1. Introduction
The first lithium batteries were already based on “Li metal” technology where metallic lithium was used as the negative electrode, achieving the highest theoretical energy densities [1]. However, the use of lithium in the metallic form coupled with an organic liquid electrolyte resulted in dendrite formation, which eventually leads to an internal short circuit and thus, a thermal runaway. The serious safety problems associated with this system stunted their growth during their years on the market. In 1991, Sony presented and marketed the first Li-ion battery (LIB) technology in which Lithium was no longer present in metallic form but only in ionic form (Li +
) in a “host” material at a higher potential than lithium metal, thus limiting the formation of dendrites [2]. Since then, LIBs have been widely developed and are now present in all portable devices requiring a rechargeable battery (mobile phone, laptop, etc.). Today, the low manufacturing cost of LIBs makes them the leading technology on the market for applications in electromobility (e-mobility). However, as e-mobility (especially Electric Vehicle, EV) is an increasing market and becoming more and more attractive for millions of customers, there is a need for higher energy density cells with increased charge–discharge and thermal performances. This could be achieved through the optimization of existing LIB chemistries.
Conventional Li-ion technology is reaching its performance limits, as there can be no compromise on lifetime or safety. The latest “advanced” Li-ion systems with a silicon anode will not exceed energy densities of 800 Wh L
−1
or 300 Wh kg
−1 on a cell scale [3][4]. In order to achieve higher energy densities, it is possible to use Li metal instead of graphite as the negative electrode. Li metal has about ten-times higher specific capacity (3.860 mAh g
on a cell scale [3,4]. In order to achieve higher energy densities, it is possible to use Li metal instead of graphite as the negative electrode. Li metal has about ten-times higher specific capacity (3.860 mAh g −1
) than graphite [5]. However, as stated previously, Li metal is not compatible with a liquid electrolyte system because of the formation of dendrites. Porous polymer-based separators do not provide a sufficient physical barrier to stop the breakthrough of dendrites. In addition, the existing liquid electrolytes are toxic and flammable due to the fluorinated salt LiPF 6
carbonate solvents. A battery system with a liquid electrolyte can cause many safety problems in the event of accidents. Its replacement with a solid electrolyte, which is also acting as a separator, would create an inert, solid system that could solve the problems mentioned above. Solid-state batteries do not have a liquid junction, which facilitates the formation of series-connected cells in a pack. The absence of this junction eliminates unnecessary volume, resulting in higher volumetric energy densities. Hence, these new all-solid state batteries (ASSB) are currently considered as the next generation of lithium batteries.
For a successful ASSB, the solid electrolyte must meet several key criteria such as (i) high ionic conductivity, (ii) wide electrochemical stable window and chemical stability, (iii) simple management of the interfaces between the components of the cell, (iv) good mechanical properties, flexibility and (v) affordable cost [6]. There have been many studies to find the most suitable solid electrolyte to make ASSBs competitive with today’s Li-ion technology.
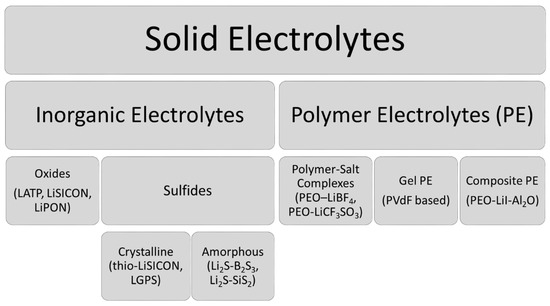
SEs are generally classified into two main groups: inorganic electrolytes and polymer electrolytes (PE). The most commonly studied SEs are given in
.
Figure 1. The most common solid electrolytes (SEs) and their examples
[7][8][7,8].
Under inorganic electrolytes, Lithium SuperIonic CONductor (LiSICON) andderivatives are widely used as oxide-type electrolytes due to their lower reactivity with water and air. However, they show lower ionic conductivity at room temperature (RT) (~10
Under inorganic electrolytes, Lithium SuperIonic CONductor (LiSICON) andderivatives are widely used as oxide-type electrolytes due to their lower reactivity with water and air. However, they show lower ionic conductivity at room temperature (RT) (~10
−7
S cm
−1
) compared to sulfide electrolytes [9]. In 1989, Aono et al. showed that Sodium (Na) SuperIonic CONductor (NaSICON)-type electrolytes such as Li 1+x
Al
x
Ti
2−x
(PO4)
3
(LATP) offer an ionic conductivity of 7 × 10
−4
S cm
−1
and a wide electrochemical window of 6 V [10]. In recent years, LATP electrolytes have been often discussed and even started to be produced by some companies [8]. An often neglected, underestimated and maybe entirely unknown fact of SEs is their lithium activity and the related stability window. Usually, solid Li-Ion conductors are still considered as inert. However, Li-Ions are partially highly mobile. Some types of Li-Ion conductors (e.g., garnet LLZO) seem to show a kinetic stability with metallic lithium. However, their reactions with H 2
O and CO
2
have been extensively reported, e.g., in [11]. This reveals a high tendency to release lithium rather than to accept or to insert it. LLZO acts, in contrary to LATP, much more as a Li-donor than a Li-acceptor.
Consequently, one has to attribute a lithium activity that is at least high enough to promote reaction with water and carbon dioxide. Therefore, it is opportune to speak about high lithium activities in such compounds. Generally, it seems to be a crucial dilemma that lithium activities, with H
2
O and CO
2
, are a necessary evil to provide at least the kinetic stability of H20 and CO2 with lithium metal. However, solid Li-ion conductors, which have a comparable sensitivity, tend to readily absorb lithium. The only way to solve this dilemma is to hypothetically block such reactions using extremely high electronic resistances. This delays the movement of electrons to allow sufficient time for the reactions to occur. However, this is impossible in reality for solid Li-Ion conductors, which always show inherent stoichiometric deviations as a consequence of preparation routes. They are not perfect crystals but powders and even perfect crystals have surfaces with different effects to those of the bulk. This is a tremendously important issue to be investigated and to be discussed for oxide-based electrolytes such as those with garnet structure, e.g., LLZO [12].
Lithium phosphorous oxy-nitride “LiPON” electrolytes are another type of inorganic oxide electrolytes with ionic conductivity of ~2 × 10
−6
S cm
−1
, which is somewhere in between LATP and LiSICON conductivity [8].
The other subcategory of inorganic electrolytes, the sulfide family generally has higher conductivities (up to 2.5 × 10
−2
S cm
−1
) than the oxides due to the higher polarizability and larger size of sulfur compared to oxygen [7]. Crystalline (glass-ceramic) sulfide electrolytes (thio-LiSICON family) are represented with the general formula Li x
M
1−δ
M
δ
′S
4
, where M represents Si, Ge, Sn and M’ represents P, Ga, Al and Zn [13]. Within this family, the crystalline sulfide electrolytes Li 10
GeP
2
S
12
(LGPS) and argyrodite-type crystallines Li
6
PS
5
X (X = Cl, Br, I) (LPS) are the most popular ones with their ionic conductivity of 1.9 × 10
−3
S cm
−1
and 6.8 × 10
−3
S cm
−1, X = Cl and Br, respectively [9][14].
, X = Cl and Br, respectively [9,14].
Amorphous (glassy)-type sulfide electrolytes are ductile and they require very high temperatures for a cell assembly to avoid the crystallization of the sulfide glasses [15].
As mentioned, SEs should have good mechanical properties, especially moderate elasticity (Young’s modulus), since they need to adjust their form with the volume change of electrodes during charging and discharging [16]. However, having a low Young’s modulus (E’) is also not enough. The material must show good strength at the same time in order to resist dendrite formation. It has been reported that, for a dendrite-free deposition, the shear modulus (G’) of an SE should be at least twice that of lithium metal (G Li = 3.4 GPa) [17][18].
Polymer electrolytes (PEs) have many particularly interesting characteristics. They are flexible (E’
PEO
= 70 MPa) [18], light, and their thickness can be controlled in the order of ten micrometers by different preparation techniques such as extrusion or pressing. The most studied PE for all-solid batteries is polyethylene oxide (PEO) coupled with a lithium salt [19]. Their conductivities lie around 10 −4
S cm
−1
depending on the lithium salt used [8]. Gel PEs are prepared with a low crystalline polymer such as poly(vinylidene fluoride)-co-hexafluoropropylene (PVdF-HFP) and an organic liquid electrolyte such as LiPF6 in EC-DMC) in the polymer matrix. Despite their good ionic conductivities (up to 6 × 10 −3
S cm
−1
) [8], they suffer from lower mechanical strength (G PEO
= 26.2 MPa) and electrode compatibilities [19].
As it can be seen, each family of SEs has its advantages and disadvantages, and each of them should be considered depending on the ASSB applications. Typically, organic liquid electrolytes for commercialized Li-Ion batteries show conductivities of about 2 × 10
−2
S cm
−1
at room temperature. Assuming a porosity of typical polyolefin separators of about 40% [20], a resulting conductivity of about 5 × 10 −3
S cm
−1
remains as a rule of thumb. SEs have to compete at least with these values, also taking into account that typical polyolefin separators have thicknesses in the range of 20–25 µm [20], which are difficult to realize with Li metal solid-state ion conductions in practice. This is a strong reason for looking at sulfide-based solid electrolytes rather than oxide-based ones since the latter do usually not exceed 5 × 10 −4
S cm
−1
even in the bulk phase. Besides, oxide-based solid Li-Ion conductors are not the focus of the present paper since sulfide- and/or phosphide-based candidates are much more promising to successfully bridge the gap to their liquid competitors. Solid polymer electrolytes (SPEs) will also be discussed due to their higher stability against Li metal anode.
2. Solid Electrolytes
2.1. Inorganic Sulfide Electrolytes
The Thio-LISICON family, a sulfur derivative of LISICON, was initially introduced by Kanno et al.
[21]. They have replaced O
2− ions with S
2− ions, which increased the mobility of Li
+ ions due to their larger size and more polarizable character. It has been shown that this substitution allows for an increase in the ion conductivity by two orders of magnitude (e.g., 2 × 10
−6 S cm
−1 for a Li
3.6Si
0.6P
0.4O
4 and 10
−4 S cm
−1 for Li
2S-SiS
2-LiI)
[21].
In recent years, one electrolyte and its derivatives have increasingly emerged as the first suitable candidate. This is, for example, LGPS with the empirical formula Li
10GeP
2S
12. Here, even conductivities in the particle of more than 10
−2 S cm
−1 are reported
[21][22][21,22]; a practical achievement was ~10
−3 S cm
−1 in a suitably prepared whole electrode
[23][24][23,24]. It is expected that a powder of this electrolyte can be compacted into films with an inert auxiliary binder so that satisfactory contact of the individual crystals with a sufficient density can be achieved in order to suppress dendrites.
Moreover, research results indicate that Li10GeP2S12 is compatible with NMC (8:1:1) cathodes, LiNi0.8Co0.1Mn0.1O2, which is considered an up-and-coming candidate for Li-ion cells. Such a cathode would then contain the solid electrolyte in the gaps of the densest NMC sphere (cubic close pack), for example, about 10–25 µm thick NCM cathode particles. The solid electrolyte must have particle sizes of at least one order of magnitude less for this to occur, an important design feature for the solid electrolyte powder. Thus, particles in the nanometer range are required.
However, there are still some challenges concerning their stability with the electrode interface and affordability. Regarding the electrochemical stability of LGPS, it is relatively limited due to the reduction of Ge
4+ in Ge
0 below 1.0 V vs. Li, and the oxidation of S
2− above 2.8 V vs. Li. Its strong reactivity at the interface with the Li metal forms products, which lead to the decomposition of LGPS, producing undesirable interphase products composed of Li
3P, Li
2S, and Li-Ge alloy
[25]. Actually, the decomposition products Li
3P and Li
2S are expected to be sufficiently good solid ionic conductors to enable ionic conductivity, but Li
xGe alloy causes unfavorable repeated volume surges during the formation and delithiation of the alloy. That is why the stability to so-called 4 and even 5 V electrodes of sulfidic compounds needs further study.
In order to overcome these stability issues, several surface modification methods have been proposed
[15][24][25][15,24,25]. For example, Zhang et al. proposed the use of a protective layer between LGPS SE and the Li metal anode, namely LiH
2PO
4 [24]. They have coated the Li metal with different concentrations of H
3PO
4 tetrahydrofuran and created an in-situ LiH
2PO
4 surface. In this way, they achieved 113.7 mAh g
−1 of a discharge capacity up to 500 cycles (at 0.1C with 80 wt% H
3PO
4). Nevertheless, the surface treatments increased the total impedance of the Li anode (more than 2.5 times). Additionally, it has been reported that long-term cycling causes a volume changing effect.
Another proposition to increase the stability of the LGPS solid electrolyte towards the Li metal anode is to prepare the cells with double-layer (bilayer) electrolytes
[26][27][28][29][30][26,27,28,29,30]. Within this approach, a Li-compatible SE-layer (mostly based on Li
2S-P
2S
5) is inserted between LGPS and Li metal. The cell characteristics prepared with bilayer electrolytes are summarized in .
Table 1. Electrochemical properties of Li10GeP2 S12 (LGPS) mono and bilayer solid electrolytes with various cell types.
As can be seen in , the cells prepared with the bilayer InSEs showed better cycle and capacity performance compared to that of single-layer LGPS electrolyte. Relatively higher cycle numbers for the electrolytes prepared with Li
2S-P
2S
5 and Li
9.6P
3S
12 can be attributed to their dendrite-resistant glass-ceramic structures (higher Shear Modulus). In case of electrolytes prepared with Li
2S-P
2O
5, the cycle number is significantly higher than that of any other cells. Many studies reported that electrolytes with oxygen atoms in their structure showed remarkable cycle stability because oxygen ions are able to suppress the side reactions between sulfide electrolyte and lithium metal
[27][28][27,28].
Another decisive factor for the commercialization of LGPS electrolytes is their price. Li
2S, P
2S
5, GeS
2 are the starting materials and the synthesis of LGPS solid electrolyte takes place between 500 and 600 °C in an inert atmosphere
[31]. As germanium is an expensive element (1300 US $/kg)
[32], the substitution of the critical element Ge has been achieved by isovalent elements such as Si or Sn (starting materials SiS
2 and SnS
2), which are much more affordable while only moderately affecting only moderately the conductivity performances
[33]. A detailed price analysis is provided in
Section 3.
As mentioned in the introduction, another interesting sulfide electrolyte is based on argyrodite-type compounds Li
6PS
5X (X = Cl, Br, I), shortly named as LPS-X solid electrolyte. Their preparation generally includes mechanical milling followed by heating at 500–600 °C in order to obtain the argyrodite phase. The starting materials are Li
2S, P
2S
5, and LiX
[34]. Deiseroth et al. presented a detailed study on the synthesis of different LPS-X electrolytes and examined their crystal structures
[34]. The highest Li-ion mobility was observed for Li
6PS
5Br electrolyte
[14][34][14,34]. On the other hand, the compounds prepared with LiI showed a conductivity of only 4.6 × 10
−7 S cm
−1 [14]. Even though Li
6PS
5I has an almost identical lattice structure compared to its relatives, it has not been clearly understood so far where the difference in the ionic conductivity values comes from. Recently, Hanghofer et al. studied substitutional disorder effects of LPS-X electrolytes by broadband impedance spectroscopy and
7Li NMR relaxation measurements
[35]. They found that the anion disorder in LPS-Cl and LPS-Br supports faster Li-ion transport. Nazar et al. also showed that the same kind of phenomena can be observed in mixed halide argyrodites LPS-X (X = Cl
0.75Br
0.25, Cl
0.5Br
0.5, and Cl
0.25Br
0.75)
[36]. For example, the electrolytes containing Cl
0.75Br
0.25 and Cl
0.5Br
0.5 showed an ionic conductivity of 3.2 × 10
−3 and 3.9 × 10
−3 S cm
−1, respectively, where this value was obtained as 3.4 × 10
3 S cm
−1 for an electrolyte containing Cl
0.25Br
0.75. This behavior was explained by the low concentrations of Br, which resulted in more conductive pathways in the material structure. In contrast, excessive concentrations effectively blocked these channels and reduced the degree of percolation and ionic conductivity. Another study, which was performed by Viallet et. al, also showed that the conductivity of LPS-Cl electrolyte can be increased by optimization of the milling time
[37]. They increased the ionic conductivity value from 2 × 10
−4 S cm
−1 to 1.33 × 10
−3 S cm
−1 by increasing the milling time from 1 to 10 h.
The cells prepared with a LGPS solid electrolyte show the same dendrite problems as cells prepared with argyrodite-type electrolytes. Although the LPS-Cl compound shows good stability to the phenomenon of lithium dissolution and deposition, it has been shown that contact losses at the interface with lithium occur when high currents are applied. High currents result in the formation of dendrites and the appearance of other degradation products (Li
2S, P
2S
x and polysulfides)
[38][39][38,39]. One of the solutions to overcome this problem is the use of LiCoO
2/L
i4Ti
5O
12 cells
[40]. Nevertheless, a breakthrough battery design has been presented by researchers from the Samsung Advanced Institute of Technology (SAIT) and the Samsung R&D Institute Japan (SRJ)
[41]. They used a silver-carbon (Ag-C) composite layer at the anode, and there was no need to handle the Li metal sheet during the cell assembly process. The cathode acted as the sole source of the lithium and while charging, Li metal deposited on the stainless steel current collector. These cells showed an energy density greater than 900 Wh l
−1 and long cycle life (1000 times). However, taking into account that silver is an expensive element and the change of Ag-distribution in the Ag-C nanocomposite layer (after 100 cycles) make the commercialization of this cell difficult. The cost/performance ratio continues to remain a challenge.
2.2. Solid Polymer Electrolytes
As the solid polymer electrolytes (SPEs) have a softer nature compared to the InSEs, they have more flexibility, and as a result, they offer better processability [42]. Moreover, this flexibility allows them to respond to volume changes within the cell due to lithiation. Cheng et al. reported that for the LiFePO 4
(LFP) cathode, the volume change is about 6.6% [43], and as the SE is directly neighboring the cathode, its flexible nature prevents the electrolyte from irreversible deformations.
The most studied polymer as a SPE is polyethylene oxide (PEO) coupled with the lithium salt, LiTFSI [44][45][46][47][48][49][50][51][52][53]. However, sole PEO-based electrolytes exhibit relatively low ionic conductivity (ca. 10
The most studied polymer as a SPE is polyethylene oxide (PEO) coupled with the lithium salt, LiTFSI [44,45,46,47,48,49,50,51,52,53]. However, sole PEO-based electrolytes exhibit relatively low ionic conductivity (ca. 10 −7
–10
−5
S cm
−1) at room temperature. In order to increase their ionic conductivity, they are prepared as composite electrolytes [54][55][56][57][58][59]. Composite SPEs contain PEO in their composition because PEO-based electrolytes are relatively more stable against Li metal and PEO can dissolve the conductive lithium salt easier due to its polar ether groups.
) at room temperature. In order to increase their ionic conductivity, they are prepared as composite electrolytes [54,55,56,57,58,59]. Composite SPEs contain PEO in their composition because PEO-based electrolytes are relatively more stable against Li metal and PEO can dissolve the conductive lithium salt easier due to its polar ether groups.
PEO-based composite SPEs are mainly used with a low-voltage LFP cathode [51][52][53][54][55][56] because PEO shows low stability at potentials greater than 3.8 V (vs. Li+/Li) and an oxidative decomposition occurs [60][61]. This limitation prevents it from using the most popular cathode materials, such as NCA or NMC, which require potentials up to 4.1–4.2 V. Nevertheless, Wakayama et al. have proposed a three-dimensional structure and built cells with LiCoO
PEO-based composite SPEs are mainly used with a low-voltage LFP cathode [51,52,53,54,55,56] because PEO shows low stability at potentials greater than 3.8 V (vs. Li+/Li) and an oxidative decomposition occurs [60,61]. This limitation prevents it from using the most popular cathode materials, such as NCA or NMC, which require potentials up to 4.1–4.2 V. Nevertheless, Wakayama et al. have proposed a three-dimensional structure and built cells with LiCoO 2
(LCO) composed of Li
7
La
3
Zr
2
O
12
(LLZ) [57]. Their cells were suitable for cycling between 3.0 and 4.2 V (up to 20 cycles). summarizes some results of ASSB cell results obtained with PEO-based composite electrolytes and a Li metal anode.
Table 2. Cell characteristics of all-solid state batteries (ASSB) cells prepared with composite polyethylene oxide (PEO) solid electrolyte.
In order to prepare a good conductive SPE, inorganic materials are introduced into the PEO matrix. Some oxides such as Al
In order to prepare a good conductive SPE, inorganic materials are introduced into the PEO matrix. Some oxides such as Al
2
O
3
, TiO
2
, ZnAl
2
O
4
, CeO, and SiO
2
in the PEO matrix increase not only the ionic conductivity values but also the electrochemical stability and the mechanical strength [54]. Among them, PEO in combination with Al 2
O
3
and SiO
2
particles is considered the most promising electrolyte [55].
The addition of lithium garnet (i.e., Li
6.75
La
3
Zr
1.75
Ta
0.25
O
12
, LLZTO) to the PEO matrix also results in higher conductivity values. Moreover, due to their stability at higher potentials, it is possible to use them with high-voltage cathodes [62]. Zhang et al. presented a study by preparing a PEO-based composite SPE without using a Li salt [62]. They have reported that the addition of nanosize LLZTO particles (with D 50
= 43nm) shifted the oxidation potential of PEO from 4.0 to 5.0 V. This behavior can be attributed to the fact that solid-state inorganic polymer ion-conducting composites, such as LLZO-PEO often show extended stability windows. However, chemical reactions are not defined electronic switches such as “on” and “off”. Thus, more precisely, the visible onset of a chemical decomposition reaction as a function of the voltage is shifted. The reason can simply be seen in the effective reactive surface. By its nature, nano-scaled LLZO yields a very high surface with a strong additional tendency to produce LiOH and Li
2
CO
3
, which are quite stable compounds. These likely cover, as LLZTO nanopowder, huge parts of the PEO and produce a shielding and coating effect, which may mislead someone into believing PEO has become more stable.
The same study also showed that dendrites coming from the Li anode were suppressed. No dendrite formation was observed after more than 700 h of cycling since the accumulation of lithium was hindered in the insulating polymer matrix. The cells containing the same composite membranes but prepared with conducting LiTFSI salt showed the dendrite formation after 25 h of cycling. The LiTFSI-free SPE also showed a high ionic conductivity (2.1 × 10
−4
S cm
−1
) at 30 °C.
As shown in
, LFP cathode can also be prepared with indium tin oxide (In
2
O
5
Sn) to increase the cell’s electronic conductivity by increasing the surface quality between the electrode and SPE.