
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Omar Kujan | + 3635 word(s) | 3635 | 2020-12-31 02:15:01 | | | |
| 2 | Dean Liu | -1852 word(s) | 1783 | 2021-01-14 04:18:41 | | |
Video Upload Options
PAs’ function as antioxidant, anti-inflammatory drugs, signal pathway regulators remain critical in many diseases.
1. Introduction
Dietary flavonoids have been reported to possess substantial anticarcinogenic and health care activities because of their antioxidant and anti-inflammatory properties. Proanthocyanidins (PAs) are considered as the key ingredient, and are abundantly available in various parts of plants, such as the fruits, barks and plant seeds. Their modes of action were evaluated through a number of studies which showed their potential role as therapeutics of cancer, cardiovascular diseases and lipid metabolic disorder, etc.
2. Structure
Flavonoids consist of 15 carbon atoms, forming structures with two aromatic rings connected by a three-carbon bridge. Dietary flavonoids can be divided into six major groups (Table 1): flavonols, flavones, flavan-3-ols, anthocyanidins, flavanones, and isoflavones[1]. The components of PAs are the monomers (+)-catechins (CC) and the isomer (−)-epicatechins (EC) in flavan-3-ols. The (+)-catechins and (−)-epicatechins connect with each other or themselves via oxidative coupling between the C4 of the heterocyclic ring and the C8 of the adjacent aromatic ring, which form proanthocyanidins B1–B4. When the C4 is connected to the C6, they form B5–B8. Type-A PAs differ in an ether bond created between the C2 from (−)-epicatechins and the C7 from the former or (+)-catechins[2]. Researchers[3] classify PAs according to the degree of polymerization (DP). Depending on the number of subunits of flavan-3-ols from 1 to 4, PAs are divided into four classes: monomers, dimers, trimers, and tetramers. PAs with DP > 4 are classified as oligomers, and DP > 10 are polymers[4].
Table 1. The basic structure of flavonoid, its main subclasses, and the majority of proanthocyanidins (PAs).
| Compound | Basic Structure |
|---|---|
| Flavonoid | 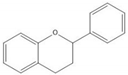 |
| Flavonol |  |
| Flavone |  |
| Flavan-3-ol |  |
| Anthocyanidin | 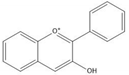 |
| Flavanone | 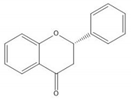 |
| Isoflavone | 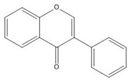 |
| (+)-Catechin | 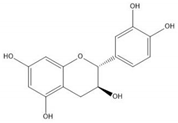 |
| (−)-Epicatechin | 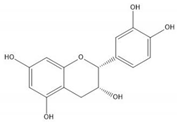 |
| Proanthocyanidin A1 | 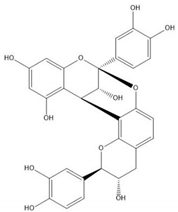 |
| Proanthocyanidin A2 | 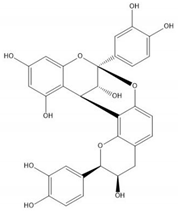 |
| Proanthocyanidin B1 | 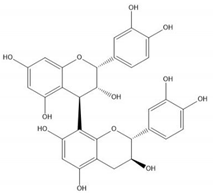 |
| Proanthocyanidin B2 | 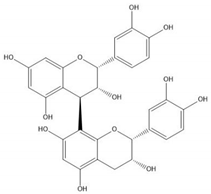 |
| Proanthocyanidin B3 | 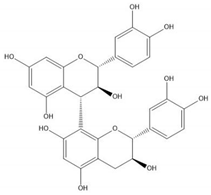 |
| Proanthocyanidin B4 | 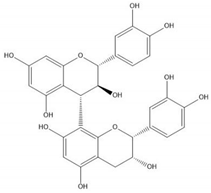 |
| Proanthocyanidin B5 | 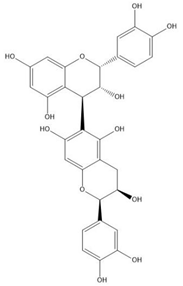 |
| Proanthocyanidin B6 | 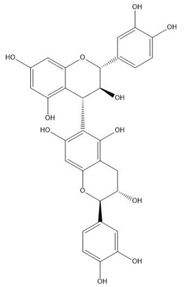 |
| Proanthocyanidin B7 | 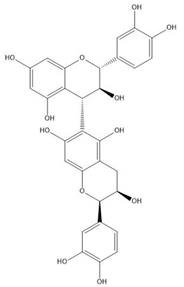 |
| Proanthocyanidin B8 | 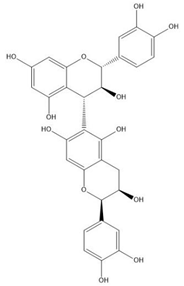 |
3. Isolation and Metabolism of PAs
PAs are found in nearly half of dietary fruits and plants, with high concentrations in the skin of fruits and leaves, even in grape seeds[5]. The methods and efficiency of PA isolation have improved with the optimization of methods and the progress of instruments. Nonetheless, the preparation procedures of proanthocyanidins from different parts of various plants or fruits are dissimilar, but predominantly contain three steps: pre-treatment, extraction, and purification[6]. Pre-treatment involves grinding fruits and plants rich in PAs into a powder first. According to the conventional extraction methods, the ground powder is then extracted by organic solvents with acetone/water (70:30, v/v) as the optimal solvent in recent years and concentrated to remove the solvent [7][8][9][10]. However, long extraction time and excessive consumption of organic solvents limit the efficiency improvement and popularization of these techniques, simultaneously with low reproducibility. Various advanced methods, such as ultrasound-assisted extraction (UAE), enzymatic extraction, ultra-high-pressure-assisted extraction, have also been reported for proanthocyanidin extraction[11][12][13][14][15]. So far, no uniform standard has been established. Based on the distinct solubility in eluent or adsorption capacity of resin, thin-layer chromatography (TLC) and Sephadex LH-20 column are currently applied to elute the crude extract into fractions rich in PAs[16][17]. Zhang et al. demonstrated that using high-speed counter-current chromatography (HSCCC) alone, or combined with semipreparative High Performance Liquid Chromatography (HPLC) could obtain PAs in different DP levels with high purity[18]. Next, the composition, structure and DP of each fraction could be analyzed by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOFMS), and even 13C-nuclear magnetic resonance (13C-NMR) analysis[19].
The DP of PAs determines their bioavailability and subseq uent pharmacology prominently. Under oral administration, the carbon bonds connecting monomers are broken due to the low pH value of gastric juice, leading to PA degradation[4]. An in vitro colonic carcinoma (caco-2) cell study demonstrated that CC, dimers and trimers could be absorbed through the intestinal epithelium[20]. Therefore, monomers and oligomers are preferentially absorbed by enterocytes for glucuronidation in the small intestine, but unabsorbed PAs with DP > 4 are fermented by colonic microbiota[9][21]. A study in mice has revealed that these proanthocyanidins with a high degree of polymerization could affect the activity of digestive enzymes and the absorption of other nutrients, suggesting that in addition to the large molecular weight and excessive OH groups, this might be another reason why polymeric proanthocyanidins were difficult to absorb[22]. Deeper metabolites sulfurated and methylated in liver by phase II enzymes then reach the target tissues for pharmacological action and are ultimately excreted through the urinary system and digestive system[21][23]. It has been reported that monomers, dimers, and their metabolites, such as catechin-glucuronides, epicatechin-glucuronides, methyl-catechin-glucuronides and methyl-epicatechin-glucuronides were detected in the serum of rats treating with grape seed proanthocyanidin extracts (GSPE)[24]. In a randomized cross-over study in humans, after ingesting a single dose of EC and PA B1 of 1 mg per kg body weight, respectively, the maximum plasma concentration of EC and its metabolites ranged from 89~190 ng/mL, while the maximum levels of PA B1 and its metabolites was 2.0 ng/mL[25]. This wide range of metabolites in blood subsequently reached the target organs and precisely exerted pharmacological effects. Furthermore, the distribution of PA metabolites could be observed in rats that ingested the hazelnut skin extract (5 g per kg body weight) whose essential component was 6.3 ± 0.54 μmol·g−1 CC, 2.4 ± 0.13 μmol·g−1 EC, 15 ± 1.3 μmol·g−1 PA dimers and 4.9 ± 0.32 μmol·g−1 PA trimers[26]. It revealed that lung, kidney, thymus, testicle and spleen were the places with a high concentration of the phase II metabolites of PAs, but not dimers and trimers. As for excretion, Zhang et al. showed that 58–78% PAs were excreted via urine and faeces, which indicated a potential low bioavailability in prototype drugs[4]. Nonetheless, supported by molecule modification technology or packaged with nanoparticles, PAs should immensely fulfil pharmacological effects.
4. Biological Properties of PAs
Well-known multifunctional substances in biological science, PAs, from low molecular weight to the high one, give significant advantages for our health[27]. Studies on PAs have been widely carried out and achieved remarkable results based on cell and animal models. However, few clinical trials have been conducted, which limits its application as a diet-therapeutic ingredient or as a clinical candidate. Estimated data from the United States Department of Agriculture (USDA)’s Continuing Survey of Food Intake by Individuals (CSFII) show that the mean daily intake of PAs in 1994–1996 was 53.6 mg/person/day excluding monomers and 57.7 mg/person/day including monomers, which is inadequate for its health benefits[28][29]. Clinical trials of PAs are still insufficient to provide substantial evidence for the recommendation of diet therapy using PAs. Such clinical trials were often based on the use of various forms of food, like complex food intake adhering to dietary guidelines, fruits, GSPE or even extracts from French Maritime Pine bark (FMPB), which were then either hard to evaluate the effectiveness or not commercially available, in order to provide a strong demonstration for the benefits of PAs as a diet-therapeutic ingredient [30][31][32][33][34]. Under these clinical trials, PAs themselves were insufficiently proved by undergoing such process for their biological properties. It might be caused by the low absorption of PAs individually and the dose used in the trial (only 130 mg)[29]. The complex form of oligomeric PAs is necessary for the following reasons: (1) As the main ingredient in such complexes like GSPE, PAs are about 39–90%, which is sufficient to have biological properties[21][27][31][35][36][37]. (2) Other ingredients have supplementary effects. For example, (−)-epicatechin and soy phospholipids are used to improve the pressure-regulating effect and enhance bioavailability, respectively [32][33][36]. The selection of the type of PA mixture still needs to be considered as PAs of different origins have different compositions[38]. So far plant or fruit extracts rich in a variety of oligomeric PAs, are the primary interventions in vitro and in vivo studies, and the purity of the PAs is up to 90%, which ensures the effectiveness of studies and the verification of pharmacological effects. Research of individual PAs is also emerging gradually, which facilitates an extended cognition and accurately defines the real active substances targeting the mechanisms of specific diseases. Up to now, however, only 4 of 40 completed human clinical trials on PAs or GSPE focused on individual PAs, leaving an essential gap in this field (www.clinicaltrials.gov). Thus, GSPE and leucoselect phytosome (LP) might be better candidates for PA employment as they are commonly used in different experiments, can be orally taken, and have a mature product such as Gravinol[32][33][39]. They are both standardized GSPE complexes (dimer B-3 and trimer C-2) that can be easily absorbed[33].
One of its pharmacological effects, antioxidant activity, is significant in preventing cardiovascular diseases or metabolic syndromes[40]. Another systematic effect is mainly about glycometabolism and lipid metabolism, respectively. Previously, some researchers found that proanthocyanidin extracts can protect β cells in the pancreas by dealing with oxidative stress, enhancing the sensitivity and secretion of insulin, or even affecting some enzyme activities in the metabolic process[41][42][43] For the past decade, clinical trials based on PA usage were mainly for the cardiovascular and lipid metabolic effect. A randomized, double-blind placebo-controlled clinical trial for eight weeks with 70 patients using grape seed extract (GSE) 200 mg or placebo were conducted to indicate that GSE increases paraoxonase (PON) activity mostly through increasing HDL-C and apo-AI levels in moderate hyperlipidemia (MMH) patients[44]. Another randomized, double-blinded study using additional PA-containing supplement, Aterofisiol, based on acetylsalicylic acid 100 mg for 25 days could ameliorate the amount of cholesterol and lipid in atherosclerosis plaque[45]. An epidemiology study in 2020 also stated that, by consuming two apples a day (about 200 mg PAs per day), PAs could lower correlated lipid index including serum cholesterol, LDL oxidation, raise HDL cholesterol, and restrict further processes happening in atherosclerosis by activation of endothelial nitric oxide synthase, prevention of platelet aggregation, and blockage of inflammatory responses [31]. These trials come up with the strength of PAs as beneficial in both prophylactic and clinical medicine. However, a phase II, placebo-controlled, randomized trial involving early breast cancer patients demonstrated that consuming GSPE (300 mg per day) for six months did not ameliorate the tissue hardness due to radiotherapy[46]. On the other hand, trials within lung cancer by using LP could reduce cancer index serum miR-19a, -19b, and -106b and an average of 55% bronchial Ki-67 in heavy active, former smokers. Phase I lung cancer chemoprevention trial with LP was shown to be succeeded by Mao, J. T. et al.[32][36]. Conflicting results from different clinical trials might be due to the different distribution of PAs in different organs and the concentration of metabolites in target tissues did not meet the pharmacological effect requirement. PAs proved their potential usage in cancers, cardiovascular diseases, endocrine diseases, osteoarticular diseases, urinary diseases and even mental disorders. Further usage in other systems still being explored[39][47][48][49][50][51][52]. In this review, we principally describe the health benefits of PAs in fatal diseases such as cancers, cardiovascular diseases, and lipid metabolism disorders based on in vitro and in vivo studies.
References
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043.
- Fine, A.M. Oligomeric proanthocyanidin complexes: History, structure, and phytopharmaceutical applications. Altern. Med. Rev. A J. Clin. Ther. 2000, 5, 144–151.
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262.
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281.
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521.
- Zeng, Y.X.; Wang, S.; Wei, L.; Cui, Y.Y.; Chen, Y.H. Proanthocyanidins: Components, Pharmacokinetics and Biomedical Properties. Am. J. Chin. Med. 2020, 48, 813–869.
- Brillouet, J.M.; Fulcrand, H.; Carrillo, S.; Rouméas, L.; Romieu, C. Isolation of Native Proanthocyanidins from Grapevine (Vitis vinifera) and Other Fruits in Aqueous Buffer. J. Agric. Food Chem. 2017, 65, 2895–2901.
- Ramsay, A.; Williams, A.R.; Thamsborg, S.M.; Mueller-Harvey, I. Galloylated proanthocyanidins from shea (Vitellaria paradoxa) meal have potent anthelmintic activity against Ascaris suum. Phytochemistry 2016, 122, 146–153.
- Tuominen, A.; Karonen, M. Variability between organs of proanthocyanidins in Geranium sylvaticum analyzed by off-line 2-dimensional HPLC-MS. Phytochemistry 2018, 150, 106–117.
- Zhang, H.; Dai, Y.; Cheng, Y.; He, Y.; Manyakara, Z.; Duan, Y.; Sun, G.; Sun, X. Influence of extremely low frequency magnetic fields on Ca2+ signaling and double messenger system in mice hippocampus and reversal function of procyanidins extracted from lotus seedpod. Bioelectromagnetics 2017, 38, 436–446.
- Chai, W.M.; Wang, R.; Wei, M.K.; Zou, Z.R.; Deng, R.G.; Liu, W.S.; Peng, Y.Y. Proanthocyanidins Extracted from Rhododendron pulchrum Leaves as Source of Tyrosinase Inhibitors: Structure, Activity, and Mechanism. PLoS ONE 2015, 10, e0145483.
- Czerwińska, M.E.; Dudek, M.K.; Pawłowska, K.A.; Pruś, A.; Ziaja, M.; Granica, S. The influence of procyanidins isolated from small-leaved lime flowers (Tilia cordata Mill.) on human neutrophils. Fitoterapia 2018, 127, 115–122.
- Fernández, K.; Vega, M.; Aspé, E. An enzymatic extraction of proanthocyanidins from País grape seeds and skins. Food Chem. 2015, 168, 7–13.
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61.
- Zhang, R.; Su, D.; Hou, F.; Liu, L.; Huang, F.; Dong, L.; Deng, Y.; Zhang, Y.; Wei, Z.; Zhang, M. Optimized ultra-high-pressure-assisted extraction of procyanidins from lychee pericarp improves the antioxidant activity of extracts. Biosci. Biotechnol. Biochem. 2017, 81, 1576–1585.
- Chai, W.M.; Wei, M.K.; Wang, R.; Deng, R.G.; Zou, Z.R.; Peng, Y.Y. Avocado Proanthocyanidins as a Source of Tyrosinase Inhibitors: Structure Characterization, Inhibitory Activity, and Mechanism. J. Agric. Food Chem. 2015, 63, 7381–7387.
- Wei, M.; Chai, W.M.; Yang, Q.; Wang, R.; Peng, Y. Novel Insights into the Inhibitory Effect and Mechanism of Proanthocyanidins from Pyracantha fortuneana Fruit on α-Glucosidase. J. Food Sci. 2017, 82, 2260–2268.
- Zhang, S.; Li, L.; Cui, Y.; Luo, L.; Li, Y.; Zhou, P.; Sun, B. Preparative high-speed counter-current chromatography separation of grape seed proanthocyanidins according to degree of polymerization. Food Chem. 2017, 219, 399–407.
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2002, 40, 599–607.
- Deprez, S.; Mila, I.; Huneau, J.F.; Tome, D.; Scalbert, A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid. Redox Signal. 2001, 3, 957–967.
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253.
- Zhong, H.; Xue, Y.; Lu, X.; Shao, Q.; Cao, Y.; Wu, Z.; Chen, G. The Effects of Different Degrees of Procyanidin Polymerization on the Nutrient Absorption and Digestive Enzyme Activity in Mice. Molecules 2018, 23, 2916.
- Urpi-Sarda, M.; Monagas, M.; Khan, N.; Llorach, R.; Lamuela-Raventós, R.M.; Jáuregui, O.; Estruch, R.; Izquierdo-Pulido, M.; Andrés-Lacueva, C. Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 7258–7267.
- Guerrero, L.; Margalef, M.; Pons, Z.; Quiñones, M.; Arola, L.; Arola-Arnal, A.; Muguerza, B. Serum metabolites of proanthocyanidin-administered rats decrease lipid synthesis in HepG2 cells. J. Nutr. Biochem. 2013, 24, 2092–2099.
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015, 59, 610–621.
- Serra, A.; Macià, A.; Romero, M.P.; Anglès, N.; Morelló, J.R.; Motilva, M.J. Distribution of procyanidins and their metabolites in rat plasma and tissues after an acute intake of hazelnut extract. Food Funct. 2011, 2, 562–568.
- Zhu, W.; Wang, R.F.; Khalifa, I.; Li, C.M. Understanding toward the Biophysical Interaction of Polymeric Proanthocyanidins (Persimmon Condensed Tannins) with Biomembranes: Relevance for Biological Effects. J. Agric. Food Chem. 2019, 67, 11044–11052.
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617.
- Hollands, W.J.; Tapp, H.; Defernez, M.; Perez Moral, N.; Winterbone, M.S.; Philo, M.; Lucey, A.J.; Kiely, M.E.; Kroon, P.A. Lack of acute or chronic effects of epicatechin-rich and procyanidin-rich apple extracts on blood pressure and cardiometabolic biomarkers in adults with moderately elevated blood pressure: A randomized, placebo-controlled crossover trial. Am. J. Clin. Nutr. 2018, 108, 1006–1014.
- Castro-Acosta, M.L.; Sanders, T.A.B.; Reidlinger, D.P.; Darzi, J.; Hall, W.L. Adherence to UK dietary guidelines is associated with higher dietary intake of total and specific polyphenols compared with a traditional UK diet: Further analysis of data from the Cardiovascular risk REduction Study: Supported by an Integrated Dietary Approach (CRESSIDA) randomised controlled trial. Br. J. Nutr. 2019, 121, 402–415.
- Koutsos, A.; Riccadonna, S.; Ulaszewska, M.M.; Franceschi, P.; Trošt, K.; Galvin, A.; Braune, T.; Fava, F.; Perenzoni, D.; Mattivi, F.; et al. Two apples a day lower serum cholesterol and improve cardiometabolic biomarkers in mildly hypercholesterolemic adults: A randomized, controlled, crossover trial. Am. J. Clin. Nutr. 2020, 111, 307–318.
- Mao, J.T.; Lu, Q.Y.; Xue, B.; Neis, P.; Zamora, F.D.; Lundmark, L.; Qualls, C.; Massie, L. A Pilot Study of a Grape Seed Procyanidin Extract for Lung Cancer Chemoprevention. Cancer Prev. Res. (Phila. PA) 2019, 12, 557–566.
- Mao, J.T.; Smoake, J.; Park, H.K.; Lu, Q.Y.; Xue, B. Grape Seed Procyanidin Extract Mediates Antineoplastic Effects against Lung Cancer via Modulations of Prostacyclin and 15-HETE Eicosanoid Pathways. Cancer Prev. Res. (Phila. PA) 2016, 9, 925–932.
- Van Dorsten, F.A.; Grün, C.H.; van Velzen, E.J.; Jacobs, D.M.; Draijer, R.; van Duynhoven, J.P. The metabolic fate of red wine and grape juice polyphenols in humans assessed by metabolomics. Mol. Nutr. Food Res. 2010, 54, 897–908.
- Odai, T.; Terauchi, M.; Kato, K.; Hirose, A.; Miyasaka, N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2844.
- Rodriguez-Mateos, A.; Weber, T.; Skene, S.S.; Ottaviani, J.I.; Crozier, A.; Kelm, M.; Schroeter, H.; Heiss, C. Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: Randomized, controlled, double-masked intervention trial. Am. J. Clin. Nutr. 2018, 108, 1229–1237.
- Vaid, M.; Singh, T.; Prasad, R.; Katiyar, S.K. Bioactive proanthocyanidins inhibit growth and induce apoptosis in human melanoma cells by decreasing the accumulation of β-catenin. Int. J. Oncol. 2016, 48, 624–634.
- Weber, H.A.; Hodges, A.E.; Guthrie, J.R.; O’Brien, B.M.; Robaugh, D.; Clark, A.P.; Harris, R.K.; Algaier, J.W.; Smith, C.S. Comparison of proanthocyanidins in commercial antioxidants: Grape seed and pine bark extracts. J. Agric. Food Chem. 2007, 55, 148–156.
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996.
- Gao, W.L.; Li, X.H.; Dun, X.P.; Jing, X.K.; Yang, K.; Li, Y.K. Grape Seed Proanthocyanidin Extract Ameliorates Streptozotocin-induced Cognitive and Synaptic Plasticity Deficits by Inhibiting Oxidative Stress and Preserving AKT and ERK Activities. Curr. Med Sci. 2020, 40, 434–443.
- Cao, J.; Yu, X.; Deng, Z. Chemical Compositions, Antiobesity, and Antioxidant Effects of Proanthocyanidins from Lotus Seed Epicarp and Lotus Seed Pot. J. Agric. Food Chem. 2018, 66, 13492–13502.
- Macho-González, A.; López-Oliva, M.E.; Merino, J.J.; García-Fernández, R.A.; Garcimartín, A.; Redondo-Castillejo, R.; Bastida, S.; Sánchez-Muniz, F.J.; Benedí, J. Carob fruit extract-enriched meat improves pancreatic beta-cell dysfunction, hepatic insulin signaling and lipogenesis in late-stage type 2 diabetes mellitus model. J. Nutr. Biochem. 2020, 84, 108461.
- Sun, P.; Wang, T.; Chen, L.; Yu, B.W.; Jia, Q.; Chen, K.X.; Fan, H.M.; Li, Y.M.; Wang, H.Y. Trimer procyanidin oligomers contribute to the protective effects of cinnamon extracts on pancreatic β-cells in vitro. Acta Pharmacol. Sin. 2016, 37, 1083–1090.
- Argani, H.; Ghorbanihaghjo, A.; Vatankhahan, H.; Rashtchizadeh, N.; Raeisi, S.; Ilghami, H. The effect of red grape seed extract on serum paraoxonase activity in patients with mild to moderate hyperlipidemia. Sao Paulo Med. J. Rev. Paul. Med. 2016, 134, 234–239.
- Amato, B.; Compagna, R.; Amato, M.; Gallelli, L.; de Franciscis, S.; Serra, R. Aterofisiol(®) in carotid plaque evolution. Drug Des. Dev. Ther. 2015, 9, 3877–3884.
- Brooker, S.; Martin, S.; Pearson, A.; Bagchi, D.; Earl, J.; Gothard, L.; Hall, E.; Porter, L.; Yarnold, J. Double-blind, placebo-controlled, randomised phase II trial of IH636 grape seed proanthocyanidin extract (GSPE) in patients with radiation-induced breast induration. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2006, 79, 45–51.
- Alrefaie, Z. Grape Seed Proanthocyanidins Attenuate Anxiety-Like Behavior in an Experimental Model of Dietary-Induced Hypercholesterolemia in Rats. Int. J. Vitam. Nutr. Res. Int. Z. Fur Vitam. Ernahr. J. Int. Vitaminol. Nutr. 2015, 85, 282–291.
- John, O.D.; Wanyonyi, S.; Mouatt, P. Achacha (Garcinia humilis) Rind Improves Cardiovascular Function in Rats with Diet-Induced Metabolic Syndrome. Nutrients 2018, 10, 1425.
- Lessiani, G.; Iodice, P.; Nicolucci, E.; Gentili, M. Lymphatic edema of the lower limbs after orthopedic surgery: Results of a randomized, open-label clinical trial with a new extended-release preparation. J. Biol. Regul. Homeost. Agents 2015, 29, 805–812.
- Toker, H.; Balci Yuce, H. Morphometric and histopathological evaluation of the effect of grape seed proanthocyanidin on alveolar bone loss in experimental diabetes and periodontitis. J. Periodontal Res. 2018, 53, 478–486.
- Tresserra-Rimbau, A.; Castro-Barquero, S.; Vitelli-Storelli, F.; Becerra-Tomas, N.; Vázquez-Ruiz, Z.; Díaz-López, A.; Corella, D. Associations between Dietary Polyphenols and Type 2 Diabetes in a Cross-Sectional Analysis of the PREDIMED-Plus Trial: Role of Body Mass Index and Sex. Antioxidants 2019, 8, 537.
- Zhou, Y.; Li, B.Y.; Li, X.L.; Wang, Y.J.; Zhang, Z.; Pei, F.; Wang, Q.Z.; Zhang, J.; Cai, Y.W.; Cheng, M.; et al. Restoration of Mimecan Expression by Grape Seed Procyanidin B2 Through Regulation of Nuclear Factor- κB in Mice With Diabetic Nephropathy. Iran. J. Kidney Dis. 2016, 10, 325–331.




