
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Emanuel M. Fernandes | + 3784 word(s) | 3784 | 2021-03-24 03:10:27 | | | |
| 2 | Emanuel M. Fernandes | Meta information modification | 3784 | 2021-04-01 12:28:25 | | | | |
| 3 | Emanuel M. Fernandes | Meta information modification | 3784 | 2021-04-01 12:43:17 | | | | |
| 4 | Emanuel M. Fernandes | Meta information modification | 3784 | 2021-04-01 12:47:14 | | | | |
| 5 | Bruce Ren | Meta information modification | 3784 | 2021-04-02 03:39:27 | | | | |
| 6 | Bruce Ren | Meta information modification | 3784 | 2021-04-08 03:21:00 | | |
Video Upload Options
Pathogenic microbes are a major source of health and environmental problems, mostly due to their easy proliferation on most surfaces. Currently, new classes of antimicrobial agents are under development to prevent microbial adhesion and biofilm formation. However, they are mostly from synthetic origin and present several disadvantages. The use of natural biopolymers such as cellulose, hemicellulose, and lignin, derived from lignocellulosic materials as antimicrobial agents has a promising potential. Lignocellulosic materials are one of the most abundant natural materials from renewable sources, and they present attractive characteristics, such as low density and biodegradability, are low-cost, high availability, and environmentally friendly.
1. Introduction
The presence of pathogenic microorganisms on the material surfaces can lead to significant healthcare and environmental problems. In recent times, different strategies have been defined to prevent the proliferation and adhesion of microorganisms on medical devices; or materials for food storage, and packaging [1][2]. Moreover, the biofilm formation on the materials surfaces can limit their functionality, leading to critical health related complications [3]. Furthermore, antibiotic-resistant microorganisms have emerged due to the extensive use of antibiotics or biocidal to impair their growth. Thus, it is necessary the development of new drug free materials that could avoid the increase of antibiotic-resistant microorganisms.
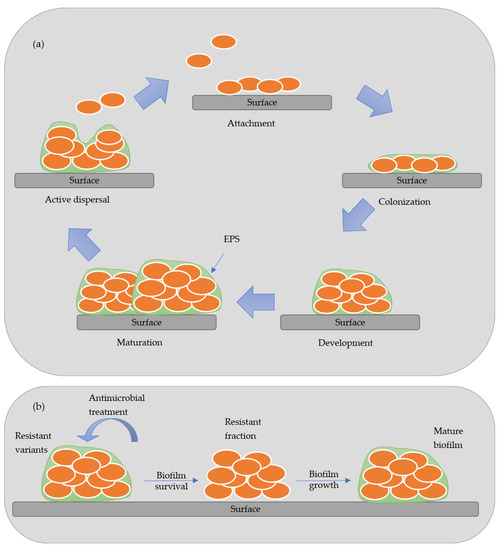
Biofilm is an aggregate of microorganisms that attaches to wet surfaces and multiplies, forming a slimy matrix of extracellular polymeric substances (EPS), thus creating an optimum environment to develop biofilms [4]. The EPS is composed of polysaccharides, proteins, lipids, and nucleic acids, forming a highly hydrated polar mixture that contributes to the three-dimensional structure of the biofilm. The biofilm formation is established in five stages: attachment, colonization, development, maturation, and active dispersal. Figure 1 presents a scheme of the stages of development of biofilm. In the attachment stage, the microorganisms are reversibly absorbed to the biotic or abiotic surface by weak van der Waals forces bonds. In contrast, in the colonization stage, stronger hydrophilic/hydrophobic bonds are established with the surfaces allowing them to proliferated and secret EPS [4][5]. In the maturation stage, a three-dimensional structure contains channels that distribute nutrients and signal molecules in the biofilm. In the last stage, called active dispersion, the cells are detached, either singly or in clumps, and colonize other locations [5]. The formation and development of biofilm depend on many factors, such as the specific bacteria strain, the properties of the material’s surface, the environmental condition (pH, temperature, and nutrients), among others [6]. Biofilms are responsible for biocorrosion, biofouling (accumulating microorganisms in surfaces), and reservoir souring, causing many constraints in different industries [4][7].
The main mechanisms of antimicrobial action by which antimicrobial compounds affect microorganism are protein synthesis inhibition, cell wall disruption, and nucleic acid inhibition. Antimicrobial compounds can act as suppressing protein synthesis targeting the ribosomal subunits or protein folding, thus inhibiting their active role. They can also disrupt cell walls, causing an increase of permeability of the membrane, leading to the leakage of intracellular constituents. In addition, they are able to inhibit nucleic acid mechanism by suppressing the replication of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) [8]. Currently, there is no deep knowledge on the mechanism of action against pathogenic microorganisms and the way they will act on surfaces.
The conventional methods to disinfect surfaces uses antimicrobial reagents, such as antibiotics, fungicides, antiviral drugs, and nonpharmaceutical chemicals [2]. Antifouling agents are also employed since they prevent the adsorption on the surface and/or kill/inhibit the growth of microbes, preventing the biofilm formation. Antimicrobial or antibacterial agents are classified as a subclass of antifouling agents, and these materials present biocidal activity [9].
The extensive use of these compounds can cause concern due to their environmental pollution potential, and the development of microbial resistance. The use of antimicrobial agents can be limited, and they cannot achieve high and durable local concentrations on the surface and providing lessen disinfection of the materials surfaces [2]. Therefore, it is important to develop new antimicrobial agents able to prevent microbe’s adhesion and proliferation on materials surfaces, and reduce their negative effects.
The antimicrobial agents are classified into two categories, organic and inorganic. The organic antimicrobial agents include natural biopolymers, for instance, the chitosan, cellulose and lignin, phenols, halogenated compounds, and quaternary ammonium salts [10][11][12]. The inorganic antimicrobial agents comprise, for example, metals, or metals bonded with phosphates, and metal oxides. The most common metallic nanoparticles or metal oxides used are silver, copper, titanium oxide, zinc oxide, magnesium, and calcium oxide [10][11][12]. In the literature, several studies explore the use of different antimicrobial agents by incorporation or applied as coatings on materials surfaces. Among them, the antimicrobial potential of natural derived lignocellulosic compounds remains still unexplored.
2. Lignocellulosic Materials and Main Compounds
Lignocellulosic materials are mainly composed of three biopolymers—cellulose, hemicellulose, and lignin—combined with smaller other components. The ratios between these compounds vary depending on the lignocellulosic material origin [13][14][15][16].
Recently, the antimicrobial activity of lignocellulosic materials has been explored. In Table 1, we present examples of the use of lignocellulosic materials main compounds as antimicrobial materials.
Table 1. Antimicrobial activity of lignocellulosic compounds.
| Compound | Origin | Antimicrobial Activity Tested Against | Application | Ref |
|---|---|---|---|---|
| Cellulose | Wood | E. coli, S. aureus | Packaging | [17] |
| E. coli, S. aureus | [18] | |||
| E. coli, P. aeruginosa, B. subtilis | Tissue engineering, wound dressing | [19] | ||
| Sugarcane Bagasse | S.aureus, T. rubrum | Skin infective | [20] | |
| Wastewater purification | [21] | |||
| Tulsi | E. coli, S. aureus, B. cereus, Ser. marcescens | Biomedical | [22] | |
| Ginger | E. coli, S. aureus, B.cereus, Sal. thyphimirium | Packaging, wound dressing, surgical material | [23] | |
| Hemicellulose | Plantago Ovata seed husk | E. coli, S. aureus, P. aeruginosa | Wound dressing, drug delivery | [24] |
| Almond gum | Actinomycetes sp, Sal. thyphimirium, K. pneumonia, L. monocytogenes, S. aureus, Sal. enterica, P. aeruginosa, B. thuringiensis, B. subtilis | Food and non-food | [25] | |
| Lignin | Softwood | S. aureus | Biomedical | [26] |
| Eucalyptus | A. niger E. coli, S. aureus, Pr. microbilis, Pr. vulgaris, P. aeruginosa, Entero. aerogenes, B. thuringiensis, Sal. enterica serotype typhmurium and Strept. mutans |
Antimicrobial additive or agent in food, textile, or chemical industry | [27] | |
| Spruce | [27] | |||
| Poplar | E. coli | Drug delivery, food packaging, wound dressing, | [28] | |
| Acacia | E. coli, S. aureus | Active packaging | [29] | |
| Apple tree pruning residues | A. niger, Sacch. cerevisiae | Food antioxidant | [30] | |
| Sugarcane Bagasse | E. coli, S. aureus, P. aeruginosa, S. epidermidis | [31] | ||
| B. aryabhattai, Klebsiella sp. | Natural antibacterial agent | [32] | ||
| S. epidermidis | Antimicrobial textile | [33] | ||
| Corn | L. monocytogenes, S. aureus, E. coli, Sal. enteritidis, C. lipolytica | Antioxidant and antimicrobial | [34] | |
| E. coli, S. aureus, B. subtilis, Sal. enterica | Natural antibacterial agent | [35] | ||
| Cotton stalks | S. aureus, K. pneumoniae | Medical and technical textiles | [36] | |
| Bamboo | E. coli, S. aureus, B. subtilis, Sal. enterica | Natural antibacterial agent | [37] |
2.1. Cellulose
Cellulose is the most abundant renewable polymer found in nature, and it is the main constituent of the cell wall. It can be biosynthesized by different organisms, such as plants, amoebae, sea animals, bacteria, and fungi [38].
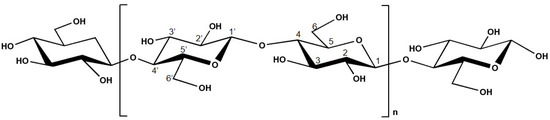
Cellulose is a linear homopolysaccharide with the molecular formula (C6H10O5)n, and it is composed of ß-D-glucopyranose (glucose) moieties linked by β-(1,4) glycosidic bonds [13][14][39][40]. The chemical structure of cellulose is presented in Figure 2. This compound possesses both well-ordered (crystalline) and disordered (amorphous) regions [13][14][39][41]. The presence of polar oxygen and hydrogen atoms in cellulose allows the formation of intermolecular and intra-molecular bonding [42].
The structure of this material is organized as microfibrils, with a diameter between 2 and 20 nm, connect together to form cellulose fibers [38][43].
Cellulose is commonly used as a raw material in different industries, such as textile, plastic, wood, cosmetics, and pharmaceutical. Cellulose presents high biocompatibility, biodegradability, non-toxicity, and high hydrophilicity. It also reveals good mechanical properties, thermal and chemical stability, chirality and allows chemical modification [44][45][46]. Cellulose can be applied in a wide range of applications, such as packaging [47][17], biomedical [22][48][49], tissue engineering [19], wound dressing [19][50][23], marine coatings [51], among others. For instance, Onofrei et al. [48], developed films composed of cellulose acetate blended with hydroxypropylcellulose. The film with a higher concentration of cellulose acetate inhibited the growth of Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus).
In work by Sun et al. [17], cellulose-based membranes were produced with cellulose fibers modified by azidation, followed by epoxidation and grafted with poly(hexamethylene guanidine) (PHMB). The antibacterial activity of the membranes against E. coli and S. aureus was investigated after each modification step. Here, membranes with PHMB grafting showed higher antibacterial activity. Furthermore, the antibacterial effect lasted up to 60 days, possibly due to the covalent connection between cellulose and PHMB. It is proposed that the membranes can be used for packaging applications.
Gogoi et al. [51], also modified nanofibrillar cellulose from Colocasia esculenta with triethanolamine and silver (Ag) nanoparticles to produce epoxy resin composites. The combination of nanofibrillar cellulose with Ag nanoparticles presented improved antibacterial activity against S. aureus and antifungal activity against Candida albicans (C. albicans). Guna et al. [22], prepared cellulose fibers from the tulsi stalk and tested the antimicrobial activity against S. aureus, E. coli, Ser. marcescens, and B. cereus. The work shows a bacteria reduction between 55% and 62% for the nanofibrillar cellulose, and a higher reduction of 90% to 98% for the tulsi stalk fiber. The same trend was also observed by Ilangovan et al. [50], where fibers made from cellulose extracted from Curcuma Longa L. residues. Gabov et al. [18], showed that beads prepared from the combination of cellulose and lignin obtained from birch wood chips presented antimicrobial activity against S. aureus. However, the beads had a high concentration of lignin in the matrix, which could have influenced the antibacterial activity. Yadav et al. [19], prepared bio-sponges from a composite of sodium alginate and cellulose extracted from mango wood scrap combined with bio-extracts from rice water and Giloy extract. The bio-sponges demonstrated good antibacterial activity against Gram-positive, B. subtilis, and Gram-negative bacteria, E. coli, and P. aeruginosa. Oliva et al. [47], isolated the cellulose from paper with concentrated sulfuric acid to produce films that were then treated with zinc oxide and carvacrol essential oil. The films made with cellulose extract showed good antibacterial activity against E. coli and S. aureus, but the ones made with the essential oil only presented a considerable reduction of the bacteria at higher concentrations (2 wt.%). On the other hand, hydrogels made with cellulose obtained from sugarcane bagasse and combined with zinc oxide nanoparticles present good antimicrobial activity against S. aureus (bacteria) and T. rubrum (fungi). It was verified that the zinc nanoparticles enhanced the already existing antimicrobial properties of the cellulose. Furthermore, Anagha et al. [21], suggested that the good antimicrobial properties allied with their excellent biocompatibility and low cytotoxic, making these hydrogels good for biomedical applications. Likewise, films made with nanocellulose obtained from oil palm or empty fruit bunches via alkaline treatment and acid hydrolysis combined with zinc oxide showed good antibacterial activity against E. coli and S. aureus higher than the activity observed for the zinc oxide particles [52]. Overall, these studies indicate that cellulose derived from different sources demonstrates good antimicrobial activity, thus inferring to the potential of these materials.
The antimicrobial activity of cellulose can be enhanced by the inclusion of inorganic nanoparticles. Li et al. [49], prepared composites by combining titanium dioxide and cellulose that presented higher inhibitory activity against E. coli than S. aureus. On the other hand, Li et al. [53] also prepared composites with silver chloride and cellulose that demonstrated good antibacterial activity against both E. coli and S. aureus. Furthermore, other studies developed composites made with cellulose, silver nanoparticles, and other commercial polymers such as Polyvinyl alcohol (PVA) [54] and polyurethane (PU) [55], also exhibit good antibacterial properties, suggesting that the addition of commercial polymers have no additional effect on the antibacterial properties of cellulose-based composite.
2.2. Hemicellulose
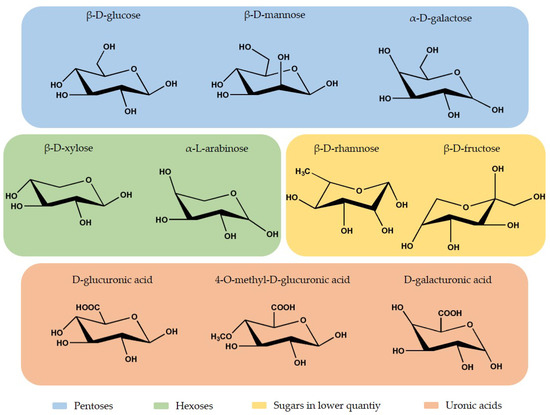
Hemicellulose is a short-chain heteropolysaccharide present in the cell wall, and it corresponds to 15% to 35% of the plant composition, depending on the plant species [56][57][58]. The heteropolymer presents an amorphous branched structure and a lower polymerization degree than cellulose, approximately 200 [58]. Hemicellulose is constituted by different monosaccharide units, hexoses (β-D-glucose, β-D-mannose, and β-D-galactose) and pentoses (β-D-xylose and α-L-arabinose) in higher quantities, and other sugars (fructose and rhamnose) in lower quantities. It also presents uronic acids, like 4-O-methyl-D-glucuronic acid, D-glucuronic acid, D-galacturonic acid, and acetyl groups [40][56][58][59][60]. In Figure 3, is possible to observe the chemical structure of the monosaccharides units present in hemicellulose.
Thus far, the antimicrobial properties of hemicellulose are less explored when compared to cellulose and lignin. Nevertheless, these polymers offer great antimicrobial potential due to their components. Ahmad et al. [24], the hemicellulose films inhibited the growth of the bacteria S. aureus, E. coli, and P. aeruginosa. Bouaziz et al. [25], the authors assessed the antibacterial activity of hemicellulose extracted from almond gum against different strains of Gram-positive (B. subtilis, B. thuringiensis, Actinomyces sp., L. monocytogenes, and S. aureus) and Gram-negative bacteria (P. aeruginosa, Sal. enterica, Salmonella typhimurium, and K. pneumoniae). Here, the hemicellulose presented higher inhibitory activity against B. thuringiensis, S. enterica, and P. aeruginosa and moderated inhibition against Actinomyces sp., Sal. thyphimirium, K. pneumonia, L. monocytogenes, and B. subtilis. However, the hemicellulose antibacterial activity was lower when compared to the positive control. Nevertheless, the work suggests that hemicellulose has a bacteriostatic behavior against the tested bacterial, showing that these materials could be used for food or non-food applications.
The antimicrobial activity of xylan is one of the most studied polymers derived from hemicellulose extract. Fu et al. [61], showed that gels using xylan, gelatin glycerol, and Nicotinamide had good antimicrobial activity against Yeast but lower activity against B. subtilis and S. aureus. Moreover, the gels displayed good cytocompatibility to be applied in cosmetic industries. Arellano-Sandoval et al. [62], also produced hydrogels with xylan extracted from bagasse and Poly(N-vinylcaprolactam) that inhibited the bacterial growth of E. coli, S. aureus, and P. aeruginosa.
2.3. Lignin
Since 1977, when Adler [63] described lignin as a highly branched polymer with different functional groups: aliphatic and phenolic hydroxyls, carboxylic, carbonyl, and methoxyl groups, the structure of lignin has significantly been studied and explored. Lignin is one of the most abundant polymers in nature, and it is an amorphous heterogeneous polymer network of phenylpropane units linked together by different, non-regular sequence bonds [13][14][64].
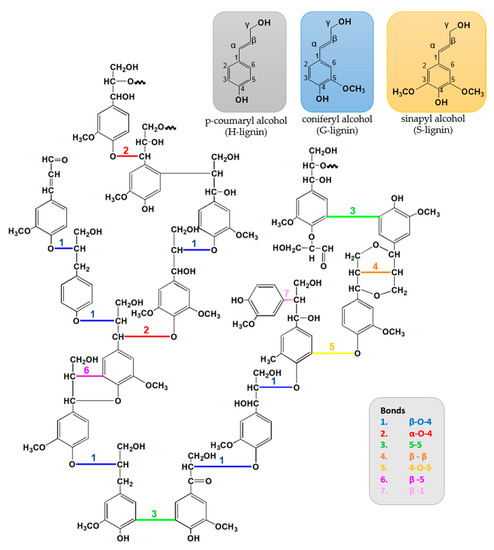
The phenylpropane units are originated from three aromatic alcohol precursors, monolignols, p-coumaryl (H-lignin), coniferyl (G-lignin), and sinapyl (S-lignin) alcohols. During the polymerization process, the monolignols units are linked by radical coupling reactions to form the three-dimensional molecular architecture with different bonds, and typically around half of these bonds are β-4-O ether connections [65][66][67][68][69]. Figure 4 presents the chemical structure of lignin with the highlighted bonds between the monolignols.
Lignin behavior is similar to a thermoplastic, presenting decomposition temperature and glass transition temperature that varies with the isolation method, absorbed water, molecular weight, and thermal history [65].
This compound is responsible for binding the lignocellulosic materials’ components, acting as a glue, making it insoluble in water. Lignin presents an essential role in woody plants; it is the major component of the vascular plant’s cell wall and confers rigidity, impermeability, resistance to microbial attack, and oxidative stress. Lignin is mainly used to generate heat and energy but is also used as a food and concrete additive, dispersants, resin, and binding material [13][14][39][64][70].
The lignin’s antimicrobial activity is influenced by different factors, such as the origin or the extraction method. Several lignin’s extracted with different methods are described in the literature, such as kraft lignin, hydrolysis lignin, organosolv lignin [71]. In the kraft process, the lignocellulosic materials are treated with a solution of sodium hydroxide (NaOH) and sodium hydrosulphide (NaHS) in a temperature range of 150 to 170 °C. After the treatment, the lignin ether bonds are cleaved, and lignin is converted to small fragments, also known as alkali-soluble lignin. For the organosolv method, lignin is dissolved in organic solvents (acetic acid, ketone, and ester), and the organosolv lignin is highly pure, sulphuric-free, and has less modification.
To understand the effect of the origin and the extraction method and how the lignin origin influence in antimicrobial properties, Gordobil et al. [27], used lignin obtained from eucalyptus and spruce by two different methods, kraft, and organosolv. The author used solutions with different lignin concentrations to evaluate antifungal activity against A. Niger and antibacterial activity against different bacteria (Table 1). It was concluded that the kraft lignin’s present higher antifungal activity than the organosolv lignins. In the kraft lignin from eucalyptus, presents a high antifungal activity for all the concentrations, however spruce lignin only acts as antifungal in lower concentrations. In this work, it is proposed that the higher antifungal activity observed in the kraft lignins could be explained by the lower carbohydrate content and by the presence of sulfur-containing derivatives. For the antibacterial tests, the authors verified that the kraft lignins present higher antibacterial activity, in some cases, higher than the commonly used antibiotic, due to their rich antioxidant and polyphenolic nature. This lignin’s revealed potential to be applied as antimicrobial additive or agent against pathogenic microorganisms in food, textile and chemical industries. As shown in a previous study, antifungal activity is not only influenced by the extraction method but also by the origin. The results obtained by García et al. [30], show that the organosolv lignin extracted from apple tree pruning residues could not show the antifungal activity against A. niger. However, the authors could demonstrate the resistance against other fungi, Saccharomyces cerevisiae.
Another promising method to extract lignin from natural fiber is the use of ionic liquids. Ionic liquids are green solvents with high thermal stability and present low toxicity [72]. In the work by Shen et al. [28], lignin extracted from poplar wood was combined with epichlorohydrin (ECH) and polyethylene glycol (PEG) to produce membranes. The lignin powder’s antibacterial activity extracted by kraft method and ionic liquid (1-ethyl-3-methylimidazolium acetate) method and the membranes were tested against E. coli. The kraft lignin powder presented 56% of bacteria reduction, while ionic liquid extracted lignin only presented 26% of bacteria reduction. The membranes produced with both types of lignin also showed a reduction of the bacteria on the surface. The developed membranes could be used for drug delivery, food packaging, and wound dressing.
Some authors use the fractionation method to extract lignin. Fractionation is a physical-chemical modification technique that allows the separation of high molecular weight lignin chains from lower molecular weight fractions [74]. In the work by Wang et al. [35] used enzymatic hydrolyzed lignin from corn stalk and performed two sequential ethanol extraction to obtain a different lignin fraction. The authors tested the antimicrobial activity of the lignin extracts against the Gram-positive bacteria, E. coli and B. subtilis, and the Gram-negative, Sal. enterica and S. aureus. The authors observed that the first extract showed higher antibacterial activity against all the bacteria, but the Gram-positive are more sensitive to the lignin extract than the Gram-negative bacteria. Likewise, they also extracted lignin from bamboo by the kraft method, and fractionated with ethanol to obtain a soluble and an insoluble fraction [37]. The kraft lignin and the lignin fractions antimicrobial activity was tested against the same bacteria used in the previous study. The insoluble phase showed low inhibition against Gram-positive bacteria. It promoted the growth of the Gram-negative bacteria probably by the low phenolic compounds content and poor water solubility, leading to the formation of insoluble particles that act as carriers for the bacteria. The soluble phase showed good growth inhibition of the growth of both types of bacteria [37]. Kaur et al. [32], modified lignin from sugarcane bagasse by three different methods, acetylation, epoxidation, and hydroxymethylation and, evaluated the antibacterial activity against the bacteria B. aryabhattai and Klebsiella sp. The author verified that lignins antibacterial activity depends on the concentration, and lignins are more effective against B. aryabhattai than Klebsiella sp. In the modified lignins, the minimum inhibitory concentration (MIC) value of the epoxy lignin is the lowest, and the acetylated lignin is the highest for both bacteria. Despite the promising results, the unmodified and modified lignin present lower antibacterial activity than standard tetracycline. The authors indicate that the modified lignins could be used as a natural antimicrobial agent.
Lignin can also be functionalized with metallic nanoparticles to improve antibacterial and antifungal activity. In the work by Aadil et al. [75], present lignin from acacia was functionalized with silver nanoparticles that displayed antibacterial efficacy against Gram-positive and Gram-negative strains. Chandna et al. [76], functionalized kraft lignin with gold (Au) and silver (Ag) nanoparticles. The bimetallic nanoparticle, composed of Au and Ag, showed better antibacterial and antifungal activity than the nanoparticle systems composed by lignin and Au or lignin and Ag.
Lignin composites have also demonstrated that the addition of lignin promotes antimicrobial activity. In the literature, it is shown the combination of different polymers such as with poly(butylene succinate) (PBS) [26], alginate [29], and poly(vinyl alcohol) (PVA) [77]. The composites obtained by Domínguez-Robles et al. [26], by the extrusion followed by injection molding of kraft lignin extracted from softwood with PBS, showed higher antimicrobial activity against S. aureus than the PBS matrix, even in a small amount (2.5 wt.%). Aadil et al. [29], also showed the antibacterial effect of lignin against S. aureus. The authors extracted lignin from Acacia wood powder and produced composite films with alginate. Lignin composites presented antibacterial activity against S. aureus, although the composites did not show any effect against E. coli. Lee et al. [77] used alkali lignin combined with PVA to produce fiber by electrospinning process. The composites with 50% and 85% of lignin presented 99.9% of reduction of S. aureus, but no effect was observed for E. coli bacteria.
In this context, the materials produced with lignin can be considered for different areas of application, such as biomedical [26][36][78], textile [33][36], packaging [28][29] and as natural antimicrobial agent [32][35][37].
3. Conclusion
Lignocellulosic materials are widely used in several production sectors such as construction, furniture, packaging, health or the automotive industry. Several studies have highlighted the potential of these natural fibers or its chemical constituents on different polymer-matrix systems. In addition, their antimicrobial effects have been recognized. In the last years, the use of lignocellulosic materials has grown mainly to their characteristics, high availability, environmentally friendly, from renewable sources, low cost, and biodegradability. This review presents an overview of the most recent advances that demonstrates the potential of the lignocellulosic based materials, cellulose, hemicellulose, lignin, and lignocellulosic fibers to be used as antimicrobial agents. In this area, the antimicrobial activity of the materials has emerged from the combination of the lignocellulosic source with antimicrobial agents from inorganic or organic origin. The potential of lignocellulosic as new drug-free polymers is extensive but this area is still virtually unexplored, especially as antimicrobial or anti-biofouling materials for industries, such as packaging, healthcare, environmental, textile, space engineering, among others. By unlocking the full potential of the antimicrobial properties of lignocellulosic materials, it will be possible to fully disclose their potential, bringing new links of knowledge between the areas involving the synthesis of natural fibers, the polymer matrices and microbiology.
References
- Franco, A.R.; Fernandes, E.M.; Rodrigues, M.T.; Rodrigues, F.J.; Gomes, M.E.; Leonor, I.B.; Kaplan, D.L.; Reis, R.L. Antimicrobial coating of spider silk to prevent bacterial attachment on silk surgical sutures. Acta Biomater. 2019, 99, 236–246.
- Song, B.; Zhang, E.; Han, X.; Zhu, H.; Shi, Y.; Cao, Z. Engineering and Application Perspectives on Designing an Antimicrobial Surface. ACS Appl. Mater. Interfaces 2020, 12, 21330–21341.
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554.
- Simões, M.; Simões, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT Food Sci. Technol. 2010, 43, 573–583.
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423.
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585.
- Xu, D.; Jia, R.; Li, Y.; Gu, T. Advances in the treatment of problematic industrial biofilms. World J. Microbiol. Biotechnol. 2017, 33.
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118.
- Choudhury, R.R.; Gohil, J.M.; Mohanty, S.; Nayak, S.K. Antifouling, fouling release and antimicrobial materials for surface modification of reverse osmosis and nanofiltration membranes. J. Mater. Chem. A 2018, 6, 313–333.
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112.
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chem. 2020, 310, 125915.
- Topuz, F.; Uyar, T. Antioxidant, antibacterial and antifungal electrospun nanofibers for food packaging applications. Food Res. Int. 2020, 130, 108927.
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685.
- Ten, E.; Vermerris, W. Functionalized Polymers from Lignocellulosic Biomass: State of the Art. Polymers 2013, 5, 600–642.
- Pauly, M.; Keegstra, K. Plant cell wall polymers as precursors for biofuels. Curr. Opin. Plant Biol. 2010, 13, 305–312.
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53.
- Sun, L.; Yang, S.; Qian, X.; An, X. High-efficacy and long term antibacterial cellulose material: Anchored guanidine polymer via double “click chemistry”. Cellulose 2020, 27, 8799–8812.
- Gabov, K.; Oja, T.; Deguchi, T.; Fallarero, A.; Fardim, P. Preparation, characterization and antimicrobial application of hybrid cellulose-lignin beads. Cellulose 2016, 24, 641–658.
- Yadav, C.; Maji, P.K. Synergistic effect of cellulose nanofibres and bio- extracts for fabricating high strength sodium alginate based composite bio-sponges with antibacterial properties. Carbohydr. Polym. 2019, 203, 396–408.
- George, D.; Maheswari, P.U.; Sheriffa Begum, K.M.M.; Arthanareeswaran, G. Biomass-Derived Dialdehyde Cellulose Cross-linked Chitosan-Based Nanocomposite Hydrogel with Phytosynthesized Zinc Oxide Nanoparticles for Enhanced Curcumin Delivery and Bioactivity. J. Agric. Food Chem. 2019, 67, 10880–10890.
- Anagha, B.; George, D.; Maheswari, P.U.; Begum, K.M.M.S. Biomass Derived Antimicrobial Hybrid Cellulose Hydrogel with Green ZnO Nanoparticles for Curcumin Delivery and its Kinetic Modelling. J. Polym. Environ. 2019, 27, 2054–2067.
- Guna, V.; Ilangovan, M.; Hu, C.; Nagananda, G.S.; Ananthaprasad, M.G.; Venkatesh, K.; Reddy, N. Antimicrobial Natural Cellulose Fibers from Hyptis suaveolens for Potential Biomedical and Textiles Applications. J. Natl. Fibers 2019, 1–10.
- Jacob, J.; Haponiuk, J.; Thomas, S.; Peter, G.; Gopi, S. Use of Ginger Nanofibers for the Preparation of Cellulose Nanocomposites and Their Antimicrobial Activities. Fibers 2018, 6, 79.
- Ahmad, N.; Tayyeb, D.; Ali, I.; KAlruwaili, N.; Ahmad, W.; Khan, A.H.; Iqbal, M.S. Development and Characterization of Hemicellulose-Based Films for Antibacterial Wound-Dressing Application. Polymers 2020, 12, 548.
- Bouaziz, F.; Koubaa, M.; Ellouz Ghorbel, R.; Ellouz Chaabouni, S. Biological properties of water-soluble polysaccharides and hemicelluloses from almond gum. Int. J. Biol. Macromol. 2017, 95, 667–674.
- Dominguez-Robles, J.; Larraneta, E.; Fong, M.L.; Martin, N.K.; Irwin, N.J.; Mutje, P.; Tarres, Q.; Delgado-Aguilar, M. Lignin/poly(butylene succinate) composites with antioxidant and antibacterial properties for potential biomedical applications. Int. J. Biol. Macromol. 2020, 145, 92–99.
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; İlk, S.; Kaya, M.; Labidi, J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 2018, 8, 24525–24533.
- Shen, X.; Berton, P.; Shamshina, J.L.; Rogers, R.D. Preparation and comparison of bulk and membrane hydrogels based on Kraft- and ionic-liquid-isolated lignins. Green Chem. 2016, 18, 5607–5620.
- Aadil, K.R.; Prajapati, D.; Jha, H. Improvement of physcio-chemical and functional properties of alginate film by Acacia lignin. Food Packag. Shelf Life 2016, 10, 25–33.
- García, A.; Spigno, G.; Labidi, J. Antioxidant and biocide behaviour of lignin fractions from apple tree pruning residues. Ind. Crops Prod. 2017, 104, 242–252.
- Sunthornvarabhas, J.; Rungthaworn, P.; Sukatta, U.; Juntratip, N.; Sriroth, K. Antimicrobial Tendency of Bagasse Lignin Extracts by Raman Peak Intensity. Sugar Tech 2020, 22, 697–705.
- Kaur, R.; Uppal, S.K.; Sharma, P. Antioxidant and Antibacterial Activities of Sugarcane Bagasse Lignin and Chemically Modified Lignins. Sugar Tech 2017, 19, 675–680.
- Sunthornvarabhas, J.; Liengprayoon, S.; Suwonsichon, T. Antimicrobial kinetic activities of lignin from sugarcane bagasse for textile product. Ind. Crops Prod. 2017, 109, 857–861.
- Dong, X.; Dong, M.; Lu, Y.; Turley, A.; Jin, T.; Wu, C. Antimicrobial and antioxidant activities of lignin from residue of corn stover to ethanol production. Ind. Crops Prod. 2011, 34, 1629–1634.
- Wang, G.; Xia, Y.; Liang, B.; Sui, W.; Si, C. Successive ethanol-water fractionation of enzymatic hydrolysis lignin to concentrate its antimicrobial activity. J. Chem. Technol. Biotechnol. 2018, 93, 2977–2987.
- Juikar, S.J.; Nadanathangam, V. Microbial Production of Nanolignin from Cotton Stalks and Its Application onto Cotton and Linen Fabrics for Multifunctional Properties. Waste Biomass Valorization 2019, 11, 6073–6083.
- Wang, G.; Pang, T.; Xia, Y.; Liu, X.; Li, S.; Parvez, A.M.; Kong, F.; Si, C. Subdivision of bamboo kraft lignin by one-step ethanol fractionation to enhance its water-solubility and antibacterial performance. Int. J. Biol. Macromol. 2019, 133, 156–164.
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications. Polymers 2010, 2, 728–765.
- Singhvi, M.S.; Chaudhari, S.; Gokhale, D.V. Lignocellulose processing: A current challenge. RSC Adv. 2014, 4, 8271–8277.
- Fernandes, E.M.; Pires, R.A.; Mano, J.F.; Reis, R.L. Bionanocomposites from lignocellulosic resources: Properties, applications and future trends for their use in the biomedical field. Prog. Polym. Sci. 2013, 38, 1415–1441.
- Thakur, V.K.; Thakur, M.K. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr. Polym. 2014, 109, 102–117.
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262.
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33.
- Gupta, V.K.; Carrott, P.J.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour. Technol. 2016, 216, 1066–1076.
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic liquid processing of cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537.
- Wang, S.; Lu, A.; Zhang, L. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206.
- Oliva, C.; Huang, W.; El Badri, S.; Lee, M.A.L.; Ronholm, J.; Chen, L.; Wang, Y. Concentrated sulfuric acid aqueous solution enables rapid recycling of cellulose from waste paper into antimicrobial packaging. Carbohydr. Polym. 2020, 241.
- Onofrei, M.D.; Dobos, A.M.; Dunca, S.; Ioanid, E.G.; Ioan, S. Biocidal activity of cellulose materials for medical implants. J. Appl. Polym. Sci. 2015, 132.
- Li, S.M.; Dong, Y.Y.; Ma, M.G.; Fu, L.H.; Sun, R.C.; Xu, F. Hydrothermal synthesis, characterization, and bactericidal activities of hybrid from cellulose and TiO(2). Carbohydr. Polym. 2013, 96, 15–20.
- Ilangovan, M.; Guna, V.; Hu, C.; Nagananda, G.S.; Reddy, N. Curcuma longa L. plant residue as a source for natural cellulose fibers with antimicrobial activity. Ind. Crops Prod. 2018, 112, 556–560.
- Gogoi, B.; Barua, S.; Sarmah, J.K.; Karak, N. In situ synthesis of a microbial fouling resistant, nanofibrillar cellulose-hyperbranched epoxy composite for advanced coating applications. Prog. Organ. Coat. 2018, 124, 224–231.
- Lefatshe, K.; Muiva, C.M.; Kebaabetswe, L.P. Extraction of nanocellulose and in-situ casting of ZnO/cellulose nanocomposite with enhanced photocatalytic and antibacterial activity. Carbohydr. Polym. 2017, 164, 301–308.
- Li, S.-M.; Fu, L.-H.; Ma, M.-G.; Zhu, J.-F.; Sun, R.-C.; Xu, F. Simultaneous microwave-assisted synthesis, characterization, thermal stability, and antimicrobial activity of cellulose/AgCl nanocomposites. Biomass Bioenergy 2012, 47, 516–521.
- Fan, L.; Zhang, H.; Gao, M.; Zhang, M.; Liu, P.; Liu, X. Cellulose nanocrystals/silver nanoparticles: In-situ preparation and application in PVA films. Holzforschung 2020, 74, 523–528.
- Liu, H.; Song, J.; Shang, S.; Song, Z.; Wang, D. Cellulose nanocrystal/silver nanoparticle composites as bifunctional nanofillers within waterborne polyurethane. ACS Appl. Mater. Interfaces 2012, 4, 2413–2419.
- Girio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Lukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800.
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289.
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86.
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A Critical Review on Hemicellulose Pyrolysis. Energy Technol. 2017, 5, 52–79.
- Sella Kapu, N.; Trajano, H.L. Review of hemicellulose hydrolysis in softwoods and bamboo. Biofuels Bioprod. Biorefining 2014, 8, 857–870.
- Fu, G.-Q.; Zhang, S.-C.; Chen, G.-G.; Hao, X.; Bian, J.; Peng, F. Xylan-based hydrogels for potential skin care application. Int. J. Biol. Macromol. 2020, 158, 244–250.
- Arellano-Sandoval, L.; Delgado, E.; Camacho-Villegas, T.A.; Bravo-Madrigal, J.; Manríquez-González, R.; Lugo-Fabres, P.H.; Toriz, G.; García-Uriostegui, L. Development of thermosensitive hybrid hydrogels based on xylan-type hemicellulose from agave bagasse: Characterization and antibacterial activity. MRS Commun. 2020, 10, 147–154.
- Adler, E. Lignin Chemistry-Past, Present and Future. Wood Sci. Technol. 1977, 11, 169–218.
- Erfani Jazi, M.; Narayanan, G.; Aghabozorgi, F.; Farajidizaji, B.; Aghaei, A.; Kamyabi, M.A.; Navarathna, C.M.; Mlsna, T.E. Structure, chemistry and physicochemistry of lignin for material functionalization. SN Appl. Sci. 2019, 1, 1–19.
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290.
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908.
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From lignin to valuable products-strategies, challenges, and prospects. Bioresour. Technol. 2019, 271, 449–461.
- Tobimatsu, Y.; Schuetz, M. Lignin polymerization: How do plants manage the chemistry so well? Curr. Opin. Biotechnol. 2019, 56, 75–81.
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092.
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335.
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249.
- Windeisen, E.; Wegener, G. Lignin as Building Unit for Polymers. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 255–265.
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Rajesh Banu, J.; Rao, C.V.; Kim, Y.G.; Yang, Y.H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300.
- Collins, M.N.; Nechifor, M.; Tanasa, F.; Zanoaga, M.; McLoughlin, A.; Strozyk, M.A.; Culebras, M.; Teaca, C.A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849.
- Aadil, K.R.; Pandey, N.; Mussatto, S.I.; Jha, H. Green synthesis of silver nanoparticles using acacia lignin, their cytotoxicity, catalytic, metal ion sensing capability and antibacterial activity. J. Environ. Chem. Eng. 2019, 7, 103296.
- Chandna, S.; Thakur, N.S.; Reddy, Y.N.; Kaur, R.; Bhaumik, J. Engineering Lignin Stabilized Bimetallic Nanocomplexes: Structure, Mechanistic Elucidation, Antioxidant, and Antimicrobial Potential. ACS Biomater. Sci. Eng. 2019, 5, 3212–3227.
- Lee, E.; Song, Y.; Lee, S. Crosslinking of lignin/poly(vinyl alcohol) nanocomposite fiber webs and their antimicrobial and ultraviolet-protective properties. Text. Res. J. 2017, 89, 3–12.
- Mehta, M.J.; Kumar, A. Ionic Liquid Stabilized Gelatin-Lignin Films: A Potential UV-Shielding Material with Excellent Mechanical and Antimicrobial Properties. Chemistry 2019, 25, 1269–1274.




