
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rodrigo Valenzuela | + 2968 word(s) | 2968 | 2021-03-22 07:04:33 | | | |
| 2 | Bruce Ren | Meta information modification | 2968 | 2021-03-31 03:24:33 | | | | |
| 3 | Bruce Ren | Meta information modification | 2968 | 2021-03-31 03:24:53 | | |
Video Upload Options
The role of docosahexaenoic acid (DHA) and arachidonic acid (AA) in neurogenesis and brain development throughout the life cycle is fundamental. DHA and AA are long-chain polyunsaturated fatty acids (LCPUFA) vital for many human physiological processes, such as signaling pathways, gene expression, structure and function of membranes, among others. DHA and AA are deposited into the lipids of cell membranes that form the gray matter representing approximately 25% of the total content of brain fatty acids. Both fatty acids have effects on neuronal growth and differentiation through the modulation of the physical properties of neuronal membranes, signal transduction associated with G proteins, and gene expression. DHA and AA have a relevant role in neuroprotection against neurodegenerative pathologies such as Alzheimer’s disease and Parkinson’s disease, which are associated with characteristic pathological expressions as mitochondrial dysfunction, neuroinflammation, and oxidative stress.
1. Introduction
Over many years there has been growing interest about n-3 and n-6 long-chain polyunsaturated fatty acids (n-3 and n-6 LCPUFA), mainly related to their role in neural development and the prevention of neurodegenerative diseases. Lipids correspond to 60% of the dry weight of the mammalian brain, mostly in the form of phospholipids [1][2]. N-3 and n-6 LCPUFA are required for proper brain growth and development, specifically docosahexaenoic acid (C22:6n-3, DHA) and arachidonic acid (C20:4n-6, AA) [3]. DHA and AA represent approximately 25% of the total content of brain fatty acids [2][4] and are constituents of the lipids of cell membranes that form the gray matter [1][5]. DHA represent up to 90% of the total n-3 LCPUFA in the brain [6]. This LCPUFA can be obtained from its dietary precursor alpha-linolenic acid (C18:3n-3, ALA), which, after a complex process of enzymatic desaturation and elongation, is converted to DHA, or can be obtained from preformed DHA of marine dietary sources [7]. DHA is mainly found in the phospholipids of synaptic terminal membranes, and the vast majority of DHA is incorporated into the structure of phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine [7][8][9]. The structural properties of DHA, such as its chain length and high unsaturation degree, give flexibility and fluidity to the neuronal plasma membrane, thus facilitating the signal transduction process into these cells [10][11]. These structural properties have a pivotal role in neuronal growth, migration, synaptogenesis, and synaptic plasticity [11][12][13]. In advanced stages of life, a decrease in plasma DHA levels is positively correlated with normal brain aging in healthy elderly individuals and also in patients diagnosed with neurogenerative diseases [14][15][16] such as Alzheimer’s disease (AD) [17][18]. It has also been observed that populations having a higher average dietary intake of DHA show a lower risk of developing cognitive impairment or AD [19][20]. Furthermore, a potential neuroprotective role of DHA in Parkinson’s disease (PD) through the increase in dopaminergic neurotransmission and the prevention of neuronal death is also actually recognized [21][22][23]. In this regard, the higher intake of n-3 LCPUFA (mostly DHA) trough supplementation may be effective to improve the nutritional status of the fatty acid and may play a role in maintaining brain health and in the prevention of brain aging symptoms [6][24][25].
AA is one of the most abundant n-6 LCPUFA in the brain and has a critical role in brain growth, being essential during the first months of life [26]. Ideally, during brain development, AA should be provided preformed in the diet because the low activity of the enzymes (Δ-5 and Δ-6 desaturases) involved in its formation from its dietary precursor linoleic acid (C18:2n-6, LA) results in a low conversion of LA to AA [27][28]. This fatty acid participates in many signaling pathways involved in cell division [29]. It is also a direct precursor of adrenic acid (C22:4n-6, AdA), a fatty acid necessary for neuronal development and enrichment of myelinic lipids [30][31]. A potential protective role of AA in neuronal aging through its participation in preserving the fluidity of the hippocampal cell membranes has been described [32][33][34][35]. However, opposite to this effect, a controversial role has been associated with AA in neuroinflammation and the genesis of cellular damage due to the pro-inflammatory action of some of AA metabolic derivatives [36][37][38]. According with this background, the present review analyzes the neurological role of DHA and AA in the extreme stages of life, emphasizing the importance of these LCPUFA during the first year of life and in the developing brain and in a context of age-related neurodegenerative disease prevention. Also, we discuss the eventual neuroprotective role of n-3 and n-6 LCPUFA in AD and PD.
2. DHA and AA: Biosynthesis, Metabolism, and Dietary Sources
ALA and LA are considered essential fatty acids because humans cannot synthesize them, so they must be supplied by the diet [39][40]. ALA is metabolized to eicosapentaenoic acid (C20:5 n-3, EPA) and subsequently to DHA, while LA is the precursor of AA [40]. ALA and LA are competitors in their respective metabolic pathways because both fatty acids are substrates for the same desaturase and elongase enzymes. In the synthesis of n-3 and n-6 LCPUFA in addition to the substrates ALA and LA, the participation of a complex enzymatic process which allows the desaturation and elongation of the 18 carbon atoms precursors (ALA and LA) is necessary [41]. This process occurs in microsomes and involves Δ-6 fatty acid desaturase 2 (FADS2), Δ-5 fatty acid desaturase 1 (FADS1), elongase 2 (ELOVL2) and elongase 5 (ELOVL5) enzymes [41]. The latter enzyme having a higher affinity for n-3 PUFA than for n-6 PUFA, favoring the transformation of ALA into DHA [42]. This less affinity of LA than ALA to desaturase and elongase enzymes has contributed to the recommendation of a 5:1 molar ratio for the dietary intake of n-6:n-3 PUFA, because when the dietary contribution of both fatty acids is similar, the formation of DHA is privileged compared to AA [43][44]. An interesting aspect about LCPUFA synthesis is the existence of polymorphisms in desaturase enzymes, which can influence both the ability to the synthesis of DHA or AA and the blood levels of these fatty acids [45]. For example, rs3834458 single nucleotide polymorphism in FADS2 may result in lower Δ-6 desaturase activity leading to higher ALA and lower DHA blood concentrations [46]. The presence of these polymorphisms can also influence lower levels of LCPUFA in breast milk, especially DHA, which could have impact on brain development [47][48][49]. The existence of polymorphisms has been associated with increased risk of developing insulin resistance, type 2 diabetes, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD), among other pathologies [50][51].
ALA is found in higher amounts in vegetable oils such as chia and flaxseed oil and in lower amounts in canola and soybean oils [52][53] The principal dietary sources of preformed DHA are fatty fish or blue fish, such as salmon, tuna, anchovy, sardine, and horse mackerel (Figure 1) [54][55][56]. The main dietary sources of LA are sunflower, soybean, and corn oils [52]. In contrast, the main dietary sources of AA are foods of animal origin, such as as beef, pork, lamb, chicken, turkey, and eggs [44]. Dietary sources of n-6 and n-3 polyunsaturated fatty acids influence brain development.
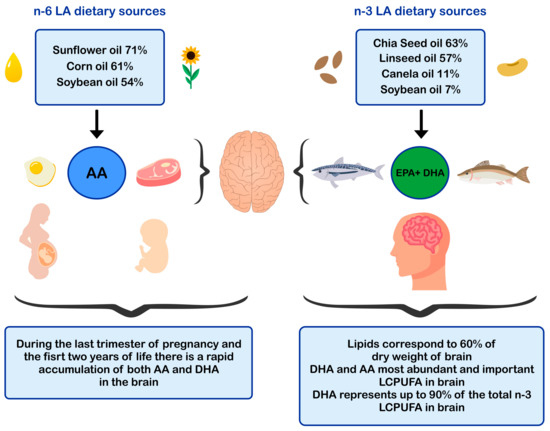
Figure 1. Dietary sources of n-6 and n-3 polyunsaturated fatty acids and impact in brain development. AA: arachidonic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; LCPUFA: long-chain polyunsaturated fatty acid; LA: linoleic acid.
3. DHA and AA in Neuronal Development and Function
DHA and AA can modulate neuronal function by influencing: (i) the physical properties of neuronal membranes by modulating ion channels and vesicular transport for endo/exocytosis of membrane-bound proteins [11][57]; (ii) signal transduction, by modulating G protein-mediated second messenger systems; and (iii) gene expression, through direct binding to transcription factors [58] or through the regulation of signaling cascades by eicosanoids derived from AA and DHA-derived docosanoids. In this sense, DHA and AA are crucial for the metabolism, growth, and differentiation of neurons [11][59] (Figure 2).
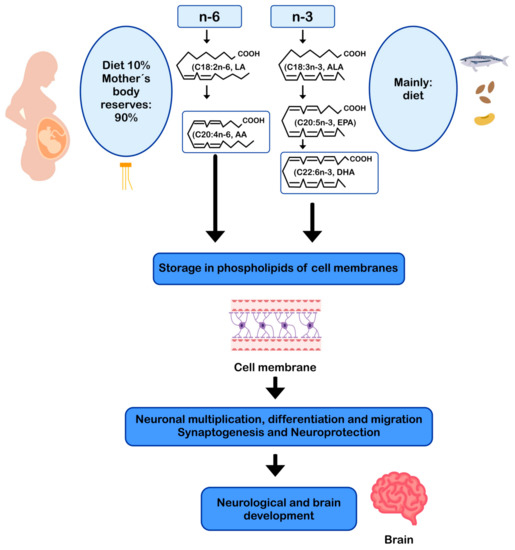
Figure 2. AA and DHA in neuronal development and function. AA: arachidonic acid; ALA: alpha-linolenic acid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; LA: linoleic acid.
3.1. DHA in Neuronal Metabolism
DHA represents more than 90% of the n-3 LCPUFA in the brain [31], mainly as part of the membrane phospholipids in the brain gray matter [6][60], constituting 35% of the total fatty acids in the synaptic membranes [61]. DHA is primarily esterified to phosphatidylethanolamine, phosphatidylserine, and to a lesser extent phosphatidylcholine in neuronal membranes. The structural properties of DHA, such as the length of its carbon chain and its six double bonds, give flexibility and fluidity to the neuronal plasma membrane, facilitating signal transduction into the cell [10][11]. The fluidity of the neuronal membrane facilitates the lateral movement of receptors, G proteins, ion channels [62], enzymes [63], and neuroreceptors, increasing the efficiency in signal transduction [64][65][66]. In the brain, DHA is involved in neuronal growth, neuronal migration, synaptogenesis, synaptic plasticity, and gene expression [11][12][13][67]. Neurons cannot form DHA from its precursor ALA, but the glial cells, (especially astrocytes) can desaturate and elongate dietary ALA to convert it into DHA, which is subsequently transferred to most neurons [68][69]. DHA can also modulate the expression of genes related to neuronal energy generation involved in the function of the respiratory chain and ATP synthesis (adenosine triphosphate synthase) [67][68][69]. That function is relevant because approximately 50% of mitochondrial ATP is consumed by the Na+/K+ ATPase pump to maintain cell homeostasis and ionic gradients, a fundamental requirement for neuronal electrical excitability [67][68][69].
3.2. AA in Neuronal Metabolism
As already mentioned, AA is one of the most abundant fatty acids in the brain [26]. This n-6 LCPUFA is indispensable for brain growth and modulation of cell division and signaling [29]. During brain development, the concentration of AA increases rapidly [70]. It has been described in animal models that approximately 70–80% of AA concentration that is reached in adulthood is the result of its cerebral accumulation in the early postnatal period [2]. AA is an immediate precursor of adrenic acid (C22:4n-6, AdA), fatty acid found in large amounts in myelinic lipids, especially in phosphatidylethanolamine and phosphatidylcholine [70][71], suggesting the fundamental role of AA as a precursor of AdA in the development of neural tissue [70]. Conversion of AA to AdA may represent an important mechanism for supplying the high demand for AdA at the brain level, which is essential for neuronal myelinic lipid enrichment [2][70]. Wijendran et al. (2002) [72] investigated the metabolism of preformed AA in newborn baboons by the administration of a single oral dose of C13-labeled AA, reporting that 79–93% of AA consumed accumulates in brain membrane lipids and approximately 5% to 16% of AA is transformed into AdA [72]. Brain accumulation of AA and AdA occurs during the first month of life and represent 17% and 8% of the total n-6 LCPUFA, respectively [72].
Another brain function of AA is directly related to its participation in phosphatidylcholine (PC) structure [71]. Some intracellular phospholipid bilayers include AA-containing PC (AA-PC), a structure that plays a role as second messenger participating in the long-term enhancement of synapses in the CA1 region of the hippocampus [73]. Using image mass spectrometry, Yang et al. (2012) [74] characterized the distribution of AA-PC within neurons in cultured upper cervical nodes, finding an increasing gradient of AA-PC along the proximal to distal axonal axis suggesting that this structure is an important source of free AA [74]. Furthermore, it has been described that free AA can activate protein kinases and ion channels and inhibit neurotransmitter recycling [29], thus contributing to better control of synaptic transmission [75]. In addition to the role of AA in modulating neuronal excitability, AA is also essential in neuronal development in part because it is directly responsible for the activation of syntaxin-3, a protein of the neuronal membrane involved in the growth and neurite repair, an essential process in neurogenesis and subsequently in synaptic transmission [76].
3.3. DHA and AA as Precursors of Endocannabinoids
Remarkably, LCPUFA levels in the brain are highly correlated with dietary intake of PUFA and LCPUFA [77]. DHA and AA are precursors of many bioactive lipid mediators identified as docosanoids and eicosanoids, respectively, which are actively involved in regulatory responses in inflammation (eicosanoids such as prostaglandins, prostacyclins, thromboxanes, leucotrienes) and in resolution of inflammation (docosanoids such as resolvins and maresins) [78]. Both LCPUFA are also involved in the formation of endocannabinoids (eCB) with regulatory effects at the central nervous system (CNS) [79]. The endocannabinoid system consists of eCB, eCB receptors CB1 and CB2, and associated anabolic and catabolic enzymes [80]. Their functions are to maintain body energy homeostasis through the nutrient availability detection and the modulation of orexinergic inputs in selective regions of the CNS [81]. PUFA derived eicosanoids and eCB have been identified as independent ligands of CB1 and CB2 receptors [80]. Hammels et al. (2019) [80] reported that CB1 ligands synthesis in CNS depends on dietary intake of AA and DHA in a model of fatty acid desaturase 2-deficient mouse [80]. Moreover, DHA and AA have been recognized as ligands of the nuclear RxR receptor in the brain, being the PUFA ratio in the western diet, a critical nutritional parameter for numerous neurodegenerative diseases [80], which could increase neuroinflammation and over-stimulation of the endocannabinoid system [82]. AA bound to phospholipids determines the formation of eCB, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), molecules involved in the regulation of neuroinflammatory responses by microglia and astrocytes [79]. On the contrary, long-term supplementation with DHA and EPA reduces AEA and 2-AG synthesis [83]. N-3 and n-6 PUFA derived eCB are synthesized by lipoxygenases (LOX), cyclooxygenase 2 (COX-2), and cytochrome P450 epoxygenases (CYP450). Nevertheless, only LOX and CYP450 metabolites have been reported for a DHA eCB derivative; n-docosahexaenoylethanolamide (DHEA), and only CYP450 metabolites for an EPA eCB derivative; eicosapentaenoyl ethanolamide (EPEA) [81]. The physiological role of the metabolites generated by LOX and CYP450 remains to be elucidated to understand how these derivatives modulate cell signaling in health and disease [81]. A better understanding of the relationship between DHA, AA, and the endocannabinoid system is expected to lead to advances in the development of their therapeutic potential and the development of more specific treatment options for the prevention and treatment of neurodegenerative diseases [79].
4. DHA and AA in Neuroprotection
The most frequent neurodegenerative age-related diseases are Alzheimer’s Disease (AD) and Parkinson’s Disease (PD) [84]. Although the etiopathogenesis and clinical characteristics of AD and PD are different, these diseases share common mechanisms of damage, such as mitochondrial dysfunction [85], neuroinflammation [86], and oxidative stress [87]. AD is characterized by dementia, memory loss, and cognitive decline, disorders that are worsened with aging [88]. In 2019, the World Alzheimer Report indicated that more than 50 million people live with dementia worldwide, a number that will increase up to 152 million by 2050 [89]. In this regard, every three seconds, a person develops dementia, and the current annual cost of dementia is estimated at US $ 1 trillion, which is estimated to double by 2030 [89].
DHA via enzyme 15-lipoxygenase (15-LOX) can be converted in oxylipins, including resolvins and neuroprotectins, which are potent lipid mediators [90][91]. Furthermore, DHA may undergo lipid peroxidation producing oxylipin metabolites, such as 4-hytoxyhexenal (4-HHE) [91][92]. These metabolites may have a role in modulating oxidative cell homeostasis by regulating the activity of the transcription factors nuclear factor kappa-B (NF-κB) and nuclear factor erythroid 2-related factor 2 (Nrf2), thus participating in the inflammatory and antioxidant response, and neuroprotection [91]. Osterman et al. (2019) [93] assigned healthy adults with low fish consumption (n = 121) to receive capsules with different doses of n-3 LCPUFA reflecting three patterns of fatty fish consumption: 1, 2, or 4 servings/week with 3.27 g of EPA + DHA (1:1.2) per serving or placebo. The authors reported that plasma oxylipins after 3 and 12 months increased linearly with the highest intake of EPA and DHA [93].
Aging is a normal process of the life cycle, and its progression is usually accompanied by the decrease of a wide range of body functions, including cognitive function, marked by decreased synaptic density, decreased neuronal survival, and loss of volume of the gray and white matter [69][94][95]. Alteration of lipid metabolism also occurs [96] and is associated with the dysfunction of fluidity and activity of brain cell membranes microdomains (or rafts) [97]. Exacerbation of alteration of lipid metabolism generates abnormal brain activity that can potentially lead to the development of neurodegenerative diseases [97][98]. Factors contributing to the early cognitive decline include diseases related to unhealthy lifestyles and metabolic syndrome [68]. Among them, atherosclerosis and hypertension are relevant because an altered blood flow generates hypoperfusion and vascular dysfunction, causing an impairment of the blood-brain barrier and triggering neurodegenerative processes that lead to cognitive deterioration and, ultimately, to the development of AD [96][97].
The pathogenesis of AD has not been fully resolved. However, it is known that the mechanisms that trigger the pathology are related to: (i) low levels of acetylcholine [99]; (ii) aggregation of insoluble β-amyloid (Aβ) peptides, a product of abnormal processing of the amyloidal precursor protein, in neuritic plaques, leading to Aβ accumulation in the CNS [100][101][102]; (iii) hyperphosphorylation of the tau protein associated with microtubules, causing intraneuronal accumulation of neurofibrillary tangles of tau protein and disruption of neuronal microtubules [103][104]; and (iv) oxidative stress, leading to inflammation, synaptic toxicity and accumulation of intraneuronal inclusions [101][105] (Figure 3). Regarding its clinical characteristics, AD is a slowly progressive disease and three stages can be recognized in its evolution: the first is characterized by memory failures; in the second, language disorders, apraxias, and Gerstmann syndrome (agraphia and agnosia) are frequently added; and in the third stage, the patient is physically disabled and prostrated [106][107].
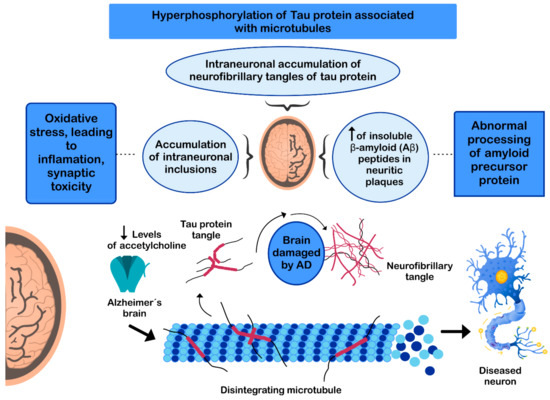
Figure 3. Mechanisms involved in the pathogenesis of Alzheimer’s disease (AD).
PD is the second most common neurodegenerative disease after AD [108], affecting 2%-3% of the population ≥65 years old [109]. The etiopathogenesis of the disease includes: (i) intracellular aggregation of α-synuclein causing the formation of Lewy bodies [110][111]; (ii) neuronal loss of the substantia nigra pars compacta (SNPc) leading to a marked striatal dopamine deficiency [112][113]; (iii) mitochondrial dysfunction, due to defects in the activity and incorrect assembly of complex I of the mitochondrial electron transport chain [114][115] (inhibition of mitochondrial complex I induces neuronal cell death in the SNPc leading to dopaminergic decrease) [112][114]; (iv) oxidative stress [112], the nigral dopaminergic neurons are particularly vulnerable to metabolic diseases and oxidative stress [116][117][118][119]; and (v) neuroinflammation [112][120][121] (Figure 4). The clinical evolution of PD is characterized by motor disturbances due to progressive nigrostriatal dopaminergic neurodegeneration that occurs in parallel with decreased levels of striatal dopamine, dopaminergic synapses, and the density of dendritic spines in mid-striated spinal neurons [122]. Other non-motor symptoms of PD include cognitive impairment [123] and gastrointestinal dysfunctions [124][125].
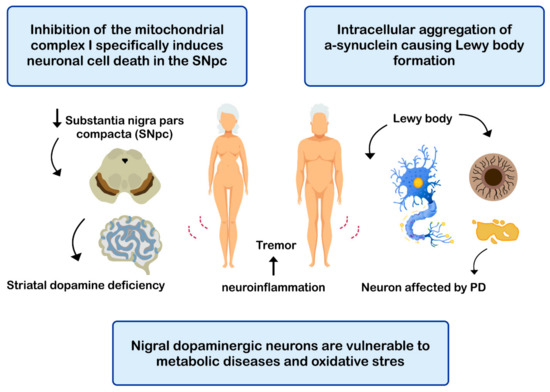
Figure 4. Mechanisms related with the pathogenesis of Parkinson’s disease (PD).
References
- Svennerholm, L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 1968, 9, 570–579.
- Crawford, M.A.; Broadhurst, C.L. The role of docosahexaenoic and the marine food web as determinants of evolution and hominid brain development: The challenge for human sustainability. Nutr. Health 2012, 21, 17–39.
- Carlson, S.E.; Colombo, J. Docosahexaenoic Acid and Arachidonic Acid Nutrition in Early Development. Adv. Pediatrics 2016, 63, 453–471.
- Belkind-Gerson, J.; Carreón-Rodríguez, A.; Contreras-Ochoa, C.O.; Estrada-Mondaca, S.; Parra-Cabrera, M.S. Fatty acids and neurodevelopment. J. Pediatr. Gastroenterol. Nutr. 2008, 47, S7–S9.
- Innis, S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43.
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrinets 2016, 8, 99.
- Brenna, J.T.; Diau, G.Y. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 247–250.
- Carrié, I.; Clément, M.; de Javel, D.; Francès, H.; Bourre, J.-M. Specific phospholipid fatty acid composition of brain regions in mice: Effects of n–3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000, 41, 465–472.
- Garcia, M.C.; Ward, G.; Ma, Y.-C.; Salem, N.; Kim, H.-Y. Effect of Docosahexaenoic Acid on the Synthesis of Phosphatidylserine in Rat Brain Microsomes and C6 Glioma Cells. J. Neurochem. 2002, 70, 24–30.
- Gawrisch, K.; Eldho, N.V.; Holte, L.L. The structure of DHA in phospholipid membranes. Lipids 2003, 38, 445–452.
- Valenzuela, R.; Morales, J.; Sanhueza, J.; Valenzuela, A. Docosahexaenoic acid (DHA), an essential fatty acid at the brain. Rev. Chil. Nutr. 2013, 40, 383–390.
- Katakura, M.; Hashimoto, M.; Shahdat, H.; Gamoh, S.; Okui, T.; Matsuzaki, K.; Shido, O. Docosahexaenoic acid promotes neuronal differentiation by regulating basic helix–loop–helix transcription factors and cell cycle in neural stem cells. Neuroscience 2009, 160, 651–660.
- Jumpsen, J.; Lien, E.L.; Goh, Y.K.; Clandinin, M.T. Small changes of dietary (n-6) and (n-3)/fatty acid content ration alter phosphatidylethanolamine and phosphatidylcholine fatty acid composition during development of neuronal and glial cells in rats. J. Nutr. 1997, 127, 724–731.
- Kyle, D.J.; Schaefer, E.; Patton, G.; Beiser, A. Low serum docosahexaenoic acid is a significant risk factor for alzheimer’s dementia. Lipids 1999, 34, S245.
- Heude, B.; Ducimetière, P.; Berr, C. Cognitive decline and fatty acid composition of erythrocyte membranes—The EVA Study. Am. J. Clin. Nutr. 2003, 77, 803–808.
- Beydoun, M.A.; Kaufman, J.S.; A Satia, J.; Rosamond, W.; Folsom, A.R. Plasma n-3 fatty acids and the risk of cognitive decline in older adults: The Atherosclerosis Risk in Communities Study. Am. J. Clin. Nutr. 2007, 85, 1103–1111.
- Conquer, J.A.; Tierney, M.C.; Zecevic, J.; Bettger, W.J.; Fisher, R.H. Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 2000, 35, 1305–1312.
- Schaefer, E.J.; Bongard, V.; Beiser, A.S.; Lamon-Fava, S.; Robins, S.J.; Au, R.; Tucker, K.L.; Kyle, D.J.; Wilson, P.W.; Wolf, P.A. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: The Framingham Heart Study. Arch. Neurol. 2006, 63, 1545–1550.
- Yurko-Mauro, K.; Alexander, D.D.; van Elswyk, M.E. Docosahexaenoic acid and adult memory: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0120391.
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Wilson, R.S.; Aggarwal, N.; Schneider, J. Consumption of Fish and n-3 Fatty Acids and Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 940–946.
- Chang, Y.L.; Chen, S.J.; Kao, C.L.; Hung, S.C.; Ding, D.C.; Yu, C.C.; Chen, Y.J.; Ku, H.H.; Lin, C.P.; Lee, K.H.; et al. Docosahexaenoic acid promotes dopaminergic differentiation in induced pluripotent stem cells and inhibits teratoma formation in rats with Parkinson-like pathology. Cell Transplant. 2012, 21, 313–332.
- Ozkan, A.; Parlak, H.; Tanriover, G.; Dilmac, S.; Ulker, S.N.; Birsen, L.; Agar, A. The protective mechanism of docosahexaenoic acid in mouse model of Parkinson: The role of heme oxygenase. Neurochem. Int. 2016, 101, 110–119.
- Mori, M.A.; Delattre, A.M.; Carabelli, B.; Pudell, C.; Bortolanza, M.; Staziaki, P.V.; Visentainer, J.V.; Montanher, P.F.; del Bel, E.A.; Ferraz, A.C. Neuroprotective effect of omega-3 polyunsaturated fatty acids in the 6-OHDA model of Parkinson’s disease is mediated by a reduction of inducible nitric oxide synthase. Nutr. Neurosci. 2018, 21, 341–351.
- Derbyshire, E. Brain Health across the Lifespan: A Systematic Review on the Role of Omega-3 Fatty Acid Supplements. Nutrients 2018, 10, 1094.
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6.
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216.
- Gao, F.; Kiesewetter, D.; Chang, L.; Rapoport, S.I.; Igarashi, M. Quantifying conversion of linoleic to arachidonic and other n-6 polyunsaturated fatty acids in unanesthetized rats. J. Lipid Res. 2010, 51, 2940–2946.
- Brenna, J.T. Arachidonic acid needed in infant formula when docosahexaenoic acid is present. Nutr. Rev. 2016, 74, 329–336.
- Harauma, A.; Hatanaka, E.; Yasuda, H.; Nakamura, M.T.; Salem, N.; Moriguchi, T. Effects of arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid on brain development using artificial rearing of delta-6-desaturase knockout mice. Prostaglandins Leukot. Essent. Fat. Acids 2017, 127, 32–39.
- Wilson, R.; Sargent, J.R.X. Lipid and fatty acid composition of brain tissue from adrenoleukodystrophy patients. J. Neurochem. 1993, 61, 290–297.
- O’Brien, J.S.; Sampson, E.L. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 545–551.
- Jahn, R.; Scheller, R.H. SNAREs—Engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643.
- Rickman, C.; Davletov, B. Arachidonic Acid Allows SNARE Complex Formation in the Presence of Munc18. Chem. Biol. 2005, 12, 545–553.
- Connell, E.; Darios, F.; Broersen, K.; Gatsby, N.; Peak-Chew, S.; Rickman, C.; Davletov, B. Mechanism of arachidonic acid action on syntaxin–Munc18. EMBO Rep. 2007, 8, 414–419.
- Latham, C.F.; Osborne, S.L.; Cryle, M.J.; Meunier, F.A. Arachidonic acid potentiates exocytosis and allows neuronal SNARE complex to interact with Munc18a. J. Neurochem. 2006, 100, 1543–1554.
- Tokuda, H.; Kontani, M.; Kawashima, H.; Kiso, Y.; Shibata, H.; Osumi, N. Differential effect of arachidonic acid and docosahexaenoic acid on age-related decreases in hippocampal neurogenesis. Neurosci. Res. 2014, 88, 58–66.
- Ho, L.; Purohit, D.; Haroutunian, V.; Luterman, J.D.; Willis, F.; Naslund, J.; Buxbaum, J.D.; Mohs, R.C.; Aisen, P.S.; Pasinetti, G.M. Neuronal Cyclooxygenase 2 Expression in the Hippocampal Formation as a Function of the Clinical Progression of Alzheimer Disease. Arch. Neurol. 2001, 58, 487–492.
- Fujimi, K.; Noda, K.; Sasaki, K.; Wakisaka, Y.; Tanizaki, Y.; Iida, M.; Kiyohara, Y.; Kanba, S.; Iwaki, T. Altered Expression of COX-2 in Subdivisions of the Hippocampus during Aging and in Alzheimer’s Disease: The Hisayama Study. Dement. Geriatr. Cogn. Disord. 2007, 23, 423–431.
- Ching, Y.K.; Chin, Y.S.; Appukutty, M.; Ramanchadran, V.; Yu, C.Y.; Ang, G.Y.; Gan, W.Y.; Chan, Y.M.; Teh, L.K.; Salleh, M.Z. Interaction of Dietary Linoleic Acid and α-Linolenic Acids with rs174547 in FADS1 Gene on Metabolic Syndrome Components among Vegetarians. Nutrinets 2019, 11, 1686.
- Rincón-Cervera, M.Á.; Valenzuela, R.; Hernandez-Rodas, M.C.; Barrera, C.; Espinosa, A.; Marambio, M.; Valenzuela, A. Vegetable oils rich in alpha linolenic acid increment hepatic n-3 LCPUFA, modulating the fatty acid metabolism and antioxidant response in rats. Prostaglandins Leukot. Essent. Fat. Acids 2016, 111, 25–35.
- Gonzalez-Soto, M.; Mutch, D.M. Diet Regulation of Long-Chain PUFA Synthesis: Role of Macronutrients, Micronutrients, and Polyphenols on Δ-5/Δ-6 Desaturases and Elongases 2/5. Adv. Nutr. 2020, 142.
- Kang, J.X. The Importance of Omega-6/Omega-3 Fatty Acid Ratio in Cell Function. World Rev. Nutr. Diet. 2003, 92, 23–36.
- Valenzuela, R.; Videla, L.A. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011, 2, 644–648.
- Food and Agriculture Organization. Fatty Acids in Human Nutrition. Report of an Expert Consultation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008; p. 91.
- Brayner, B.; Kaur, G.; Keske, M.A.; Livingstone, K.M. FADS Polymorphism, Omega-3 Fatty Acids and Diabetes Risk: A Sys-tematic Review. Nutrients 2018, 13, 10758.
- Chen, X.; Wu, Y.; Zhang, Z.; Zheng, X.; Wang, Y.; Yu, M.; Liu, G. Effects of the rs3834458 Single Nucleotide Polymorphism in FADS2 on Levels of n-3 Long-chain Polyunsaturated Fatty Acids: A Meta-analysis. Prostaglandins Leukot. Essent. Fat. Acids 2019, 150, 1–6.
- Xie, L.; Innis, S.M. Genetic Variants of the FADS1 FADS2 Gene Cluster Are Associated with Altered (n-6) and (n-3) Essential Fatty Acids in Plasma and Erythrocyte Phospholipids in Women during Pregnancy and in Breast Milk during Lactation. J. Nutr. 2008, 138, 2222–2228.
- Steer, C.D.; Smith, G.D.; Emmett, P.M.; Hibbeln, J.R.; Golding, J. FADS2 Polymorphisms Modify the Effect of Breastfeeding on Child IQ. PLoS ONE 2010, 5, e11570.
- Miliku, K.; Duan, Q.L.; Moraes, T.J.; Becker, A.B.; Mandhane, P.J.; E Turvey, S.; Lefebvre, D.L.; Sears, M.R.; Subbarao, P.; Field, C.J.; et al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD Cohort Study. Am. J. Clin. Nutr. 2019, 110, 1370–1383.
- Koletzko, B.; Reischl, E.; Tanjung, C.; Gonzalez-Casanova, I.; Ramakrishnan, U.; Meldrum, S.; Simmer, K.; Heinrich, J.; Demmelmair, H. FADS1 and FADS2 Polymorphisms Modulate Fatty Acid Metabolism and Dietary Impact on Health. Annu. Rev. Nutr. 2019, 39, 21–44.
- Xu, Y.; Zhao, Z.; Liu, S.; Xiao, Y.; Miao, M.; Dong, Q.; Xin, Y. Association of Nonalcoholic Fatty Liver Disease and Coronary Artery Disease with FADS2 rs3834458 Gene Polymorphism in the Chinese Han Population. Gastroenterol. Res. Pr. 2019, 2019, 1–7.
- Valenzuela, R.; Morales, G.; González, M.; Morales, J.; Sanhueza, J.; Valenzuela, A. N-3 long chain polyunsaturated fatty acids and cardiovascular disease. Rev. Chil. Nutr. 2014, 41, 319–327.
- Morales, J.; Valenzuela, R.; González, D.; González, M.; Tapia, G.; Sanhueza, J. New dietary sources of alpha-linolenic acid: A critical view. Rev. Chil. Nutr. 2012, 39, 79–87.
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662.
- EFSA Panel on Dietetic Products. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, pol-yunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461.
- Harris, W.S.; Mozaffarian, D.; Lefevre, M.; Toner, C.D.; Colombo, J.; Cunnane, S.C.; Holden, J.M.; Klurfeld, D.M.; Morris, M.C.; Whelan, J. Towards Establishing Dietary Reference Intakes for Eicosapentaenoic and Docosahexaenoic Acids. J. Nutr. 2009, 139, 804S–819S.
- Koletzko, B.; Lien, E.; Agostoni, C.; Böhles, H.; Campoy, C.; Cetin, I.; Decsi, T.; Dudenhausen, J.W.; Dupont, C.; Forsyth, S.; et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. J. Peérinat. Med. 2008, 36, 5–14.
- Talamonti, E.; Sasso, V.; To, H.; Haslam, R.P.; Napier, J.A.; Ulfhake, B.; Pernold, K.; Asadi, A.; Hessa, T.; Jacobsson, A.; et al. Impairment of DHA synthesis alters the expression of neuronal plasticity markers and the brain inflammatory status in mice. FASEB J. 2019, 34, 2024–2040.
- Wainwright, P.E.; Xing, H.-C.; Mutsaers, L.; McCutcheon, D.; Kyle, D. Arachidonic Acid Offsets the Effects on Mouse Brain and Behavior of a Diet with a Low (n-6):(n-3) Ratio and Very High Levels of Docosahexaenoic Acid. J. Nutr. 1997, 127, 184–193.
- Giusto, N.M.; Salvador, G.A.; Castagnet, P.I.; Pasquaré, S.J.; Ilincheta de Boschero, M.G. Age-associated changes in central nervous system glycerolipid composition and metabolism. Neurochem. Res. 2002, 27, 1513–1523.
- Innis, S.M. Biology of metabolism in growing animals. In Essential Fatty Acid Metabolism During Early Development; Burrin, D.G., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2005; pp. 235–274.
- Poling, J.S.; Karanian, J.W.; Salem, N.; Vicini, S. Time- and voltage-dependent block of delayed rectifier potassium channels by docosahexaenoic acid. Mol. Pharmacol. 1995, 47, 381–390.
- Strokin, M.; Chechneva, O.; Reymann, K.G.; Reiser, G. Neuroprotection of rat hippocampal slices exposed to oxygen-glucose dep-rivation by enrichment with docosahexaenoic acid and by inhibition of hydrolysis of docosahexaenoic acid-containing phos-pholipids by calcium independent phospholipase A2. Neuroscience 2006, 140, 547–553.
- De Lion, S.; Chalon, S.; Guilloteau, D.; Besnard, J.-C.; Durand, G. α-Linolenic Acid Dietary Deficiency Alters Age-Related Changes of Dopaminergic and Serotoninergic Neurotransmission in the Rat Frontal Cortex. J. Neurochem. 2002, 66, 1582–1591.
- Cheng, L.; Hu, T.; Shi, H.; Chen, X.; Wang, H.; Zheng, K.; Huang, X.-F.; Yu, Y. DHA reduces hypothalamic inflammation and improves central leptin signaling in mice. Life Sci. 2020, 257, 118036.
- Chalon, S.; Delion-Vancassel, S.; Belzung, C.; Guilloteau, D.; Leguisquet, A.M.; Besnard, J.C.; Durand, G. Dietary fish oil affects mon-oaminergic neurotransmission and behavior in rats. J. Nutr. 1998, 128, 2512–2519.
- Feltham, B.A.; Louis, X.L.; Eskin, A.M.N.; Suh, M. Docosahexaenoic Acid: Outlining the Therapeutic Nutrient Potential to Combat the Prenatal Alcohol-Induced Insults on Brain Development. Adv. Nutr. 2020, 11, 724–735.
- Singh, R.B.; Gupta, S.; Dherange, P.; de Meester, F.; Wilczynska, A.; Alam, S.E.; Pella, D.; Wilson, D.W. Metabolic syndrome: A brain disease. Can. J. Physiol. Pharmacol. 2012, 90, 1171–1183.
- Masliah, E.; Crews, L.; Hansen, L. Synaptic remodeling during aging and in Alzheimer’s disease. J. Alzheimers Dis. 2006, 9, 91–99.
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138.
- Hsieh, A.T.; Anthony, J.C.; Diersen-Schade, D.A.; Rumsey, S.C.; Lawrence, P.; Li, C.; Nathanielsz, P.W.; Brenna, J.T. The Influence of Moderate and High Dietary Long Chain Polyunsaturated Fatty Acids (LCPUFA) on Baboon Neonate Tissue Fatty Acids. Pediatr. Res. 2007, 61, 537–545.
- Wijendran, V.; Lawrence, P.; Diau, G.-Y.; Boehm, G.; Nathanielsz, P.; Brenna, J. Significant utilization of dietary arachidonic acid is for brain adrenic acid in baboon neonates. J. Lipid Res. 2002, 43, 762–767.
- Alashmali, S.M.; Kitson, A.P.; Lin, L.; Lacombe, R.J.S.; Bazinet, R.P. Maternal dietary n-6 polyunsaturated fatty acid deprivation does not exacerbate post-weaning reductions in arachidonic acid and its mediators in the mouse hippocampus. Nutr. Neurosci. 2017, 22, 223–234.
- Yang, H.-J.; Sugiura, Y.; Ikegami, K.; Konishi, Y.; Setou, M. Axonal Gradient of Arachidonic Acid-containing Phosphatidylcholine and Its Dependence on Actin Dynamics. J. Biol. Chem. 2012, 287, 5290–5300.
- Lauritzen, L.; Hansen, H.S.; Jørgensen, M.H.; Michaelsen, K.F. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid. Res. 2001, 40, 1–94.
- Darios, F.; Davletov, B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nat. Cell Biol. 2006, 440, 813–817.
- Novak, E.M.; Dyer, R.A.; Innis, S.M. High dietary omega-6 fatty acids contribute to reduced docosahexaenoic acid in the developing brain and inhibit secondary neurite growth. Brain Res. 2008, 1237, 136–145.
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflam-mation. Immunity 2014, 40, 315–327.
- Dyall, S.C. Interplay between n-3 and n-6 Long-Chain Polyunsaturated Fatty Acids and the Endocannabinoid System in Brain Protection and Repair. Lipids 2017, 52, 885–900.
- Hammels, I.; Binczek, E.; Schmidt-Soltau, I.; Jenke, B.; Thomas, A.; Vogel, M.; Thevis, M.; Filipova, D.; Papadopoulos, S.; Stoffel, W. Novel CB1-ligands maintain homeostasis of the endocannabinoid system in ω3- and ω6-long-chain-PUFA deficiency. J. Lipid Res. 2019, 60, 1396–1409.
- Watson, J.E.; Kim, J.S.; Das, A. Emerging class of omega-3 fatty acid endocannabinoids & their derivatives. Prostaglandins Other Lipid Mediat. 2019, 143, 106337.
- Simopoulos, A. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379.
- Wood, J.T.; Williams, J.S.; Pandarinathan, L.; Janero, D.R.; Lammi-Keefe, C.J.; Makriyannis, A. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J. Lipid Res. 2010, 51, 1416–1423.
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 11, 889–909.
- Camilleri, A.; Vassallo, N. The Centrality of Mitochondria in the Pathogenesis and Treatment of Parkinson’s Disease. CNS Neurosci. Ther. 2014, 20, 591–602.
- Sanchez-Guajardo, V.; Tentillier, N.; Romero-Ramos, M. The relation between α-synuclein and microglia in Parkinson’s disease: Recent developments. Neuroscience 2015, 302, 47–58.
- Xie, A.; Gao, J.; Xu, L.; Meng, D. Shared Mechanisms of Neurodegeneration in Alzheimer’s Disease and Parkinson’s Disease. BioMed Res. Int. 2014, 2014, 1–8.
- Zhang, C.; Du, Q.Y.; Chen, L.D. Design, synthesis and evaluation of novel tacrine-multialkoxybenzene hybrids as multi-targeted compounds against Alzheimer’s disease. Eur. J. Med. Chem. 2016, 116, 200–209.
- Alzheimer’s Disease International. World Alzheimer Report 2019: Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2019.
- Shalini, S.-M.; Ho, C.F.-Y.; Ng, Y.-K.; Tong, J.-X.; Ong, E.-S.; Herr, D.R.; Dawe, G.S.; Ong, W.-Y. Distribution of Alox15 in the Rat Brain and Its Role in Prefrontal Cortical Resolvin D1 Formation and Spatial Working Memory. Mol. Neurobiol. 2018, 55, 1537–1550.
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahex-aenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent Fatty Acids. 2018, 136, 3–13.
- Ishikado, A.; Morino, K.; Nishio, Y.; Nakagawa, F.; Mukose, A.; Sono, Y.; Yoshioka, N.; Kondo, K.; Sekine, O.; Yoshizaki, T.; et al. 4-Hydroxy Hexenal Derived from Docosahexaenoic Acid Protects Endothelial Cells via Nrf2 Activation. PLoS ONE 2013, 8, e69415.
- Ostermann, A.I.; West, A.L.; Schoenfeld, K.; Browning, L.M.; Walker, C.G.; A Jebb, S.; Calder, P.C.; Schebb, N.H. Plasma oxylipins respond in a linear dose-response manner with increased intake of EPA and DHA: Results from a randomized controlled trial in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1251–1263.
- Van Skike, C.E.; Lin, A.L.; Roberts-Burbank, R.; Halloran, J.J.; Hernandez, S.F.; Cuvillier, J.; Soto, V.Y.; Hussong, S.A.; Jahrling, J.B.; Javors, M.A.; et al. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell. 2020, 19, e13057.
- Tisserand, D.J.; Pruessner, J.C.; Sanz-Arigita, E.J.; van Boxtel, M.P.; Evans, A.C.; Jolles, J.; Uylings, H.B. Regional frontal cortical volumes decrease differentially in aging: An MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage 2002, 17, 657–669.
- Marin, R.; Fabelo, N.; Fernández-Echevarría, C.; Canerina-Amaro, A.; Rodríguez-Barreto, D.; Quinto-Alemany, D.; Mesa-Herrera, F.; Díaz, M. Lipid Raft Alterations in Aged-Associated Neuropathologies. Curr. Alzheimer Res. 2016, 13, 973–984.
- Sonnino, S.; Aureli, M.; Grassi, S.; Mauri, L.; Prioni, S.; Prinetti, A. Lipid Rafts in Neurodegeneration and Neuroprotection. Mol. Neurobiol. 2014, 50, 130–148.
- Santos, G.; Díaz, M.; Torres, N.V. Lipid Raft Size and Lipid Mobility in Non-raft Domains Increase during Aging and Are Exac-erbated in APP/PS1 Mice Model of Alzheimer’s Disease. Predictions from an Agent-Based Mathematical Model. Front Physiol. 2016, 7, 90.
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s disease. Mech. Ageing. Dev. 2001, 122, 1961–1969.
- Hardy, J. The amyloid hypothesis for Alzheimer’s disease: A critical reappraisal. J. Neurochem. 2009, 110, 1129–1134.
- Martín, V.; Fabelo, N.; Santpere, G.; Puig, B.; Marín, R.; Ferrer, I.; Díaz, M. Lipid alterations in lipid rafts from Alzheimer’s disease human brain cortex. J. Alzheimers Dis. 2010, 19, 489–502.
- García-Ayllón, M.-S.; Small, D.H.; Avila, J.; Saez-Valero, J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with P-tau and β-amyloid. Front. Mol. Neurosci. 2011, 4, 22.
- Gao, Y.; Tan, L.; Yu, J.T.; Tan, L. Tau in Alzheimer’s Disease: Mechanisms and Therapeutic Strategies. Curr. Alzheimer Res. 2018, 15, 283–300.
- Gauthier, S.; Feldman, H.H.; Schneider, L.S.; Wilcock, G.K.; Frisoni, G.B.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Wischik, D.J.; et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: A randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 2016, 388, 2873–2884.
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121.
- Robert, C.; Wilson, C.S.; Lipton, R.B.; Arreto, C.-D. Evolution of the Research Literature and the Scientific Community of Alzheimer’s Disease from 1983–2017: A 35-Year Survey. J. Alzheimer’s Dis. 2020, 75, 1105–1134.
- Jellinger, K.A.; Logroscino, G.; Rizzo, G.; Copetti, M.; Arcuti, S.; Martino, D.; Fontana, A. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Author Response. Neurology 2016, 87, 237–238.
- Muller, M.; Tang, M.X.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement. Geriatr. Cogn. Disord. 2007, 24, 185–192.
- Twelves, D.; Perkins, K.S.; Counsell, C. Systematic review of incidence studies of Parkinson’s disease. Mov. Disord. 2003, 18, 19–31.
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers. 2017, 23, 17013.
- Kim, W.S.; Kågedal, K.; Halliday, G.M. Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Res. Ther. 2014, 6, 73.
- Przedborski, S. The two-century journey of Parkinson disease research. Nat. Rev. Neurosci. 2017, 18, 251–259.
- Warner, T.T.; Schapira, A.H. Genetic and Environmental Factors in the Cause of Parkinson’s Disease. Ann. Neurol. 2003, 53 (Suppl. 3), S16–S23; discussion S23–S25.
- Bose, A.; Beal, M.F. Mitochondrial dysfunction in Parkinson’s disease. J. Neurochem. 2016, 139, 216–231.
- Keeney, P.M.; Xie, J.; Capaldi, R.A.; Bennett, J.P., Jr. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 2006, 10, 5256–5264.
- Bolam, J.P.; Pissadaki, E.K. Living on the edge with too many mouths to feed: Why dopamine neurons die. Mov. Disord. 2012, 27, 1478–1483.
- Pissadaki, E.K.; Bolam, J.P. The energy cost of action potential propagation in dopamine neurons: Clues to susceptibility in Parkinson’s disease. Front Comput. Neurosci. 2013, 7, 13.
- Surmeier, D.J.; Guzman, J.N.; Sanchez-Padilla, J.; Schumacker, P.T. The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson’s disease. Neuroscience 2011, 198, 221–231.
- Mosharov, E.V.; Larsen, K.E.; Kanter, E.; Phillips, K.A.; Wilson, K.; Schmitz, Y.; Krantz, D.E.; Kobayashi, K.; Edwards, R.H.; Sulzer, D. Interplay between cytosolic dopamine, calcium, and alpha-synuclein causes selective death of substantia nigra neurons. Neuron 2009, 62, 218–229.
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397.
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783.
- Rodrigues, T.M.; Castro-Caldas, A.; Ferreira, J.J. Pharmacological interventions for daytime sleepiness and sleep disorders in Parkinson’s disease: Systematic review and meta-analysis. Parkinsonism Relat Disord. 2016, 27, 25–34.
- Wissel, B.D.; Dwivedi, A.K.; Merola, A.; Chin, D.; Jacob, C.; Duker, A.P.; Vaughan, J.E.; Lovera, L.; la Faver, K.; Levy, A.; et al. Functional neurological disorders in Parkinson disease. J. Neurol. Neurosurg. Psychiatry 2018, 89, 566–571.
- Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Curr. Treat. Options Neurol. 2018, 20, 54.
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639.




