E. faecalis and
Staphylococcus hominis isolated from breast milk against MCF-7 breast cancer cell line revealed significant decrease in cellular proliferation in concentration- and time-dependent style. Morphological signals of apoptosis such as cell shrinkage, cell death, and membrane blebbing were observed in over one-third of the tumor cells [
182].
In short, the available data on possible probiotics isolated from human milk is abundant. Further, focused evaluation of safety and efficacy of this rich milieu of microbes is needed to determine which members are good alternative nutraceuticals with health-promoting profiles or promising therapeutic indices.