1. Introduction
The first lithium batteries were already based on “Li metal” technology where metallic lithium was used as the negative electrode, achieving the highest theoretical energy densities [
1]. However, the use of lithium in the metallic form coupled with an organic liquid electrolyte resulted in dendrite formation, which eventually leads to an internal short circuit and thus, a thermal runaway. The serious safety problems associated with this system stunted their growth during their years on the market. In 1991, Sony presented and marketed the first Li-ion battery (LIB) technology in which Lithium was no longer present in metallic form but only in ionic form (Li
+) in a “host” material at a higher potential than lithium metal, thus limiting the formation of dendrites [
2]. Since then, LIBs have been widely developed and are now present in all portable devices requiring a rechargeable battery (mobile phone, laptop, etc.). Today, the low manufacturing cost of LIBs makes them the leading technology on the market for applications in electromobility (e-mobility). However, as e-mobility (especially Electric Vehicle, EV) is an increasing market and becoming more and more attractive for millions of customers, there is a need for higher energy density cells with increased charge–discharge and thermal performances. This could be achieved through the optimization of existing LIB chemistries.
Conventional Li-ion technology is reaching its performance limits, as there can be no compromise on lifetime or safety. The latest “advanced” Li-ion systems with a silicon anode will not exceed energy densities of 800 Wh L
−1 or 300 Wh kg
−1 on a cell scale [
3,
4]. In order to achieve higher energy densities, it is possible to use Li metal instead of graphite as the negative electrode. Li metal has about ten-times higher specific capacity (3.860 mAh g
−1) than graphite [
5]. However, as stated previously, Li metal is not compatible with a liquid electrolyte system because of the formation of dendrites. Porous polymer-based separators do not provide a sufficient physical barrier to stop the breakthrough of dendrites. In addition, the existing liquid electrolytes are toxic and flammable due to the fluorinated salt LiPF
6 carbonate solvents. A battery system with a liquid electrolyte can cause many safety problems in the event of accidents. Its replacement with a solid electrolyte, which is also acting as a separator, would create an inert, solid system that could solve the problems mentioned above. Solid-state batteries do not have a liquid junction, which facilitates the formation of series-connected cells in a pack. The absence of this junction eliminates unnecessary volume, resulting in higher volumetric energy densities. Hence, these new all-solid state batteries (ASSB) are currently considered as the next generation of lithium batteries.
For a successful ASSB, the solid electrolyte must meet several key criteria such as (i) high ionic conductivity, (ii) wide electrochemical stable window and chemical stability, (iii) simple management of the interfaces between the components of the cell, (iv) good mechanical properties, flexibility and (v) affordable cost [
6]. There have been many studies to find the most suitable solid electrolyte to make ASSBs competitive with today’s Li-ion technology.
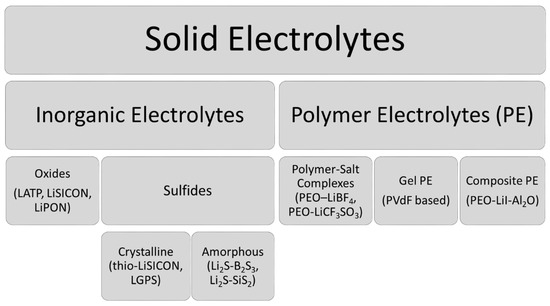
SEs are generally classified into two main groups: inorganic electrolytes and polymer electrolytes (PE). The most commonly studied SEs are given in .
Figure 1. The most common solid electrolytes (SEs) and their examples [
7,
8].
Under inorganic electrolytes, Lithium SuperIonic CONductor (LiSICON) andderivatives are widely used as oxide-type electrolytes due to their lower reactivity with water and air. However, they show lower ionic conductivity at room temperature (RT) (~10
−7 S cm
−1) compared to sulfide electrolytes [
9]. In 1989, Aono et al. showed that Sodium (Na) SuperIonic CONductor (NaSICON)-type electrolytes such as Li
1+xAl
xTi
2−x (PO4)
3 (LATP) offer an ionic conductivity of 7 × 10
−4 S cm
−1 and a wide electrochemical window of 6 V [
10]. In recent years, LATP electrolytes have been often discussed and even started to be produced by some companies [
8]. An often neglected, underestimated and maybe entirely unknown fact of SEs is their lithium activity and the related stability window. Usually, solid Li-Ion conductors are still considered as inert. However, Li-Ions are partially highly mobile. Some types of Li-Ion conductors (e.g., garnet LLZO) seem to show a kinetic stability with metallic lithium. However, their reactions with H
2O and CO
2 have been extensively reported, e.g., in [
11]. This reveals a high tendency to release lithium rather than to accept or to insert it. LLZO acts, in contrary to LATP, much more as a Li-donor than a Li-acceptor.
Consequently, one has to attribute a lithium activity that is at least high enough to promote reaction with water and carbon dioxide. Therefore, it is opportune to speak about high lithium activities in such compounds. Generally, it seems to be a crucial dilemma that lithium activities, with H
2O and CO
2, are a necessary evil to provide at least the kinetic stability of H20 and CO2 with lithium metal. However, solid Li-ion conductors, which have a comparable sensitivity, tend to readily absorb lithium. The only way to solve this dilemma is to hypothetically block such reactions using extremely high electronic resistances. This delays the movement of electrons to allow sufficient time for the reactions to occur. However, this is impossible in reality for solid Li-Ion conductors, which always show inherent stoichiometric deviations as a consequence of preparation routes. They are not perfect crystals but powders and even perfect crystals have surfaces with different effects to those of the bulk. This is a tremendously important issue to be investigated and to be discussed for oxide-based electrolytes such as those with garnet structure, e.g., LLZO [
12].
Lithium phosphorous oxy-nitride “LiPON” electrolytes are another type of inorganic oxide electrolytes with ionic conductivity of ~2 × 10
−6 S cm
−1, which is somewhere in between LATP and LiSICON conductivity [
8].
The other subcategory of inorganic electrolytes, the sulfide family generally has higher conductivities (up to 2.5 × 10
−2 S cm
−1) than the oxides due to the higher polarizability and larger size of sulfur compared to oxygen [
7]. Crystalline (glass-ceramic) sulfide electrolytes (thio-LiSICON family) are represented with the general formula Li
xM
1−δM
δ′S
4, where M represents Si, Ge, Sn and M’ represents P, Ga, Al and Zn [
13]. Within this family, the crystalline sulfide electrolytes Li
10GeP
2 S
12 (LGPS) and argyrodite-type crystallines Li
6PS
5X (X = Cl, Br, I) (LPS) are the most popular ones with their ionic conductivity of 1.9 × 10
−3 S cm
−1 and 6.8 × 10
−3 S cm
−1, X = Cl and Br, respectively [
9,
14].
Amorphous (glassy)-type sulfide electrolytes are ductile and they require very high temperatures for a cell assembly to avoid the crystallization of the sulfide glasses [
15].
As mentioned, SEs should have good mechanical properties, especially moderate elasticity (Young’s modulus), since they need to adjust their form with the volume change of electrodes during charging and discharging [
16]. However, having a low Young’s modulus (E’) is also not enough. The material must show good strength at the same time in order to resist dendrite formation. It has been reported that, for a dendrite-free deposition, the shear modulus (G’) of an SE should be at least twice that of lithium metal (G
Li = 3.4 GPa) [
17,
18].
Polymer electrolytes (PEs) have many particularly interesting characteristics. They are flexible (E’
PEO = 70 MPa) [
18], light, and their thickness can be controlled in the order of ten micrometers by different preparation techniques such as extrusion or pressing. The most studied PE for all-solid batteries is polyethylene oxide (PEO) coupled with a lithium salt [
19]. Their conductivities lie around 10
−4 S cm
−1 depending on the lithium salt used [
8]. Gel PEs are prepared with a low crystalline polymer such as poly(vinylidene fluoride)-co-hexafluoropropylene (PVdF-HFP) and an organic liquid electrolyte such as LiPF6 in EC-DMC) in the polymer matrix. Despite their good ionic conductivities (up to 6 × 10
−3 S cm
−1) [
8], they suffer from lower mechanical strength (G
PEO = 26.2 MPa) and electrode compatibilities [
19].
As it can be seen, each family of SEs has its advantages and disadvantages, and each of them should be considered depending on the ASSB applications. Typically, organic liquid electrolytes for commercialized Li-Ion batteries show conductivities of about 2 × 10
−2 S cm
−1 at room temperature. Assuming a porosity of typical polyolefin separators of about 40% [
20], a resulting conductivity of about 5 × 10
−3 S cm
−1 remains as a rule of thumb. SEs have to compete at least with these values, also taking into account that typical polyolefin separators have thicknesses in the range of 20–25 µm [
20], which are difficult to realize with Li metal solid-state ion conductions in practice. This is a strong reason for looking at sulfide-based solid electrolytes rather than oxide-based ones since the latter do usually not exceed 5 × 10
−4 S cm
−1 even in the bulk phase. Besides, oxide-based solid Li-Ion conductors are not the focus of the present paper since sulfide- and/or phosphide-based candidates are much more promising to successfully bridge the gap to their liquid competitors. Solid polymer electrolytes (SPEs) will also be discussed due to their higher stability against Li metal anode.