
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rafael Ballan | + 3844 word(s) | 3844 | 2021-01-12 10:25:30 | | | |
| 2 | Lily Guo | + 99 word(s) | 3943 | 2021-01-31 15:01:01 | | |
Video Upload Options
Inflammatory bowel disease (IBD) is a chronic inflammation of the gastrointestinal tract (GIT), including Crohn’s disease (CD) and ulcerative colitis (UC), which differ in the location and lesion extensions. Both diseases are associated with microbiota dysbiosis, with a reduced population of butyrate-producing species, abnormal inflammatory response, and micronutrient deficiency (e.g., vitamin D hypovitaminosis). Vitamin D (VitD) is involved in immune cell differentiation, gut microbiota modulation, gene transcription, and barrier integrity. Vitamin D receptor (VDR) regulates the biological actions of the active VitD (1α,25-dihydroxyvitamin D3), and is involved in the genetic, environmental, immune, and microbial aspects of IBD. VitD deficiency is correlated with disease activity and its administration targeting a concentration of 30 ng/mL may have the potential to reduce disease activity. Moreover, VDR regulates functions of T cells and Paneth cells and modulates release of antimicrobial peptides in gut microbiota-host interactions. Meanwhile, beneficial microbial metabolites, e.g., butyrate, upregulate the VDR signaling.
1. Introduction
Inflammatory bowel disease (IBD) is defined as a chronic inflammation of the gastrointestinal tract (GIT) that affects more than six million people worldwide[1][2]. The most common types are Crohn’s disease (CD) and ulcerative colitis (UC) [1]. CD is a segmental, asymmetrical, and transmural inflammation that may affect the whole GIT, but is more frequently observed in the ileum and colon. UC is related to mucosal inflammation from the rectum to the proximal colon[1][3][4]. In fact, IBD has a great impact on the physical, psychological, and social aspects of life, and depression and anxiety are usually increased in these patients. Thus, the management of IBD is of utmost importance for the quality of life of the patients[2].
Several factors are associated with the risk of IBD development, such as country development degree, smoking, sex, age, use of antibiotics or oral contraceptives, lower serum levels of vitamin D, and diet[2][5]. IBD may be triggered by an abnormal immune response to gut commensal bacteria in genetically predisposed individuals and is associated with an impaired intestinal barrier function and a less diverse gut microbiota composition[6][7][8].
The gut microbiota is comprised of more than 2000 metagenomic species (MGS) of bacteria distributed throughout the GIT[9]. The population density increases from the stomach to the colon, reaching 1010–1012 CFU (colony forming units)/mL at the end of the large intestine. Innumerous functions are attributed to the gut microbiota, like metabolism of nutrients from the diet, fiber fermentation, SCFA (short-chain fatty acids) production, vitamin production, barrier function and tight junctions regulation, antimicrobial compounds secretion, immune regulatory, among others [9][10]. Microbial metabolites released by the gut microbiota circulate and may affect the proper function of other organs and systems of the body. Therefore, strategies that address the gut microbiota modulation, improvement of the gut barrier function, and decrease in the intestinal mucosa inflammation are of the greatest significance for IBD treatment [11][12].
Micronutrient deficiencies are often observed in IBD patients, and mostly low levels of vitamin D and zinc, even during disease remission[13]. Observational studies have reported that low levels of vitamin D are directly associated with increased disease activity, mucosal inflammation, clinical relapse, and quality of life. Thus, vitamin D deficiency might be both, the cause, and a consequence of IBD[13][14]. In fact, chronic diarrhea, nutrients malabsorption, low exposure to sunlight, and reduced consumption of vitamin D-fortified foods, like dairy products, are frequent in IBD patients, which may lead to vitamin D deficiency[15].
In this review, we explore vitamin D deficiency and gut dysbiosis in IBD, and the potential use of vitamin D in the management of the disease. In addition, we discuss the epigenetic factors and probiotics involved in IBD and vitamin D/VDR mechanisms.
2. Vitamin D Critical Role in IBD
2.1. Mechanisms of Action of Vitamin D
Vitamin D is a fat-soluble vitamin that can be found in two different chemical structures: cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2). It can be obtained either by exposure of the skin to UVB (ultraviolet B) rays from the sun, when the 7-dehydrocholesterol, present in the skin, is converted to cholecalciferol, or by the consumption of some fatty fishes, sun-exposed mushrooms, fortified foods—mostly dairy products, or even by supplements. Vitamin D is transported to the liver by the circulation and transformed into 25(OH)D (25-hydroxyvitamin D), the main circulation form and vitamin D status marker, by the enzyme 25-hydroxylase (CYP2R1). Nonetheless, the 25-hydroxyvitamin D should have another hydroxylation in the kidneys by the enzyme 1-α-hydroxylase (CYP27B1), where it is converted to calcitriol or 1,25-(OH)2D (1,25-dihydroxyvitamin D), the active form of the vitamin[16] (Figure 1).
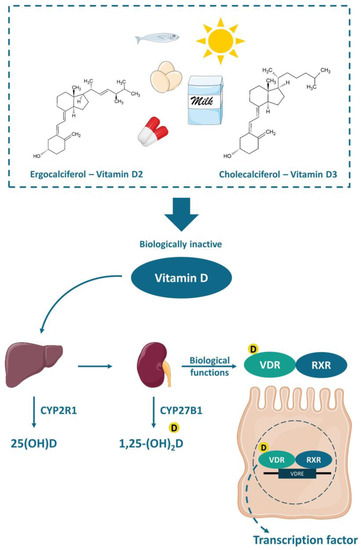
Figure 1. Chemical structure and activation of vitamin D. The vitamin D obtained by the exposure of skin to sunlight or consumed in food or supplements is transported to the liver and converted to 25(OH)D (25-hydroxyvitamin D) by the enzyme 25-hydroxylase (CYP2R1). Thereafter, a second hydroxylation occurs in the kidneys by the enzyme 1-α-hydroxylase (CYP27B1) generating the active vitamin D (1,25-(OH)2D or 1,25-dihydroxyvitamin D), which biological functions are mediated by the VDR (vitamin D receptor). The VDR bounded to 1,25-(OH)2D forms a heterodimer with the retinoic acid receptor (RXR), which in turn attaches to the vitamin D-response element (VDRE) acting as a nuclear transcription regulator.
The functions of calcitriol in the body are mediated by the nuclear receptor VDR. VDR is expressed in various tissues (e.g., skin, parathyroid gland, adipocyte, small intestines, and colon). The VDR bounded to 1,25-(OH)2D forms a heterodimer with the retinoic acid receptor (RXR), which migrates to the cell nucleus and binds to the vitamin D-response element (VDRE) in the promoter regions of target genes, acting as a nuclear transcription regulator[16][17][18](Figure 1). The VDRE is found in many genes, explaining the mechanisms associated with vitamin D, like autophagy[19], cell proliferation [20], intestinal barrier function [21][22], gut microbiota modulation[19][23][24], and immune functions[25][26], besides the most well-known mechanism, regarding calcium homeostasis and bone health [17][18][23].
Vitamin D immunomodulatory effects are directly related to antigen presenter cells (e.g., macrophages and dendritic cells) and T-cells functions. It seems that 1,25-(OH)2D modulates the T-cell differentiation, shifting from a pro-inflammatory Th1 immune response to an anti-inflammatory Th2 immune response, increasing the secretion of IL-4 while decreasing the secretion of IL-2 and IFN-γ. Moreover, 1,25-(OH)2D may inhibit dendritic cell differentiation and IL-12 production while increasing IL-10. Additionally, the lack of 1,25-(OH)2D harms regulatory T-cells (Tregs) differentiation and weakens its functions, which may trigger autoimmune diseases[27][28][29].
There is no consensus about the ideal circulating level of vitamin D. According to the Institute of Medicine (IOM), for the majority of the population, a minimum 25(OH)D serum level of 20 ng/mL (50 nmol/L) is considered enough, in case of a minimum sun exposure. Meanwhile, the risk of vitamin D deficiency is considered when the 25(OH)D serum level is below 12 ng/mL (30 nmol/L)[30]. Nevertheless, the Clinical Practice Guideline from the Endocrine Society defined vitamin D deficiency as serum level of 25(OH)D below 20 ng/mL (50 nmol/L) and values between 21–29 ng/mL (525–725 nmol/L) are considered as vitamin D insufficiency[31]. These thresholds of vitamin D serum levels were established for bone health. However, it is known that vitamin D deficiency may also be related to certain types of cancer, cardiovascular diseases and hypertension, type 2 diabetes and metabolic syndrome, autoimmune diseases (e.g., type 1 diabetes, rheumatoid arthritis, IBD, CD, systemic lupus erythematosus, and multiple sclerosis), and infectious diseases (e.g., tuberculosis and upper respiratory infections), autism, depression, and others[16][29][30][31]. A recent review has summarized 130 studies and demonstrated an inverse association between vitamin D and the development of several autoimmune diseases, such as CD, UC, SLE, thyrotoxicosis, type 1 diabetes, iridocyclitis, psoriasis vulgaris, seropositive RA, polymyalgia rheumatic, and MS. These studies support that vitamin D plays an important role on different aspects of the immune system. Furthermore, it is important to point out that the exposure to sunlight is the most effective natural source of vitamin D. However, people usually avoid sunlight exposure or use sunscreen due to skin cancer risk and it is difficult to reach the minimum required through the diet, thus supplementation is often necessary [16][30][31].
2.2. Implications of Vitamin D Deficiency in Inflammatory Bowel Diseases
Vitamin D deficiency in IBD patients has widely been discussed in numerous studies. It is common for patients with IBD to self-impose dietary restrictions, which is generally associated with insufficient macro and micronutrients in the diet[32]. One study compared patients with inactive or average CD with healthy controls. An inadequate nutrient intake due to the exclusion of food groups, such as milk, vegetables, and grains in CD group was observed[33]. More than a third of the individuals with IBD had BMI (body mass index) above 25, showing malnutrition accompanied by obesity, which may be due to physical inactivity or treatment with corticosteroids. The main micronutrient deficiencies observed in patients with IBD are zinc, iron, vitamin B12, and vitamin D, contributing to a critical condition and influencing on well-being[34][35].
In the meta-analysis conducted by Gubatan et al., the relationship between low levels of vitamin D and the risks of clinically active disease, mucosal inflammation, clinical relapse, and low quality of life scores among 8316 IBD patients from observational studies was evaluated. Low levels of 25(OH)D were significantly associated with an increase in the clinically active disease [UC (pooled OR 1.47, 95% CI 1.03–2.09, p = 0.03, I2 = 0%); CD (pooled OR 1.66, 95% CI 1.36–2.02, p < 0.00001, I2 = 0%)] and clinical relapse [UC (pooled OR 1.20, 95% CI 1.01–1.43, p = 0.04, I2 = 0%); CD (pooled OR 1.35, 95% CI 1.14–1.59, p = 0.0004, I2 = 0%)]. Meanwhile, low vitamin D levels were associated with increased mucosal inflammation and low quality of life scores only in CD patients. In fact, mucosal inflammation may lead to malabsorption of vitamin D in CD, thus low levels of vitamin D could be considered as an inflammation biomarker for CD. Accordingly, MacMaster et al. observed that around 30% of 93 IBD patients in remission presented vitamin D deficiency[13]. Together, the use of standard medications may affect the absorption and use of micronutrients. Sulfasalazine, for example, is a folic acid antagonist, which may lead to anemia when used for a long period. Glucocorticoids decrease the absorption and use of calcium, zinc, and phosphorus and impair vitamin D metabolism[36].
According to an integrative review conducted by Rocha et al., malnutrition is associated to hospitalization of patients affected by the disease. Moreover, nutritional status may influence hospitalization in IBD, although no comparison with adequate nutritional status was evaluated[35]. Low or insufficient levels of vitamin D have already been linked to an increased need for hospitalization and surgery in IBD, when compared to normal serum levels[37][38]. This highlights the importance of maintaining levels considered as adequate for vitamin D, since its anti-inflammatory effect is very well studied, and these patients can benefit their well-being.
Supplementation of vitamin D in IBD patients is challenging due to nutrients malabsorption issues, and higher doses are often necessary to achieve the recommended circulating level (above 20 ng/mL, according to IOM). Nevertheless, it seemed to be a promising complementary treatment that may improve inflammation markers, such as high-sensitivity C-reactive protein (hs-CRP) and erythrocyte sedimentation rate (ESR), suppressing the Th1 immune response, while reduced clinical disease activity index[14][39][40][41][42][43][44][45].
Despite these challenges, Myint et al. published a guide for clinical practice, as a standard of care, aiming to achieve a concentration of 30 ng/mL of 25(OH)D in patients with IBD, using the following protocol: administration of 50,000 IU/week of ergocalciferol or 2000–4000 IU/day of cholecalciferol (2000 IU/day for 25[OH]D < 30 ng/mL or 4000 IU/day for 25[OH]D < 20 ng/mL), recheck after 8–12 weeks, and if the level of 30 ng/mL is reached, provide maintenance dose between 1000–2000 IU/day of cholecalciferol for the few next months and its discontinuation when the disease is quiescent. The authors explain that although it is not yet known whether vitamin D positively influences disease activity, if it helps, reaching the higher 25(OH)D concentration would have a positive impact, otherwise, reaching moderately high concentrations of 25(OH)D would not be as harmful as vitamin D deficiency would be. It is noteworthy that the authors highlight the methodological limitations and heterogeneity of the observational studies available so far, in addition to the high variability of the strength of association between vitamin D levels and IBD activity, which could be influenced by factors such as genetics, disease subtype or environment, and therefore requires further investigation[15]. However, the status of VDR in IBD patients is not considered in regular treatments. If patients with IBD have genetic variations of VDR or dysfunction of VDR in its biological roles, the supplementation of vitamin D may not work as expected.
2.3. Vitamin D and Gut Microbiota Modulation
IBD is characterized by an abnormal immune response to gut commensal bacteria in genetically predisposed individuals, which presents less diverse and imbalanced gut microbiota composition, with less abundance of butyrate producer’s species [7][8][11]. As discussed earlier, vitamin D status is implicated in the severity of IBD while the supplementation seemed to improve the disease status. Nonetheless, it has been suggested that this protective effect is directly related to the gut microbiota (Figure 2).
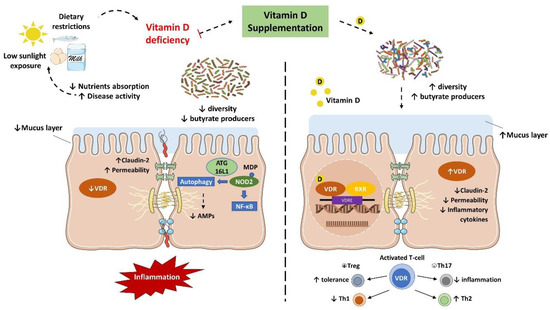
Figure 2. Vitamin D/VDR is involved in the genetic, environmental, immune, and microbial aspects of inflammatory bowel disease (IBD). Thus, the vitamin D supplement and activation of VDR could be considered as a multifunctional factor in IBD treatment.
Several in vivo studies have shown that vitamin D supplementation and VDR anti-inflammatory effect are directly associated with the gut microbiota. In fact, the downregulation of VDR or the inability to produce the active form of vitamin D were associated with a decrease in Lactobacillus in the gut microbiota, while Proteobacteria was increased. Meanwhile, butyrate may improve the VDR signaling, which together with the fact that prokaryotes do not express the VDR highlights that the gut microbiota plays a key role in the host intestinal immune response related to vitamin D mechanisms[46][47].
Interestingly, Du et al. reported that in inflammation conditions, the colonic VDR downregulation is associated with an increased expression of local CYP27B1, as a homeostatic protective effect to reduce inflammation and improve VDR signaling. Notably, the gut microbiota plays a critical role in this process. In fact, mice submitted to antibiotic treatment failed to upregulate the CYP27B1 while developed more severe colitis. Meanwhile, LPS treatment stimulated the upregulation of CYP27B1 as well, reinforcing the role of VDR in the barrier function and anti-inflammatory and anti-infection pathways [48].
VDR is known to negatively regulate bacterial-stimulated NF–κB activity[49], and this mechanism may also be an important contributor to intestinal homeostasis and host protection from bacterial invasion and infection. In an experimental colitis model, it was demonstrated that mice with the gut epithelium VDR deletion developed a more severe clinical colitis and worsened epithelial cell apoptosis, leading to an increased intestinal mucosa permeability[21], and promoted the Th1 and Th17 (T helper 17) mucosal response[50]. It suggests that the downregulation of the colonic VDR observed in patients with IBD may be related to impaired barrier functions in the intestine.
The VDR is also implicated in the anti-bacterial functions of Paneth cells. Lu et al. remarkably revealed that the downregulation of VDR and ATG16L1 genes were observed in small intestines tissue from CD patients, as well as a lower percentage and abnormal Paneth cells[51]. Meanwhile, mice with Paneth cells VDR knockout showed a reduction in the relative abundance of beneficial bacteria (e.g., Lactobacillus) in the gut microbiota and AMPs release, while were more prone to Salmonella infection and DSS-induced colitis[51]. These findings confirm that Paneth cell abnormalities result in a reduced bacterial clearance ability through AMPs, and together with a reduction in autophagy responses could explain the association of dysbiosis and Paneth cell abnormalities observed in individuals with IBD.
Furthermore, higher levels of vitamin D are related to increased serum cathelicidin and reduced inflammation in UC patients. In the meantime, vitamin D may improve the cathelicidin antimicrobial activity against E. coli in vitro while showed a protective effect to induced colitis in vivo[52].
There is a lack of human studies evaluating the anti-inflammatory effect of vitamin D associated with its potential in gut microbiota modulation as an adjuvant treatment for IBD management, some of them will be discussed hereafter and are detailed in Table 1. Still, studies with healthy populations have shown promising outcomes about vitamin D and VDR functions in modulating the gut microbiota and improving the immune response[25][26][53][54].
Table 1. Summary of published human studies outcomes evaluating vitamin D3 and its modulation of microbiota in inflammatory bowel disease.
| Soy Products | Treatments (Bacterial Fermentation) | Health Benefits | References |
|---|---|---|---|
| Starter culture | |||
| Fermented soybean | Bacillus subtilis SHZ, B. subtilis MTCC 5480 | Antioxidant | [62,63] |
| Douchi qu | B. subtilis natto, B. subtilis B1 | ACE inhibitory | [64] |
| Cheonggukjang | B. licheniformis SCD 111067P | Antihypertensive, Antidiabetic | [65,66] |
| Soy Products | Treatments (Fungi Fermentation) | Health Benefits | References |
| Starter culture | |||
| Douchi qu | Aspergillus oryzae, Mucor wutungkiao | ACE inhibitory | [64] |
| Douchi | Aspergillus oryzae, Aspergillus egyptiacus | Antioxidant, Antihypertensive | [67,68] |
| Meju | Aspergillus oryzae | Antimicrobial | [69] |
| Soy Products | Heat Treatments | Effects | References |
| Soymilk | Microwave-assisted extraction | Increase protein content, viscosity, protein solubility, and digestibility | [70] |
| Raw soybean | Gamma irradiation | Increase total phenolic content, Decrease tannins and trypsin inhibitors | [71] |
| Soybeans | Infrared treatment | Inactivated both trypsin inhibitors and lipoxygenase | [72] |
In a pioneering prospective pilot study, Garg et al., 2018 assessed the impacts of vitamin D replacement (40,000 IU, once weekly) over 8 weeks in 25 vitamin D-deficient patients with and without UC in the intestinal microbiota and inflammatory markers. Participants were divided into three groups, active or inactive UC and non-IBD controls. At the end of the intervention, all groups showed an increase in serum concentrations of 25(OH)D, without significant differences. Although no changes in alpha diversity were observed before and after vitamin D replacement in all groups, a subtle reduction in the mucolytic bacteria Ruminococcus gnavus was observed. Only participants with UC had an increase in the abundance of Enterobacteriaceae, with no significant change in E. coli and invasive Fusobacterium nucleatum. Despite these findings, the UC group had improved inflammatory markers, such as fecal calprotectin, albumin, and platelet count, together with disease activity. Although a reduction in Enterobacteriaceae was expected, this increase does not imply a worsening in overall profile of microbiota, since this family of bacteria comprises other not harmful and commensal bacteria. These are very interesting results despite the small sample size of the study[39].
Schaffler et al. reported that vitamin D3 supplementation (total of 300,000 IU after 4 weeks) altered the gut microbiota composition only in remission CD patients (n = 7), and no changes were noted in the healthy controls with vitamin D deficiency (n = 10). Throughout 4 weeks, an increase in the abundance of beneficial bacteria like Alistipes, Parabacteroides, Roseburia, and Faecalibacterium was observed, even though it was transient. The authors suggested that 4 weeks might have been a too short intervention period to detect a greater change. However, these results suggest that vitamin D administration has potential as an adjuvant therapy for CD patients[55]. It is noteworthy that the reduced abundance of the Faecalibacterium genus is commonly associated with both diseases, UC and CD. Its characteristic of producing butyrate has already been shown to be a way to reduce inflammation and promote a balance between Th17 and Treg[56].
In an interesting cohort study, the possible connection between the seasonality of serum vitamin D levels and changes in the microbiome was evaluated. The target population was composed by adults (n = 87) with IBD (CD or UC), who lived in regions far from the equator, and both the intestinal mucosa and the fecal samples microbiome were evaluated. After confirming the differences in the concentrations of 25(OH)D, which were higher in periods with higher sun exposure (summer/autumn), some changes in the microbial composition were also observed. In the summer/autumn period, an increase in the abundance of Pediococcus spp., Clostridium spp., and Escherichia/Shigella spp. was observed. In contrast, inflammation-related bacterial genera such as Eggerthella lenta, Fusobacterium spp., Helicobacter spp., and Faecalibacterium prausnitzii showed lower relative abundance. Unlike other studies, low levels of vitamin D were associated with a more balanced composition of the microbiome. It should be noted that it was not a randomized controlled trial (RTC), but vitamin D levels were correlated with changes in the microbiome in individuals with IBD[57].
There is still limited evidence of vitamin D3 in modulating gut microbiota in human IBD. The study designs are heterogeneous together with a substantially small number of patients enrolled in human trials, resulting in inconsistent and controversial outcomes. It is difficult to state an effective dose so far. Meanwhile, it has been suggested that higher doses of vitamin D supplementation may increase the secretion of antimicrobial peptides, contributing to the gut microbiota modulation. Further human clinical trials, with appropriate intervention design, evaluating the impact of vitamin D on the gut microbiota of IBD patients are needed to better understand the mechanisms involved and support the indication of use as a complementary treatment. It is also needed to consider the influence of gender, age, ethnicity, genetics, metabolic disorders (e.g., obesity, diabetes, and NAFLD), and IBD subtype.
3. Conclusions and Future Directions
Vitamin D/VDR deficiency could be considered as a multifunctional susceptibility factor and is critical in the development and treatment of IBD. According to the guide to clinical practice, the administration of 50,000 IU/week of ergocalciferol or 2000–4000 IU/day of cholecalciferol to patients with IBD aiming to reach levels of 30 ng/mL that could potentially have a positive impact on the disease activity. Vitamin D administration leads to a shift of the intestinal bacterial composition in CD patients, but not in healthy controls. Meanwhile, beneficial microbial metabolites, such as butyrate, may have the potential to regulate the VDR functions.
So far, vitamin D supplementation contributes to the reduction of inflammation in individuals with IBD and can promote changes in the human microbiota. However, studies reported have several limitations, such as the small sample, the unmatched methodology, or even the lack of definition of what would be the composition of a healthy microbiota. Surely, VDR is a crucial factor for gut microbiota homeostasis, having a great impact on the metabolome profile as well. In addition, its proper functions influence several genes associated with inflammation, barrier function, cancer, autophagy, among others. The downregulation of VDR is related to an upregulation of intestinal CYP27B as a homeostatic anti-inflammatory response, while the VDR function is implicated in regulatory T cells and Paneth cells differentiation, as well as in antimicrobial peptides release, and the gut microbiota seemed to play a critical role in these processes. Therefore, more studies to assess the microbiota and microbial metabolites in IBD are needed.
Finally, a different profile of miRNA is expressed in CD, UC, or healthy control individuals and epigenetics markers revealed to be a highly sensitive, specific, and precise tool for IBD diagnosis, therefore a promising and less invasive alternative when compared with endoscopy and biopsies, which are employed nowadays. Moreover, vitamin D plays a role in IBD regulating transcription factors associated with barrier function and immune responses. The exact mechanisms are not well understood and more studies are needed to explore the therapeutic potential of vitamin D/VDR in the gut microbiota modulation and anti-inflammatory effects in IBD at the metabolic, immunological, and epigenetic levels.
VDR is identified as the first human gene to shape the gut microbiome[23]. However, the variations of the Vdr gene in human IBD are still unknown. In the future study, we need considering the status of VDR in the patients of IBD, in addition to the serum 25(OH) vitamin D concentration. We need well-designed therapeutic studies to examine whether enhanced vitamin D will restore functions of VDR and microbiome in inhibiting chronic inflammation, as well as to test the appropriate dose by considering the influence of gender, age, ethnicity, genetics, and metabolic disorders in the IBD subtype.
References
- AGA Patient Information Section. Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2017, 15, A21, doi:10.1016/S1542-3565(17)30640-7.
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30, doi:10.1016/S2468-1253(19)30333-4.
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755, doi:10.1016/S0140-6736(16)31711-1.
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770, doi:10.1016/s0140-6736(16)32126-2.
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-analyses. Gastroenterology 2019, 157, 647–659, doi:10.1053/j.gastro.2019.04.016.
- Levine, A.; Boneh, R.S.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738, doi:10.1136/gutjnl-2017-315866.
- Ryan, F.J.; Ahern, A.M.; Fitzgerald, R.S.; Laserna-Mendieta, E.J.; Power, E.M.; Clooney, A.G.; O’Donoghue, K.W.; McMurdie, P.J.; Iwai, S.; Crits-Christoph, A.; et al. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat. Commun. 2020, 11, 1512, doi:10.1038/s41467-020-15342-5.
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511, doi:10.1038/s41579-019-0213-6.
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504, doi:10.1038/s41586-019-0965-1.
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493, doi:10.1007/s00018-018-2943-4.
- Stange, E.F.; Schroeder, B.O. Microbiota and mucosal defense in IBD: An update. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 963–976, doi:10.1080/17474124.2019.1671822.
- Salvucci, E. The human-microbiome superorganism and its modulation to restore health. Int. J. Food Sci. Nutr. 2019, 70, 781–795, doi:10.1080/09637486.2019.1580682.
- MacMaster, M.J.; Damianopoulou, S.; Thomson, C.; Talwar, D.; Stefanowicz, F.; Catchpole, A.; Gerasimidis, K.; Gaya, D.R. A prospective analysis of micronutrient status in quiescent inflammatory bowel disease. Clin. Nutr. 2020, 40, 327–331, 10.1016/j.clnu.2020.05.010, doi:10.1016/j.clnu.2020.05.010.
- Gubatan, J.; Chou, N.D.; Nielsen, O.H.; Moss, A.C. Systematic review with meta-analysis: Association of vitamin D status with clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol. Ther. 2019, 50, 1146–1158, doi:10.1111/apt.15506.
- Myint, A.; Sauk, J.S.; Limketkai, B.N. The role of vitamin D in inflammatory bowel disease: A guide for clinical practice. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 539–552, doi:10.1080/17474124.2020.1775580.
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165, doi:10.1007/s11154-017-9424-1.
- Bakke, D.; Chatterjee, I.; Agrawal, A.; Dai, Y.; Sun, J. Regulation of Microbiota by Vitamin D Receptor: A Nuclear Weapon in Metabolic Diseases. Nucl. Receptor. Res. 2018, 5, 101377, doi:10.11131/2018/101377.
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)(2)vitamin D(3): Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559, doi:10.1016/j.beem.2011.05.010.
- Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut 2015, 64, 1082–1094, doi:10.1136/gutjnl-2014-307436.
- Jin, D.; Zhang, Y.G.; Wu, S.; Lu, R.; Lin, Z.; Zheng, Y.; Chen, H.; Cs-Szabo, G.; Sun, J. Vitamin D receptor is a novel transcriptional regulator for Axin1. J. Steroid Biochem. Mol. Biol. 2017, 165, 430–437, doi:10.1016/j.jsbmb.2016.09.002.
- Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.; Claud, E.C.; Sun, J. Lack of Vitamin D Receptor Leads to Hyperfunction of Claudin-2 in Intestinal Inflammatory Responses. Inflamm. Bowel. Dis. 2019, 25, 97–110, doi:10.1093/ibd/izy292.
- Zhang, Y.G.; Wu, S.; Lu, R.; Zhou, D.; Zhou, J.; Carmeliet, G.; Petrof, E.; Claud, E.C.; Sun, J. Tight junction CLDN2 gene is a direct target of the vitamin D receptor. Sci. Rep. 2015, 5, 10642, doi:10.1038/srep10642.
- Wang, J.; Thingholm, L.B.; Skieceviciene, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.A.; Ruhlemann, M.C.; Szymczak, S.; et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016, 48, 1396–1406, doi:10.1038/ng.3695.
- Zhang, Y.G.; Lu, R.; Wu, S.; Chatterjee, I.; Zhou, D.; Xia, Y.; Sun, J. Vitamin D Receptor Protects Against Dysbiosis and Tumorigenesis via the JAK/STAT Pathway in Intestine. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 729–746, doi:10.1016/j.jcmgh.2020.05.010.
- Bashir, M.; Prietl, B.; Tauschmann, M.; Mautner, S.I.; Kump, P.K.; Treiber, G.; Wurm, P.; Gorkiewicz, G.; Hogenauer, C.; Pieber, T.R. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur. J. Nutr. 2016, 55, 1479–1489, doi:10.1007/s00394-015-0966-2.
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338, doi:10.1006/abbi.1999.1605.
- Cantorna, M.T. IBD: Vitamin D and IBD: Moving towards clinical trials. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 322–323, doi:10.1038/nrgastro.2016.72.
- Lim, W.C.; Hanauer, S.B.; Li, Y.C. Mechanisms of disease: Vitamin D and inflammatory bowel disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 308–315, doi:10.1038/ncpgasthep0215.
- Szodoray, P.; Nakken, B.; Gaal, J.; Jonsson, R.; Szegedi, A.; Zold, E.; Szegedi, G.; Brun, J.G.; Gesztelyi, R.; Zeher, M.; et al. The complex role of vitamin D in autoimmune diseases. Scand. J. Immunol. 2008, 68, 261–269, doi:10.1111/j.1365-3083.2008.02127.x.
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011; doi:10.17226/13050.
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930, doi:10.1210/jc.2011-0385.
- Lim, H.S.; Kim, S.K.; Hong, S.J. Food Elimination Diet and Nutritional Deficiency in Patients with Inflammatory Bowel Disease. Clin. Nutr. Res. 2018, 7, 48–55, doi:10.7762/cnr.2018.7.1.48.
- Guerreiro, C.S.; Cravo, M.; Costa, A.R.; Miranda, A.; Tavares, L.; Moura-Santos, P.; Vidal, P.M.; Leitao, C.N. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: A case-control study. Am. J. Gastroenterol. 2007, 102, 2551–2556, doi:10.1111/j.1572-0241.2007.01439.x.
- Massironi, S.; Rossi, R.E.; Cavalcoli, F.A.; Valle, S.D.; Fraquelli, M.; Conte, D. Nutritional deficiencies in inflammatory bowel disease: Therapeutic approaches. Clin. Nutr. 2013, 32, 904–910, doi:10.1016/j.clnu.2013.03.020.
- Rocha, R.; Sousa, U.H.; Reis, T.L.M.; Santana, G.O. Nutritional status as a predictor of hospitalization in inflammatory bowel disease: A review. World J. Gastrointest. Pharmacol. Ther. 2019, 10, 50–56, doi:10.4292/wjgpt.v10.i2.50.
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017, 8646495, doi:10.1155/2017/8646495.
- Ananthakrishnan, A.N.; Cagan, A.; Gainer, V.S.; Cai, T.; Cheng, S.C.; Savova, G.; Chen, P.; Szolovits, P.; Xia, Z.; De Jager, P.L.; et al. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm. Bowel. Dis. 2013, 19, 1921–1927, doi:10.1097/MIB.0b013e3182902ad9.
- Kabbani, T.A.; Koutroubakis, I.E.; Schoen, R.E.; Ramos-Rivers, C.; Shah, N.; Swoger, J.; Regueiro, M.; Barrie, A.; Schwartz, M.; Hashash, J.G.; et al. Association of Vitamin D Level With Clinical Status in Inflammatory Bowel Disease: A 5-Year Longitudinal Study. Am. J. Gastroenterol. 2016, 111, 712–719, doi:10.1038/ajg.2016.53.
- Garg, M.; Hendy, P.; Ding, J.N.; Shaw, S.; Hold, G.; Hart, A. The Effect of Vitamin D on Intestinal Inflammation and Faecal Microbiota in Patients with Ulcerative Colitis. J. Crohns Colitis 2018, 12, 963–972, doi:10.1093/ecco-jcc/jjy052.
- Garg, M.; Rosella, O.; Rosella, G.; Wu, Y.; Lubel, J.S.; Gibson, P.R. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin. Nutr. 2018, 37, 1375–1382, doi:10.1016/j.clnu.2017.06.011.
- Guzman-Prado, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B. Vitamin D Therapy in Adults With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel. Dis. 2020, 26, 1819–1830, doi:10.1093/ibd/izaa087.
- Mechie, N.C.; Mavropoulou, E.; Ellenrieder, V.; Petzold, G.; Kunsch, S.; Neesse, A.; Amanzada, A. Serum vitamin D but not zinc levels are associated with different disease activity status in patients with inflammatory bowel disease. Medicine 2019, 98, e15172, doi:10.1097/MD.0000000000015172.
- Mentella, M.C.; Scaldaferri, F.; Pizzoferrato, M.; Gasbarrini, A.; Miggiano, G.A.D. The Association of Disease Activity, BMI and Phase Angle with Vitamin D Deficiency in Patients with IBD. Nutrients 2019, 11, 2583, doi:10.3390/nu11112583.
- Sharifi, A.; Hosseinzadeh-Attar, M.J.; Vahedi, H.; Nedjat, S. A randomized controlled trial on the effect of vitamin D3 on inflammation and cathelicidin gene expression in ulcerative colitis patients. Saudi. J. Gastroenterol. 2016, 22, 316–323, doi:10.4103/1319-3767.187606.
- Sharifi, A.; Vahedi, H.; Nedjat, S.; Rafiei, H.; Hosseinzadeh-Attar, M.J. Effect of single-dose injection of vitamin D on immune cytokines in ulcerative colitis patients: A randomized placebo-controlled trial. APMIS 2019, 127, 681–687, doi:10.1111/apm.12982.
- Jin, D.; Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Dong, H.; Sun, J. Lack of Vitamin D Receptor Causes Dysbiosis and Changes the Functions of the Murine Intestinal Microbiome. Clin. Ther. 2015, 37, 996–1009, doi:10.1016/j.clinthera.2015.04.004.
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J. Nutr. 2013, 143, 1679–1686, doi:10.3945/jn.113.180794.
- Du, J.; Wei, X.; Ge, X.; Chen, Y.; Li, Y.C. Microbiota-Dependent Induction of Colonic Cyp27b1 Is Associated with Colonic Inflammation: Implications of Locally Produced 1,25-Dihydroxyvitamin D3 in Inflammatory Regulation in the Colon. Endocrinology 2017, 158, 4064–4075, doi:10.1210/en.2017-00578.
- Wu, S.; Liao, A.P.; Xia, Y.; Li, Y.C.; Li, J.D.; Sartor, R.B.; Sun, J. Vitamin D receptor negatively regulates bacterial-stimulated NF-kappaB activity in intestine. Am. J. Pathol. 2010, 177, 686–697, doi:10.2353/ajpath.2010.090998.
- He, L.; Liu, T.; Shi, Y.; Tian, F.; Hu, H.; Deb, D.K.; Chen, Y.; Bissonnette, M.; Li, Y.C. Gut Epithelial Vitamin D Receptor Regulates Microbiota-Dependent Mucosal Inflammation by Suppressing Intestinal Epithelial Cell Apoptosis. Endocrinology 2018, 159, 967–979, doi:10.1210/en.2017-00748.
- Lu, R.; Zhang, Y.; Xia, Y.; Zhang, J.; Kaser, A.; Blumberg, R.; Sun, J. Paneth cell alertness to pathogens maintained by vitamin D receptors. Gastroenterology 2020, doi:10.1053/j.gastro.2020.11.015.
- Gubatan, J.; Mehigan, G.A.; Villegas, F.; Mitsuhashi, S.; Longhi, M.S.; Malvar, G.; Csizmadia, E.; Robson, S.; Moss, A.C. Cathelicidin Mediates a Protective Role of Vitamin D in Ulcerative Colitis and Human Colonic Epithelial Cells. Inflamm. Bowel. Dis. 2020, 26, 885–897, doi:10.1093/ibd/izz330.
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.; Ferreira, S.R. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism 2017, 69, 76–86, doi:10.1016/j.metabol.2017.01.007.
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020, 40, 551–556, doi:10.21873/anticanres.13984.
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel. Dis. 2018, 24, 1926–1940, doi:10.1093/ibd/izy182.
- Schaffler, H.; Herlemann, D.P.; Klinitzke, P.; Berlin, P.; Kreikemeyer, B.; Jaster, R.; Lamprecht, G. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J. Dig. Dis. 2018, 19, 225–234, doi:10.1111/1751-2980.12591.
- Soltys, K.; Stuchlikova, M.; Hlavaty, T.; Gaalova, B.; Budis, J.; Gazdarica, J.; Krajcovicova, A.; Zelinkova, Z.; Szemes, T.; Kuba, D.; et al. Seasonal changes of circulating 25-hydroxyvitamin D correlate with the lower gut microbiome composition in inflammatory bowel disease patients. Sci. Rep. 2020, 10, 6024, doi:10.1038/s41598-020-62811-4.




