
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Duygu Kaus | + 3702 word(s) | 3702 | 2021-03-09 06:41:43 | | | |
| 2 | Bruce Ren | Meta information modification | 3702 | 2021-03-16 02:31:51 | | |
Video Upload Options
Electrolytes are key components in electrochemical storage systems, which provide an ion-transport mechanism between the cathode and anode of a cell. As battery technologies are in continuous development, there has been growing demand for more efficient, reliable and environmentally friendly materials. Solid-state lithium ion batteries (SSLIBs) are considered as next-generation energy storage systems and solid electrolytes (SEs) are the key components for these systems. Compared to liquid electrolytes, SEs are thermally stable (safer), less toxic and provide a more compact (lighter) battery design. However, the main issue is the ionic conductivity, especially at low temperatures. So far, there are two popular types of SEs: (1) inorganic solid electrolytes (InSEs) and (2) polymer electrolytes (PEs). Among InSEs, sulfide-based SEs are providing very high ionic conductivities (up to 10−2 S/cm) and they can easily compete with liquid electrolytes (LEs). On the other hand, they are much more expensive than LEs. PEs can be produced at less cost than InSEs but their conductivities are still not sufficient for higher performances.
1. Introduction
The first lithium batteries were already based on “Li metal” technology where metallic lithium was used as the negative electrode, achieving the highest theoretical energy densities [1]. However, the use of lithium in the metallic form coupled with an organic liquid electrolyte resulted in dendrite formation, which eventually leads to an internal short circuit and thus, a thermal runaway. The serious safety problems associated with this system stunted their growth during their years on the market. In 1991, Sony presented and marketed the first Li-ion battery (LIB) technology in which Lithium was no longer present in metallic form but only in ionic form (Li+) in a “host” material at a higher potential than lithium metal, thus limiting the formation of dendrites [2]. Since then, LIBs have been widely developed and are now present in all portable devices requiring a rechargeable battery (mobile phone, laptop, etc.). Today, the low manufacturing cost of LIBs makes them the leading technology on the market for applications in electromobility (e-mobility). However, as e-mobility (especially Electric Vehicle, EV) is an increasing market and becoming more and more attractive for millions of customers, there is a need for higher energy density cells with increased charge–discharge and thermal performances. This could be achieved through the optimization of existing LIB chemistries.
Conventional Li-ion technology is reaching its performance limits, as there can be no compromise on lifetime or safety. The latest “advanced” Li-ion systems with a silicon anode will not exceed energy densities of 800 Wh L−1 or 300 Wh kg−1 on a cell scale [3][4]. In order to achieve higher energy densities, it is possible to use Li metal instead of graphite as the negative electrode. Li metal has about ten-times higher specific capacity (3.860 mAh g−1) than graphite [5]. However, as stated previously, Li metal is not compatible with a liquid electrolyte system because of the formation of dendrites. Porous polymer-based separators do not provide a sufficient physical barrier to stop the breakthrough of dendrites. In addition, the existing liquid electrolytes are toxic and flammable due to the fluorinated salt LiPF6 carbonate solvents. A battery system with a liquid electrolyte can cause many safety problems in the event of accidents. Its replacement with a solid electrolyte, which is also acting as a separator, would create an inert, solid system that could solve the problems mentioned above. Solid-state batteries do not have a liquid junction, which facilitates the formation of series-connected cells in a pack. The absence of this junction eliminates unnecessary volume, resulting in higher volumetric energy densities. Hence, these new all-solid state batteries (ASSB) are currently considered as the next generation of lithium batteries.
For a successful ASSB, the solid electrolyte must meet several key criteria such as (i) high ionic conductivity, (ii) wide electrochemical stable window and chemical stability, (iii) simple management of the interfaces between the components of the cell, (iv) good mechanical properties, flexibility and (v) affordable cost [6]. There have been many studies to find the most suitable solid electrolyte to make ASSBs competitive with today’s Li-ion technology.
SEs are generally classified into two main groups: inorganic electrolytes and polymer electrolytes (PE). The most commonly studied SEs are given in Figure 1.
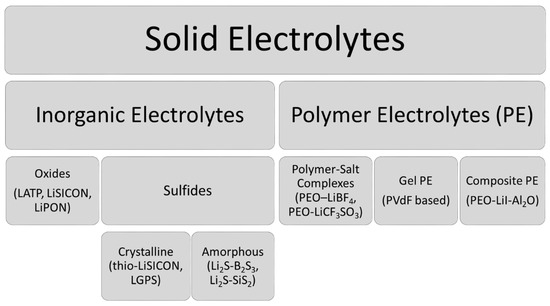
Under inorganic electrolytes, Lithium SuperIonic CONductor (LiSICON) andderivatives are widely used as oxide-type electrolytes due to their lower reactivity with water and air. However, they show lower ionic conductivity at room temperature (RT) (~10−7 S cm−1) compared to sulfide electrolytes [9]. In 1989, Aono et al. showed that Sodium (Na) SuperIonic CONductor (NaSICON)-type electrolytes such as Li1+xAlxTi2−x (PO4)3 (LATP) offer an ionic conductivity of 7 × 10−4 S cm−1 and a wide electrochemical window of 6 V [10]. In recent years, LATP electrolytes have been often discussed and even started to be produced by some companies [8]. An often neglected, underestimated and maybe entirely unknown fact of SEs is their lithium activity and the related stability window. Usually, solid Li-Ion conductors are still considered as inert. However, Li-Ions are partially highly mobile. Some types of Li-Ion conductors (e.g., garnet LLZO) seem to show a kinetic stability with metallic lithium. However, their reactions with H2O and CO2 have been extensively reported, e.g., in [11]. This reveals a high tendency to release lithium rather than to accept or to insert it. LLZO acts, in contrary to LATP, much more as a Li-donor than a Li-acceptor.
Consequently, one has to attribute a lithium activity that is at least high enough to promote reaction with water and carbon dioxide. Therefore, it is opportune to speak about high lithium activities in such compounds. Generally, it seems to be a crucial dilemma that lithium activities, with H2O and CO2, are a necessary evil to provide at least the kinetic stability of H20 and CO2 with lithium metal. However, solid Li-ion conductors, which have a comparable sensitivity, tend to readily absorb lithium. The only way to solve this dilemma is to hypothetically block such reactions using extremely high electronic resistances. This delays the movement of electrons to allow sufficient time for the reactions to occur. However, this is impossible in reality for solid Li-Ion conductors, which always show inherent stoichiometric deviations as a consequence of preparation routes. They are not perfect crystals but powders and even perfect crystals have surfaces with different effects to those of the bulk. This is a tremendously important issue to be investigated and to be discussed for oxide-based electrolytes such as those with garnet structure, e.g., LLZO [12].
Lithium phosphorous oxy-nitride “LiPON” electrolytes are another type of inorganic oxide electrolytes with ionic conductivity of ~2 × 10−6 S cm−1, which is somewhere in between LATP and LiSICON conductivity [8].
The other subcategory of inorganic electrolytes, the sulfide family generally has higher conductivities (up to 2.5 × 10−2 S cm−1) than the oxides due to the higher polarizability and larger size of sulfur compared to oxygen [7]. Crystalline (glass-ceramic) sulfide electrolytes (thio-LiSICON family) are represented with the general formula LixM1−δMδ′S4, where M represents Si, Ge, Sn and M’ represents P, Ga, Al and Zn [13]. Within this family, the crystalline sulfide electrolytes Li10GeP2 S12 (LGPS) and argyrodite-type crystallines Li6PS5X (X = Cl, Br, I) (LPS) are the most popular ones with their ionic conductivity of 1.9 × 10−3 S cm−1 and 6.8 × 10−3 S cm−1, X = Cl and Br, respectively [9][14].
Amorphous (glassy)-type sulfide electrolytes are ductile and they require very high temperatures for a cell assembly to avoid the crystallization of the sulfide glasses [15].
As mentioned, SEs should have good mechanical properties, especially moderate elasticity (Young’s modulus), since they need to adjust their form with the volume change of electrodes during charging and discharging [16]. However, having a low Young’s modulus (E’) is also not enough. The material must show good strength at the same time in order to resist dendrite formation. It has been reported that, for a dendrite-free deposition, the shear modulus (G’) of an SE should be at least twice that of lithium metal (GLi = 3.4 GPa) [17][18].
Polymer electrolytes (PEs) have many particularly interesting characteristics. They are flexible (E’PEO = 70 MPa) [18], light, and their thickness can be controlled in the order of ten micrometers by different preparation techniques such as extrusion or pressing. The most studied PE for all-solid batteries is polyethylene oxide (PEO) coupled with a lithium salt [19]. Their conductivities lie around 10−4 S cm−1 depending on the lithium salt used [8]. Gel PEs are prepared with a low crystalline polymer such as poly(vinylidene fluoride)-co-hexafluoropropylene (PVdF-HFP) and an organic liquid electrolyte such as LiPF6 in EC-DMC) in the polymer matrix. Despite their good ionic conductivities (up to 6 × 10−3 S cm−1) [8], they suffer from lower mechanical strength (GPEO = 26.2 MPa) and electrode compatibilities [19].
As it can be seen, each family of SEs has its advantages and disadvantages, and each of them should be considered depending on the ASSB applications. Typically, organic liquid electrolytes for commercialized Li-Ion batteries show conductivities of about 2 × 10−2 S cm−1 at room temperature. Assuming a porosity of typical polyolefin separators of about 40% [20], a resulting conductivity of about 5 × 10−3 S cm−1 remains as a rule of thumb. SEs have to compete at least with these values, also taking into account that typical polyolefin separators have thicknesses in the range of 20–25 µm [20], which are difficult to realize with Li metal solid-state ion conductions in practice. This is a strong reason for looking at sulfide-based solid electrolytes rather than oxide-based ones since the latter do usually not exceed 5 × 10−4 S cm−1 even in the bulk phase. Besides, oxide-based solid Li-Ion conductors are not the focus of the present paper since sulfide- and/or phosphide-based candidates are much more promising to successfully bridge the gap to their liquid competitors. Solid polymer electrolytes (SPEs) will also be discussed due to their higher stability against Li metal anode.
2. Solid Electrolytes
2.1. Inorganic Sulfide Electrolytes
| Solid Inorganic Electrolyte | Cathode | Energy Density (Wh kg−1) |
Full Cell Capacity (mAh g−1) | Cycle | Reference |
|---|---|---|---|---|---|
| Li10GeP2S12 | LCO-LiNbO3 | - | 114 (at 0.12 C) | 8 | [22] |
| Li10GeP2S12//Li9.6P3S12 | LCO-LiNbO3 | 33 | 114 (at 0.12 C) | 30 | [30] |
| Li10GeP2S12//Li2S-P2S5 | NiS-CNT | - | 170 | 150 | [29] |
| Li10GeP2S12//Li2S-P2O5 | LCO-Li7P3S11 | 17 | 647 (at 0.06C) | 1000 | [28] |
| Li10GeP2S12//Li3P0.98Sb0.02S3.95O0.05 | LCO-LiNbO3 | 14 | 134 (at 0.09C) | 50 | [27] |
| Li3.15Ge0.15P0.85S4//Li2S-P2S5 | LCO-Al2O3 | 7 | 121 (at 0.13C) | 25 | [26] |
2.2. Solid Polymer Electrolytes
As the solid polymer electrolytes (SPEs) have a softer nature compared to the InSEs, they have more flexibility, and as a result, they offer better processability [42]. Moreover, this flexibility allows them to respond to volume changes within the cell due to lithiation. Cheng et al. reported that for the LiFePO4 (LFP) cathode, the volume change is about 6.6% [43], and as the SE is directly neighboring the cathode, its flexible nature prevents the electrolyte from irreversible deformations.
The most studied polymer as a SPE is polyethylene oxide (PEO) coupled with the lithium salt, LiTFSI [44][45][46][47][48][49][50][51][52][53]. However, sole PEO-based electrolytes exhibit relatively low ionic conductivity (ca. 10−7–10−5 S cm−1) at room temperature. In order to increase their ionic conductivity, they are prepared as composite electrolytes [54][55][56][57][58][59]. Composite SPEs contain PEO in their composition because PEO-based electrolytes are relatively more stable against Li metal and PEO can dissolve the conductive lithium salt easier due to its polar ether groups.
PEO-based composite SPEs are mainly used with a low-voltage LFP cathode [51][52][53][54][55][56] because PEO shows low stability at potentials greater than 3.8 V (vs. Li+/Li) and an oxidative decomposition occurs [60][61]. This limitation prevents it from using the most popular cathode materials, such as NCA or NMC, which require potentials up to 4.1–4.2 V. Nevertheless, Wakayama et al. have proposed a three-dimensional structure and built cells with LiCoO2 (LCO) composed of Li7La3Zr2O12 (LLZ) [57]. Their cells were suitable for cycling between 3.0 and 4.2 V (up to 20 cycles). Table 2 summarizes some results of ASSB cell results obtained with PEO-based composite electrolytes and a Li metal anode.
| Solid Polymer Electrolyte | Cathode | Energy Density (Wh kg−1) |
Full Cell Capacity (mAh g−1) | Cycle | Ref. |
|---|---|---|---|---|---|
| Ca–CeO2/LiTFSI/PEO | LFP | - | 157 (at 0.2C) | 40 | [54] |
| PEO/LiTFSI/ Al-Li6.75La3Zr1.75Ta0.25 O12 |
LFP-In2O5Sn | 334 (Wh l−1) | 155 (at 0.01C) | 10 | [55] |
| poly(-LiMTFSI)-b-PEO-b-poly(LiMTFSI) | LFP | 168 | 158 (at 0.1C) | 300 | [56] |
| PEO-LiTFSI | LCO-Li7La3Zr2O12 | 141 | 136 (at 0.05C) | 20 | [57] |
| poly(LiTFSI)-b-PEO-b-poly(LiTFSI) | LFP | 282 | 158 (at 0.36C) | 1400 | [58] |
| poly(STFSILi)-b-PEO-b-poly(STFSILi) | LFP | 120 | 162 (at 0.07C) | - | [59] |
In order to prepare a good conductive SPE, inorganic materials are introduced into the PEO matrix. Some oxides such as Al2O3, TiO2, ZnAl2O4, CeO, and SiO2 in the PEO matrix increase not only the ionic conductivity values but also the electrochemical stability and the mechanical strength [54]. Among them, PEO in combination with Al2O3 and SiO2 particles is considered the most promising electrolyte [55].
The addition of lithium garnet (i.e., Li6.75La3Zr1.75Ta0.25 O12, LLZTO) to the PEO matrix also results in higher conductivity values. Moreover, due to their stability at higher potentials, it is possible to use them with high-voltage cathodes [62]. Zhang et al. presented a study by preparing a PEO-based composite SPE without using a Li salt [62]. They have reported that the addition of nanosize LLZTO particles (with D50 = 43nm) shifted the oxidation potential of PEO from 4.0 to 5.0 V. This behavior can be attributed to the fact that solid-state inorganic polymer ion-conducting composites, such as LLZO-PEO often show extended stability windows. However, chemical reactions are not defined electronic switches such as “on” and “off”. Thus, more precisely, the visible onset of a chemical decomposition reaction as a function of the voltage is shifted. The reason can simply be seen in the effective reactive surface. By its nature, nano-scaled LLZO yields a very high surface with a strong additional tendency to produce LiOH and Li2CO3, which are quite stable compounds. These likely cover, as LLZTO nanopowder, huge parts of the PEO and produce a shielding and coating effect, which may mislead someone into believing PEO has become more stable.
The same study also showed that dendrites coming from the Li anode were suppressed. No dendrite formation was observed after more than 700 h of cycling since the accumulation of lithium was hindered in the insulating polymer matrix. The cells containing the same composite membranes but prepared with conducting LiTFSI salt showed the dendrite formation after 25 h of cycling. The LiTFSI-free SPE also showed a high ionic conductivity (2.1 × 10−4 S cm−1) at 30 °C.
As shown in Table 2, LFP cathode can also be prepared with indium tin oxide (In2O5Sn) to increase the cell’s electronic conductivity by increasing the surface quality between the electrode and SPE.
References
- Whittingham, M.S. Electrical energy storage and intercalation chemistry. Science 1976, 192, 1126–1127.
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106.
- Xiang, H.; Zhang, K.; Ji, G.; Lee, J.Y.; Zou, C.; Chen, X.; Wu, J. Graphene/nanosized silicon composites for Lithium battery anodes with improved cycling stability. Carbon 2011, 49, 1787–1796.
- Chakrapani, V.; Rusli, F.; Filler, M.A.; Kohl, P.A. Silicon nanowire anode: Improved battery life with capacity-limited cycling. J. Power Sources 2012, 205, 433–438.
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845.
- Wanga, L.; Zhoua, Z.; Yan, X.; Hou, F.; Wen, L.; Luo, W.; Liang, J.; Dou, S.X. Engineering of Lithium-metal anodes towards a safe and stable battery. Energy Storage Mater. 2018, 14, 22–48.
- Lau, J.; DeBlock, R.H.; Butts, D.M.; Ashby, D.S.; Choi, C.S.; Dunn, B.S. Sulfide Solid Electrolytes for Lithium Battery Applications. Adv. Energy Mater. 2018, 8, 1800933.
- TechVision Group. Innovations in Solid State Batteries: Need for Safer Alternatives Drives Innovations in Solid State Batteries; Frost & Sullivan: Santa Clara, CA, USA, 2018.
- Cao, C.; Li, Z.-B.; Wang, X.-L.; Zhao, X.-B.; Han, W.-Q. Recent Advances in Inorganic Solid Electrolytes for Lithium Batteries. Front. Energy Res. 2014, 2, 25.
- Aono, H.; Sugimoto, E.; Sadaoka, Y.; Imanaka, N.; Adachi, G.-Y. Ionic Conductivity of the Lithium Titanium Phosphate (Li1 + X M X Ti2 − X (PO4)3 M = Al Sc Y and La) Systems. J. Electrochem. Soc. 1989, 136, 590.
- Xia, W.; Xu, B.; Duan, H.; Guo, Y.; Kang, H.; Li, H.; Liu, H. Ionic Conductivity and Air Stability of Al-Doped Li7La3Zr1O12 Sintered in Alumina and Pt Crucibles. ACS Appl. Mater. Interfaces 2016, 8, 5335–5342.
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical Challenges and Future Perspectives of All-Solid-State Lithium-Metal Batteries. Chem 2019, 5, 753–785.
- Minafra, N.; Culver, S.P.; Li, C.; Senyshyn, A.; Zeier, W.G. Influence of the Lithium Substructure on the Diffusion Pathways and Transport Properties of the Thio-LISICON Li 4 Ge 1– x Sn x S 4. Chem. Mater. 2019, 31, 3794–3802.
- Rao, R.P.; Adams, S. Studies of Lithium argyrodite solid electrolytes for all-solid-state batteries. Phys. Status Solidi A 2011, 208, 1804–1807.
- Tatsumisago, M.; Nagao, M.; Hayashi, A. Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable Lithium batteries. J. Asian Ceram. Soc. 2013, 1, 17–25.
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide Solid Electrolyte with Favorable Mechanical Property for All-Solid-State Lithium Battery. Sci. Rep. 2013, 3, 1–5.
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252.
- Barai, P.; Higa, K.; Srinivasan, V. Lithium dendrite growth mechanisms in polymer electrolytes and prevention strategies. Phys. Chem. Chem. Phys. 2017, 19, 20493–20505.
- Fergus, J.W. Ceramic and polymeric solid electrolytes for Lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569.
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of Lithium-ion battery separators. Nat. Energy 2019, 4, 16–25.
- Kanno, R.; Murayama, M. Lithium Ionic Conductor Thio-LISICON: The Li2 S-GeS2-P 2 S 5 System. J. Electrochem. Soc. 2001, 148, A742.
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A Lithium superionic conductor. Nat. Mater. 2011, 10, 682–686.
- Zhang, Q.; Hu, J.; Chu, Y.; Wan, W.; Zhao, L.; Zhu, Y. Electrochemical performance of sulfide solid electrolyte Li10GeP2S12 synthesized by a new method. Mater. Lett. 2019, 248, 153–156.
- Zhang, Z.; Chen, S.; Yang, J.; Wang, J.; Yao, L.; Yao, X.; Cui, P.; Xu, X. Interface Re-Engineering of Li10GeP2S12 Electrolyte and Lithium anode for All-Solid-State Lithium Batteries with Ultralong Cycle Life. ACS Appl. Mater. Interfaces 2018, 10, 2556–2565.
- Wenzel, S.; Randau, S.; Leichtweiß, T.; Weber, D.A.; Sann, J.; Zeier, W.G.; Janek, J. Direct Observation of the Interfacial Instability of the Fast Ionic Conductor Li10GeP2S12 at the Lithium Metal Anode. Chem. Mater. 2016, 28, 2400–2407.
- Woo, J.H.; Trevey, J.E.; Cavanagh, A.S.; Choi, Y.S.; Kim, S.C.; George, S.M.; Oh, K.H.; Lee, S.H. Nanoscale Interface Modification of LiCoO2 by Al2O3 Atomic Layer Deposition for Solid-State Li Batteries. J. Electrochem. Soc. 2012, 159, A1120.
- Xie, D.; Chen, S.; Zhang, Z.; Ren, J.; Yao, L.; Wu, L.; Yao, X.; Xu, X. High ion conductive Sb2O5-doped β-Li3PS4 with excellent stability against Li for all-solid-state Lithium batteries. J. Power Sources 2018, 389, 140–147.
- Yao, X.; Liu, D.; Wang, C.; Long, P.; Peng, G.; Hu, Y.S.; Li, H.; Chen, L.; Xu, X. High-Energy All-Solid-State Lithium Batteries with Ultralong Cycle Life. Nano Lett. 2016, 16, 7148–7154.
- Zhang, Q.; Peng, G.; Mwizerwa, J.P.; Wan, H.; Cai, L.; Xu, X.; Yao, X. Nickel sulfide anchored carbon nanotubes for all-solid-state Lithium batteries with enhanced rate capability and cycling stability. J. Mater. Chem. A 2018, 6, 12098–12105.
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 1–7.
- Kato, Y.; Hori, S.; Kanno, R. Li10GeP2S12—Type Superionic Conductors: Synthesis, Structure, and Ionic Transportation. Adv. Energy Mater. 2020, 10.
- Ruiz, A.G.; Sola, P.C.; Palmerola, N.M. Advanced Material and Device Applications with Germanium: Germanium: Current and Novel Recovery Processes; InTech Open: London, UK, 2018.
- Kato, Y.; Saito, R.; Sakano, M.; Mitsui, A.; Hirayama, M.; Kanno, R. Synthesis, structure and Lithium ionic conductivity of solid solutions of Li10(Ge1−xMx)P2S12 (M = Si, Sn). J. Power Sources 2014, 271, 60–64.
- Deiseroth, H.J.; Kong, S.T.; Eckert, H.; Vannahme, J.; Reiner, C.; Zaiß, T.; Schlosser, M. Li6PS5X: A Class of Crystalline Li-Rich Solids With an Unusually High Li+ Mobility. Angew. Chem. 2008, 120, 767–770.
- Hanghofer, I.; Brinek, M.; Eisbacher, S.L.; Bitschnau, B.; Volck, M.; Hennige, V.; Hanzu, I.; Rettenwander, D.; Wilkening, H.M.R. Substitutional disorder: Structure and ion dynamics of the argyrodites Li6PS5Cl, Li6PS5Br and Li6PS5I. Phys. Chem. Chem. Phys. 2019, 21, 8489–8507.
- Zhou, L.; Park, K.H.; Sun, X.; Lalѐre, F.; Adermann, T.; Hartmann, P.; Nazar, L.F. Solvent-engineered design of argyrodite Li6PS5X (X = Cl, Br, I) solid electrolytes with high ionic conductivity. ACS Energy Lett. 2018, 4, 265–270.
- Boulineau, S.; Courty, M.; Tarascon, J.; Viallet, V. Mechanochemical synthesis of Li-argyrodite Li6PS5X (X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ion. 2012, 221, 1–5.
- Kasemchainan, J.; Zekoll, S.; Jolly, D.S.; Ning, Z.; Hartley, G.O.; Marrow, J.; Bruce, P.G. Critical stripping current leads to dendrite formation on plating in Lithium anode solid electrolyte cells. Nat. Mater 2019, 18, 1105–1111.
- Zhou, Y.; Doerrer, C.; Kasemchainan, J.; Bruce, P.G.; Pasta, M.; Hardwick, L.J. Observation of Interfacial Degradation of Li6PS5Cl against Lithium Metal and LiCoO2 via In Situ Electrochemical Raman Microscopy. Batter Supercaps 2020, 3, 647–652.
- Viallet, V.; Tarascon, J.-M.; Boulineau, S. Improvement of Electrochemical Performances of All-Solid-State Argyrodite-Based Lithium Batteries. In Proceedings of the 17th International Meeting on Lithium Batteries, Como, Italy, 10–14 June 2014; p. 757.
- Lee, Y.G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state Lithium metal batteries enabled by silver–carbon composite anodes. Nat. Energy 2020, 5, 299–308.
- Wu, Z.; Xie, Z.; Yoshida, A.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Utmost limits of various solid electrolytes in all-solid-state Lithium batteries: A critical review. Renew. Sustain. Energy Rev. 2019, 109, 367–385.
- Cheng, S.H.S.; He, K.Q.; Liu, Y.; Zha, J.W.; Kamruzzaman, M.; Ma, R.L.W.; Dang, Z.M.; Li, R.K.; Chung, C.Y. Electrochemical performance of all-solid-state Lithium batteries using inorganic Lithium garnets particulate reinforced PEO/LiClO4 electrolyte. Electrochim. Acta 2017, 253, 430–438.
- Gorecki, W.; Jeannin, M.; Belorizky, E.; Roux, C.; Armand, M. Physical properties of solid polymer electrolyte PEO(LiTFSI) complexes. J. Phys. Condens. Matter 1995, 7, 6823.
- Rey, I.; Lassègues, J.C.; Grondin, J.; Servant, L. Infrared and Raman study of the PEO-LiTFSI polymer electrolyte. Electrochim. Acta 1998, 43, 1505–1510.
- Bouchet, R.; Lascaud, S.; Rosso, M. An EIS Study of the Anode Li/PEO-LiTFSI of a Li Polymer Battery. J. Electrochem. Soc. 2003, 150, A1385.
- Stolwijk, N.A.; Wiencierz, M.; Heddier, C.; Kösters, J. What can we learn from ionic conductivity measurements in polymer electrolytes? A case study on poly(ethylene oxide) (PEO)-NaI and PEO-LiTFSI. J. Phys. Chem. B 2012, 116, 3065–3074.
- Marzantowicz, M.; Dygas, J.R.; Krok, F. Impedance of interface between PEO:LiTFSI polymer electrolyte and blocking electrodes. Electrochim. Acta 2008, 53, 7417–7425.
- Molinari, N.; Mailoa, J.P.; Kozinsky, B. Effect of Salt Concentration on Ion Clustering and Transport in Polymer Solid Electrolytes: A Molecular Dynamics Study of PEO–LiTFSI. Chem. Mater. 2018, 30, 6298–6306.
- Utpalla, P.; Sharma, S.K.; Sudarshan, K.; Sahu, M.; Pujari, P.K. Investigation of the free volume characteristics of PEO based solid state polymer electrolyte by means of positron annihilation spectroscopy. Solid State Ion. 2019, 339, 114990.
- Wan, J.; Xie, J.; Kong, X.; Liu, Z.; Liu, K.; Shi, F.; Pei, A.; Chen, H.; Chen, W.; Chen, J. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for Lithium batteries. Nat. Nanotechnol. 2019, 14, 705–711.
- Qiu, J.; Yang, L.; Sun, G.; Yu, X.; Li, H.; Chen, L. A stabilized PEO-based solid electrolyte via a facile interfacial engineering method for a high voltage solid-state Lithium metal battery. Chem. Commun. 2020, 56, 5633–5636.
- Wurster, V.; Engel, C.; Graebe, H.; Ferber, T.; Jaegermann, W.; Hausbrand, R. Characterization of the Interfaces in LiFePO4/PEO-LiTFSI Composite Cathodes and to the Adjacent Layers. J. Electrochem. Soc. 2019, 166, A5410.
- Chen, H.; Adekoya, D.; Hencz, L.; Ma, J.; Chen, S.; Yan, C.; Zhao, H.; Cui, G.; Zhang, S. Stable Seamless Interfaces and Rapid Ionic Conductivity of Ca—CeO2/LiTFSI/PEO Composite Electrolyte for High—Rate and High—Voltage All—Solid—State Battery. Adv. Energy Mater. 2020, 10.
- Chen, R.J.; Zhang, Y.B.; Liu, T.; Xu, B.Q.; Lin, Y.H.; Nan, C.W.; Shen, Y. Addressing the Interface Issues in All-Solid-State Bulk-Type Lithium Ion Battery via an All-Composite Approach. ACS Appl. Mater. Interfaces 2017, 9, 9654–9661.
- Porcarelli, L.; Aboudzadeh, M.A.; Rubatat, L.; Nair, J.R.; Shaplov, A.S.; Gerbaldi, C.; Mecerreyes, D. Single-ion triblock copolymer electrolytes based on poly(ethylene oxide) and methacrylic sulfonamide blocks for Lithium metal batteries. J. Power Sources 2017, 364, 191–199.
- Wakayama, H.; Yonekura, H.; Kawai, Y. Three-Dimensional Bicontinuous Nanocomposite from a Self-Assembled Block Copolymer for a High-Capacity All-Solid-State Lithium Battery Cathode. Chem. Mater. 2016, 28, 4453–4459.
- Hovington, P.; Lagacé, M.; Guerfi, A.; Bouchard, P.; Mauger, A.; Julien, C.M.; Armand, M.; Zaghib, K. New Lithium metal polymer solid state battery for an ultrahigh energy: Nano C-LiFePO4 versus nano Li1.2V3O8. Nano Lett. 2015, 15, 2671–2678.
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.P.; Phan, T.N.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for Lithium-metal batteries. Nat. Mater. 2013, 12, 452–457.
- Randau, S.; Weber, D.A.; Kötz, O.; Koerver, R.; Braun, P.; Weber, A.; Ivers-Tiffée, E.; Adermann, T.; Kulisch, J.; Zeier, W.G.; et al. Benchmarking the performance of all-solid-state Lithium batteries. Nat. Energy 2020, 5, 259–270.
- Homann, G.; Stolz, L.; Nair, J.; Laskovic, I.C.; Winter, M.; Kasnatscheew, J. Poly(Ethylene Oxide)-based Electrolyte for Solid-State-Lithium-Batteries with High Voltage Positive Electrodes: Evaluating the Role of Electrolyte Oxidation in Rapid Cell Failure. Sci. Rep. 2020, 10, 1–9.
- Zhang, J.; Zhao, N.; Zhang, M.; Li, Y.; Chu, P.K.; Guo, X.; Di, Z.; Wang, X.; Li, H. Flexible and ion-conducting membrane electrolytes for solid-state Lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy 2016, 28, 447–454.




