Pathogenic microbes are a major source of health and environmental problems, mostly due to their easy proliferation on most surfaces. Currently, new classes of antimicrobial agents are under development to prevent microbial adhesion and biofilm formation. However, they are mostly from synthetic origin and present several disadvantages. The use of natural biopolymers such as cellulose, hemicellulose, and lignin, derived from lignocellulosic materials as antimicrobial agents has a promising potential. Lignocellulosic materials are one of the most abundant natural materials from renewable sources, and they present attractive characteristics, such as low density and biodegradability, are low-cost, high availability, and environmentally friendly.
- lignocellulosic materials
- natural fibers
- bacteria
- fungi
- biofilm
- cellulose
- hemicellulose
- lignin
- biopolymers
1. Introduction
The presence of pathogenic microorganisms on the material surfaces can lead to significant healthcare and environmental problems. In recent times, different strategies have been defined to prevent the proliferation and adhesion of microorganisms on medical devices; or materials for food storage, and packaging [1][2]. Moreover, the biofilm formation on the materials surfaces can limit their functionality, leading to critical health related complications [3]. Furthermore, antibiotic-resistant microorganisms have emerged due to the extensive use of antibiotics or biocidal to impair their growth. Thus, it is necessary the development of new drug free materials that could avoid the increase of antibiotic-resistant microorganisms.
Figure 1 presents a scheme of the stages of development of biofilm. In the attachment stage, the microorganisms are reversibly absorbed to the biotic or abiotic surface by weak van der Waals forces bonds. In contrast, in the colonization stage, stronger hydrophilic/hydrophobic bonds are established with the surfaces allowing them to proliferated and secret EPS [4][5]. In the maturation stage, a three-dimensional structure contains channels that distribute nutrients and signal molecules in the biofilm. In the last stage, called active dispersion, the cells are detached, either singly or in clumps, and colonize other locations [5]. The formation and development of biofilm depend on many factors, such as the specific bacteria strain, the properties of the material’s surface, the environmental condition (pH, temperature, and nutrients), among others [6]. Biofilms are responsible for biocorrosion, biofouling (accumulating microorganisms in surfaces), and reservoir souring, causing many constraints in different industries [4][7].
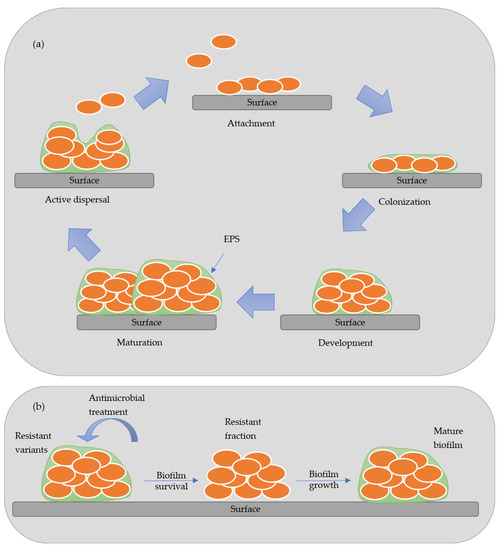
The antimicrobial agents are classified into two categories, organic and inorganic. The organic antimicrobial agents include natural biopolymers, for instance, the chitosan, cellulose and lignin, phenols, halogenated compounds, and quaternary ammonium salts [10][11][12]. The inorganic antimicrobial agents comprise, for example, metals, or metals bonded with phosphates, and metal oxides. The most common metallic nanoparticles or metal oxides used are silver, copper, titanium oxide, zinc oxide, magnesium, and calcium oxide [10][11][12]. In the literature, several studies explore the use of different antimicrobial agents by incorporation or applied as coatings on materials surfaces. Among them, the antimicrobial potential of natural derived lignocellulosic compounds remains still unexplored.
2. Lignocellulosic Materials and Main Compounds
Lignocellulosic materials are mainly composed of three biopolymers—cellulose, hemicellulose, and lignin—combined with smaller other components. The ratios between these compounds vary depending on the lignocellulosic material origin [13][14][15][16].
Table 1.
| Compound | Origin | Antimicrobial Activity Tested Against | Application | Ref | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellulose | Wood | E. coli | , | S. aureus | Packaging | [17] | [27] | |||||||||||||
| E. coli | , | S. aureus | [18] | [35] | ||||||||||||||||
| E. coli | , | P. aeruginosa | , | B. subtilis | Tissue engineering, wound dressing | [19] | [31] | |||||||||||||
| Sugarcane Bagasse | S.aureus | , | T. rubrum | Skin infective | [20] | [36] | ||||||||||||||
| Wastewater purification | [21] | [37] | ||||||||||||||||||
| Tulsi | E. coli | , | S. aureus | , | B. cereus | , | Ser. marcescens | Biomedical | [22] | [28] | ||||||||||
| Ginger | E. coli | , | S. aureus | , | B.cereus | , | Sal. thyphimirium | Packaging, wound dressing, surgical material | [23] | [33] | ||||||||||
| Hemicellulose | Plantago Ovata seed husk | E. coli | , | S. aureus | , | P. aeruginosa | Wound dressing, drug delivery | [24] | [38] | |||||||||||
| Almond gum | Actinomycetes sp | , | Sal. thyphimirium | , | K. pneumonia | , | L. monocytogenes | , | S. aureus | , | Sal. enterica | , | P. aeruginosa | , | B. thuringiensis | , | B. subtilis | Food and non-food | [25] | [39] |
| Lignin | Softwood | S. aureus | Biomedical | [26] | [40] | |||||||||||||||
| Eucalyptus | A. niger | E. coli | , | S. aureus | , | Pr. microbilis | , | Pr. vulgaris | , | P. aeruginosa | , | Entero. aerogenes | , | B. thuringiensis | , | Sal. enterica serotype typhmurium and Strept. mutans | Antimicrobial additive or agent in food, textile, or chemical industry | [27] | [41] | |
| Spruce | [27] | [41] | ||||||||||||||||||
| Poplar | E. coli | Drug delivery, food packaging, wound dressing, | [28] | [42] | ||||||||||||||||
| Acacia | E. coli | , | S. aureus | Active packaging | [29] | [43] | ||||||||||||||
| Apple tree pruning residues | A. niger | , | Sacch. cerevisiae | Food antioxidant | [30] | [44] | ||||||||||||||
| Sugarcane Bagasse | E. coli | , | S. aureus | , | P. aeruginosa | , | S. epidermidis | [31] | [45] | |||||||||||
| B. aryabhattai | , | Klebsiella | sp. | Natural antibacterial agent | [32] | [46] | ||||||||||||||
| S. epidermidis | Antimicrobial textile | [33] | [47] | |||||||||||||||||
| Corn | L. monocytogenes | , | S. aureus | , | E. coli | , | Sal. enteritidis | , | C. lipolytica | Antioxidant and antimicrobial | [34] | [48] | ||||||||
| E. coli | , | S. aureus | , | B. subtilis | , | Sal. enterica | Natural antibacterial agent | [35] | [49] | |||||||||||
| Cotton stalks | S. aureus | , | K. pneumoniae | Medical and technical textiles | [36] | [50] | ||||||||||||||
| Bamboo | E. coli | , | S. aureus | , | B. subtilis | , | Sal. enterica | Natural antibacterial agent | [37] | [51] |
2.1. Cellulose
Cellulose is the most abundant renewable polymer found in nature, and it is the main constituent of the cell wall. It can be biosynthesized by different organisms, such as plants, amoebae, sea animals, bacteria, and fungi [38].
6
10
5
n, and it is composed of ß-D-glucopyranose (glucose) moieties linked by β-(1,4) glycosidic bonds [13][14][39][40]. The chemical structure of cellulose is presented in
Figure 2. This compound possesses both well-ordered (crystalline) and disordered (amorphous) regions [13][14][39][41]. The presence of polar oxygen and hydrogen atoms in cellulose allows the formation of intermolecular and intra-molecular bonding [42].
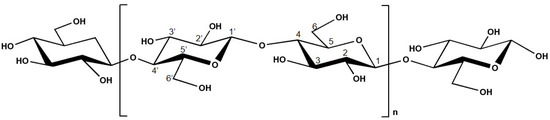
The structure of this material is organized as microfibrils, with a diameter between 2 and 20 nm, connect together to form cellulose fibers [38][43].
Cellulose is commonly used as a raw material in different industries, such as textile, plastic, wood, cosmetics, and pharmaceutical. Cellulose presents high biocompatibility, biodegradability, non-toxicity, and high hydrophilicity. It also reveals good mechanical properties, thermal and chemical stability, chirality and allows chemical modification [44][45][46]. Cellulose can be applied in a wide range of applications, such as packaging [47][17], biomedical [22][48][49], tissue engineering [19], wound dressing [19][50][23], marine coatings [51], among others. For instance, Onofrei et al. [48], developed films composed of cellulose acetate blended with hydroxypropylcellulose. The film with a higher concentration of cellulose acetate inhibited the growth of
Escherichia coli
E. coli
Staphylococcus aureus
S. aureus
In work by Sun et al. [17], cellulose-based membranes were produced with cellulose fibers modified by azidation, followed by epoxidation and grafted with poly(hexamethylene guanidine) (PHMB). The antibacterial activity of the membranes against
E. coli
S. aureus
Gogoi et al. [51], also modified nanofibrillar cellulose from
Colocasia esculenta
S. aureus
Candida albicans
C. albicans). Guna et al. [22], prepared cellulose fibers from the tulsi stalk and tested the antimicrobial activity against
S. aureus
E. coli
Ser. marcescens
B. cereus. The work shows a bacteria reduction between 55% and 62% for the nanofibrillar cellulose, and a higher reduction of 90% to 98% for the tulsi stalk fiber. The same trend was also observed by Ilangovan et al. [50], where fibers made from cellulose extracted from
Curcuma Longa L. residues. Gabov et al. [18], showed that beads prepared from the combination of cellulose and lignin obtained from birch wood chips presented antimicrobial activity against
S. aureus. However, the beads had a high concentration of lignin in the matrix, which could have influenced the antibacterial activity. Yadav et al. [19], prepared bio-sponges from a composite of sodium alginate and cellulose extracted from mango wood scrap combined with bio-extracts from rice water and Giloy extract. The bio-sponges demonstrated good antibacterial activity against Gram-positive,
B. subtilis
E. coli
P. aeruginosa. Oliva et al. [47], isolated the cellulose from paper with concentrated sulfuric acid to produce films that were then treated with zinc oxide and carvacrol essential oil. The films made with cellulose extract showed good antibacterial activity against
E. coli
S. aureus
S. aureus
T. rubrum (fungi). It was verified that the zinc nanoparticles enhanced the already existing antimicrobial properties of the cellulose. Furthermore, Anagha et al. [21], suggested that the good antimicrobial properties allied with their excellent biocompatibility and low cytotoxic, making these hydrogels good for biomedical applications. Likewise, films made with nanocellulose obtained from oil palm or empty fruit bunches via alkaline treatment and acid hydrolysis combined with zinc oxide showed good antibacterial activity against
E. coli
S. aureus
The antimicrobial activity of cellulose can be enhanced by the inclusion of inorganic nanoparticles. Li et al. [49], prepared composites by combining titanium dioxide and cellulose that presented higher inhibitory activity against
E. coli
S. aureus
E
coli
aureus
2.2. Hemicellulose
Hemicellulose is a short-chain heteropolysaccharide present in the cell wall, and it corresponds to 15% to 35% of the plant composition, depending on the plant species [56][57][58]. The heteropolymer presents an amorphous branched structure and a lower polymerization degree than cellulose, approximately 200 [58]. Hemicellulose is constituted by different monosaccharide units, hexoses (β-D-glucose, β-D-mannose, and β-D-galactose) and pentoses (β-D-xylose and α-L-arabinose) in higher quantities, and other sugars (fructose and rhamnose) in lower quantities. It also presents uronic acids, like 4-O-methyl-D-glucuronic acid, D-glucuronic acid, D-galacturonic acid, and acetyl groups [40][56][58][59][60]. In
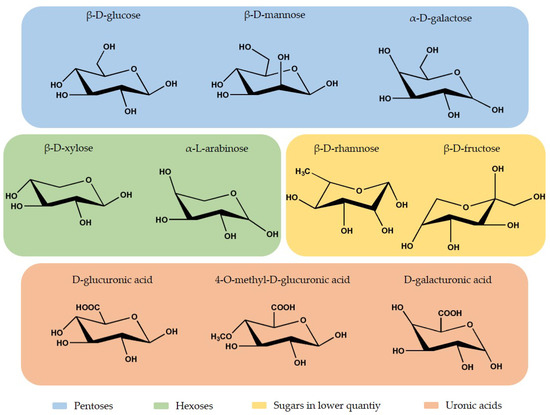
Thus far, the antimicrobial properties of hemicellulose are less explored when compared to cellulose and lignin. Nevertheless, these polymers offer great antimicrobial potential due to their components. Ahmad et al. [24], the hemicellulose films inhibited the growth of the bacteria
S. aureus
E. coli
P. aeruginosa. Bouaziz et al. [25], the authors assessed the antibacterial activity of hemicellulose extracted from almond gum against different strains of Gram-positive (
B. subtilis
B. thuringiensis
Actinomyces
L. monocytogenes
S. aureus
P. aeruginosa
Sal. enterica
Salmonella typhimurium
K. pneumoniae
B. thuringiensis
S. enterica
P. aeruginosa
Actinomyces
Sal
thyphimirium
K. pneumonia
L. monocytogenes
B. subtilis
B. subtilis
S. aureus.
E. coli
S. aureus
P. aeruginosa
2.3. Lignin
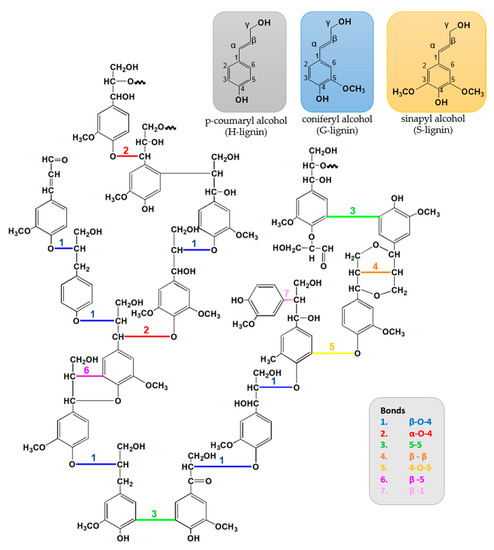
Since 1977, when Adler [63] described lignin as a highly branched polymer with different functional groups: aliphatic and phenolic hydroxyls, carboxylic, carbonyl, and methoxyl groups, the structure of lignin has significantly been studied and explored. Lignin is one of the most abundant polymers in nature, and it is an amorphous heterogeneous polymer network of phenylpropane units linked together by different, non-regular sequence bonds [13][14][64].
The phenylpropane units are originated from three aromatic alcohol precursors, monolignols, p-coumaryl (H-lignin), coniferyl (G-lignin), and sinapyl (S-lignin) alcohols. During the polymerization process, the monolignols units are linked by radical coupling reactions to form the three-dimensional molecular architecture with different bonds, and typically around half of these bonds are β-4-O ether connections [65][66][67][68][69].
This compound is responsible for binding the lignocellulosic materials’ components, acting as a glue, making it insoluble in water. Lignin presents an essential role in woody plants; it is the major component of the vascular plant’s cell wall and confers rigidity, impermeability, resistance to microbial attack, and oxidative stress. Lignin is mainly used to generate heat and energy but is also used as a food and concrete additive, dispersants, resin, and binding material [13][14][39][64][70].
To understand the effect of the origin and the extraction method and how the lignin origin influence in antimicrobial properties, Gordobil et al. [27], used lignin obtained from eucalyptus and spruce by two different methods, kraft, and organosolv. The author used solutions with different lignin concentrations to evaluate antifungal activity against
A. Niger
Table 1). It was concluded that the kraft lignin’s present higher antifungal activity than the organosolv lignins. In the kraft lignin from eucalyptus, presents a high antifungal activity for all the concentrations, however spruce lignin only acts as antifungal in lower concentrations. In this work, it is proposed that the higher antifungal activity observed in the kraft lignins could be explained by the lower carbohydrate content and by the presence of sulfur-containing derivatives. For the antibacterial tests, the authors verified that the kraft lignins present higher antibacterial activity, in some cases, higher than the commonly used antibiotic, due to their rich antioxidant and polyphenolic nature. This lignin’s revealed potential to be applied as antimicrobial additive or agent against pathogenic microorganisms in food, textile and chemical industries. As shown in a previous study, antifungal activity is not only influenced by the extraction method but also by the origin. The results obtained by García et al. [30], show that the organosolv lignin extracted from apple tree pruning residues could not show the antifungal activity against
A. niger
Saccharomyces cerevisiae
Another promising method to extract lignin from natural fiber is the use of ionic liquids. Ionic liquids are green solvents with high thermal stability and present low toxicity [72]. In the work by Shen et al. [28], lignin extracted from poplar wood was combined with epichlorohydrin (ECH) and polyethylene glycol (PEG) to produce membranes. The lignin powder’s antibacterial activity extracted by kraft method and ionic liquid (1-ethyl-3-methylimidazolium acetate) method and the membranes were tested against
E. coli
Some authors use the fractionation method to extract lignin. Fractionation is a physical-chemical modification technique that allows the separation of high molecular weight lignin chains from lower molecular weight fractions [74]. In the work by Wang et al. [35] used enzymatic hydrolyzed lignin from corn stalk and performed two sequential ethanol extraction to obtain a different lignin fraction. The authors tested the antimicrobial activity of the lignin extracts against the Gram-positive bacteria,
E. coli and B. subtilis
Sal. enterica
S. aureus. The authors observed that the first extract showed higher antibacterial activity against all the bacteria, but the Gram-positive are more sensitive to the lignin extract than the Gram-negative bacteria. Likewise, they also extracted lignin from bamboo by the kraft method, and fractionated with ethanol to obtain a soluble and an insoluble fraction [37]. The kraft lignin and the lignin fractions antimicrobial activity was tested against the same bacteria used in the previous study. The insoluble phase showed low inhibition against Gram-positive bacteria. It promoted the growth of the Gram-negative bacteria probably by the low phenolic compounds content and poor water solubility, leading to the formation of insoluble particles that act as carriers for the bacteria. The soluble phase showed good growth inhibition of the growth of both types of bacteria [37]. Kaur et al. [32], modified lignin from sugarcane bagasse by three different methods, acetylation, epoxidation, and hydroxymethylation and, evaluated the antibacterial activity against the bacteria
B. aryabhattai
Klebsiella
B. aryabhattai
Klebsiella
Lignin composites have also demonstrated that the addition of lignin promotes antimicrobial activity. In the literature, it is shown the combination of different polymers such as with poly(butylene succinate) (PBS) [26], alginate [29], and poly(vinyl alcohol) (PVA) [77]. The composites obtained by Domínguez-Robles et al. [26], by the extrusion followed by injection molding of kraft lignin extracted from softwood with PBS, showed higher antimicrobial activity against
S. aureus than the PBS matrix, even in a small amount (2.5 wt.%). Aadil et al. [29], also showed the antibacterial effect of lignin against
S. aureus
S. aureus
E. coli
S. aureus
E. coli
In this context, the materials produced with lignin can be considered for different areas of application, such as biomedical [26][36][78], textile [33][36], packaging [28][29] and as natural antimicrobial agent [32][35][37].
3. Conclusion
Lignocellulosic materials are widely used in several production sectors such as construction, furniture, packaging, health or the automotive industry. Several studies have highlighted the potential of these natural fibers or its chemical constituents on different polymer-matrix systems. In addition, their antimicrobial effects have been recognized. In the last years, the use of lignocellulosic materials has grown mainly to their characteristics, high availability, environmentally friendly, from renewable sources, low cost, and biodegradability. This review presents an overview of the most recent advances that demonstrates the potential of the lignocellulosic based materials, cellulose, hemicellulose, lignin, and lignocellulosic fibers to be used as antimicrobial agents. In this area, the antimicrobial activity of the materials has emerged from the combination of the lignocellulosic source with antimicrobial agents from inorganic or organic origin. The potential of lignocellulosic as new drug-free polymers is extensive but this area is still virtually unexplored, especially as antimicrobial or anti-biofouling materials for industries, such as packaging, healthcare, environmental, textile, space engineering, among others. By unlocking the full potential of the antimicrobial properties of lignocellulosic materials, it will be possible to fully disclose their potential, bringing new links of knowledge between the areas involving the synthesis of natural fibers, the polymer matrices and microbiology.
