| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manuel Izquierdo | + 1857 word(s) | 1857 | 2020-04-14 08:48:43 | | | |
| 2 | Rita Xu | -542 word(s) | 1315 | 2020-04-16 09:33:35 | | | | |
| 3 | Manuel Izquierdo | Meta information modification | 1315 | 2020-04-16 10:38:28 | | | | |
| 4 | Rita Xu | -28 word(s) | 1287 | 2020-10-29 09:26:18 | | |
Video Upload Options
Exosomes are extracellular vesicles (EV) of endosomal origin (multivesicular bodies, MVB) constitutively released by many different eukaryotic cells by fusion of MVB to the plasma membrane. However, inducible exosome secretion controlled by cell surface receptors is restricted to very few cell types and a limited number of cell surface receptors. Among these, exosome secretion is induced in T lymphocytes and B lymphocytes when stimulated at the immune synapse (IS) via T-cell receptor (TCR) and B-cell receptor (BCR), respectively. IS formation by T and B lymphocytes constitutes a crucial event involved in antigen-specific, cellular and humoral immune responses. Upon IS formation by T and B lymphocytes with antigen-presenting cells (APC) the convergence of MVB towards the microtubule organization center (MTOC), and MTOC polarization to the IS, are involved in polarized exosome secretion at the synaptic cleft. This specialized mechanism provides the immune system with a finely-tuned strategy to increase the specificity and efficiency of crucial secretory effector functions of B and T lymphocytes. Since inducible exosome secretion by antigen-receptors is a critical and unique feature of the immune system this entry considers the study of the traffic events leading to polarized exosome secretion at the IS and some of their biological consequences.
1. Introduction
To constitute an IS, T lymphocytes must recognize processed antigenic peptides loaded onto MHC molecules present on the cell surface of professional antigen-presenting cells (APC) or pathogen-infected cells. TCR interaction with peptide-MHC-I complexes (pMHC-I) induces naïve CD8+ cytotoxic T lymphocytes (CTL) activation (priming), whereas TCR interaction with peptide-MHC-II complexes (pMHC-II) leads to CD4+ Th lymphocyte activation (Figure 1) [1]. Primed CTL form IS with target cells resulting in a specific killing. In addition, mature IS formation can induce T lymphocyte anergy or activation-induced apoptosis (AICD)[2].
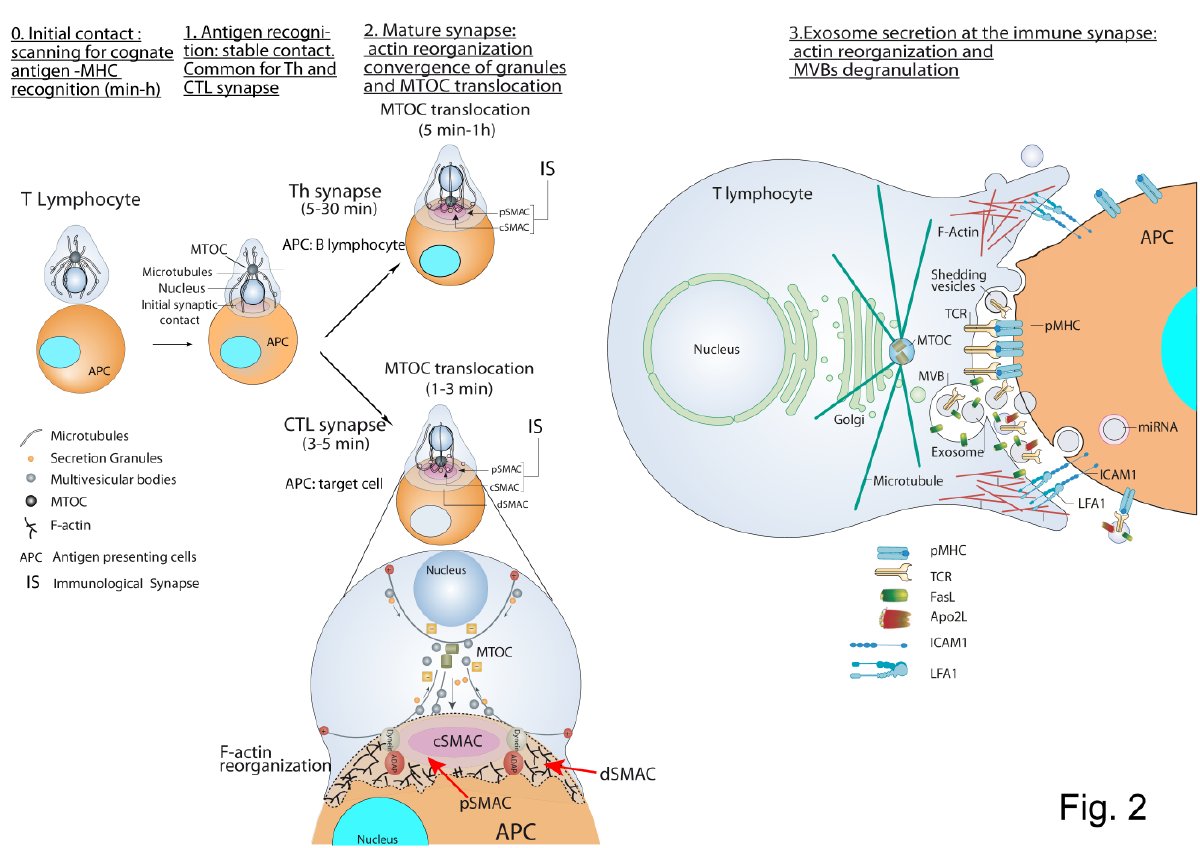
Figure 1. T lymphocyte—antigen-presenting cells (APC) immune synapse (IS) and polarized secretion. Stages 0 and 1 are common for both Th and cytotoxic T lymphocytes (CTL) IS. After the initial scanning contact of TCR with pMHC on APC, Th effector T lymphocytes (upper panel) form mature IS with antigen-presenting B lymphocytes within several minutes. This IS lasts many hours during which de novo cytokine (i.e., IL-2, IFN-γ) production and secretion occur, which require continuous T-cell receptors (TCR) signaling. Primed effector CTL (lower panel) establish more transient, mature IS after scanning their target cells (i.e., a virus-infected cell), and deliver their lethal hits within a few minutes. Secretory lysosomes (lytic granules) are very rapidly transported (within very few minutes) towards the microtubule organization center (MTOC) (in the minus “–“ direction) and, almost simultaneously, the MTOC polarizes towards the central supramolecular activation complex (cSMAC) of the IS, an F-actin poor area that constitutes a secretory domain. MTOC translocation to the IS appears to be dependent on dynein anchored to the Adhesion and Degranulation Promoting Adapter Protein (ADAP) at the peripheral SMAC (pSMAC), which pulls MTOC in the minus direction. In both types of IS (lower zoom panel), the initial F-actin reorganization in the cell-to-cell contact area, followed by a decrease in F-actin at the cSMAC and an accumulation at the distal SMAC (dSMAC) appears to be involved in granule secretion. In stage 3, MVB fusion with the plasma membrane occurs in both types of IS and leads to TCR-containing exosome polarized secretion at the IS. The exosomes released in Th IS contain proapoptotic FasL and Apo2L and can induce target cell death or Th cell death (AICD). TCR–containing shedding microvesicles have been described in Th IS.
2. CTL-Target Cell Immune Synapse
CTL-target cell IS induces the rapid polarization (from seconds to few minutes) of CTL MTOC and lytic granules (secretory granules or secretory lysosomes-SL-with MVB structure) towards the central supramolecular activation cluster (cSMAC) at the IS (Figure 1). Lytic granules fusion with the plasma membrane (degranulation) induces the secretion of certain cytotoxic factors such as perforin and granzymes to the synaptic cleft, triggering target cell apoptosis [3]. Upon degranulation, FasL located at the secretory granule limiting membrane becomes exposed to the plasma membrane at the IS and induces target cell Fas crosslinking leading to target cell apoptosis [4][5][6][7].
Another consequence of degranulation is ILV secretion as nanosize EV at the CTL-target cell synaptic cleft, first described by Peters et al. [8]. Although the vesicles secreted by CTL were not referred at that time as exosomes, their formation and mode of exocytosis justifies such a nomenclature [8][9].
Subsequent publications demonstrated that T lymphocyte stimulation of T lymphoblasts (including CD4+ and CD8+ lymphocytes) with activation agonists produced non-directional secretion of nanosize EV (quoted as microvesicles) carrying pro-apoptotic FasL and Apo2L [10] via MVB-mediated degranulation [11], providing an alternative mechanism of TCR-controlled AICD that does not necessarily imply cell-to-cell contact [11][12]. Moreover, it was shown that upon TCR triggering T lymphoblasts secrete exosomes [12][13] containing TCR/CD3 [13], extending the early observations obtained in CTL forming synapses [8]. CTL MTOC reorientation is initially guided by a diacylglycerol (DAG) gradient centered at the IS [14], generated by TCR-stimulated phospholipase C (PLC). DAG phosphorylation by diacylglycerol kinase α(DGKα) is involved in the spatiotemporal control of the DAG gradient [15][16] and MTOC polarization to the IS in CTL [14]. DAG activates, among others, several members of the PKC and PKD families [17], such as PKCδ, which is necessary for the polarization of lytic granules and cytotoxicity in mouse CTL [18][19].
3. Th Immune Synapse
Polarized secretion upon Th IS formation has been less studied than polarized CTL secretion [20]. Th IS are more stable and longer (from minutes up to several hours) than CTL IS (few minutes) [21][22]. Th IS are required for both directional and continuous cytokine secretion [21][22]. These cytokines are contained in secretory vesicles and IL-2, IFN-γ-containing secretory vesicles undergo polarized traffic to the F-actin poor area at cSMAC [23][24][25][26] as CTL lytic granules. Although the identity of the cytokine-containing secretory vesicles has not been characterized yet [27][28] they, most probably, are not MVB [28] (Figure 1).
Early reports in CD4+ Jurkat cells and T lymphoblasts demonstrated that stimulation with activation agonists [10] or anti-TCR [12][13] induced exosome secretion. Stimulation with a heterologous receptor agonist, that mimics TCR-derived signals leading to full T cell activation [29] and AICD [30], also induced exosome secretion in CD4+ Jurkat cells [12], suggesting that exosome secretion is a general consequence of T lymphocyte activation. IS formation by CD4+ Jurkat cells and superantigen-coated Raji B cells acting as an APC, which constitutes a well-established IS model [31][32][33], induces polarized MVB traffic towards the IS, MVB degranulation and exosome release [34][35] (Figure 1, right side panel). In this Th-APC IS model, a positive role of TCR-triggered DAG and its regulator DGKα [15], in polarized MVB traffic towards the IS was demonstrated [34][36][37]. As DGKα also controls late endosomes polarized traffic during invasive migration [38], and MTOC and lytic granules polarized traffic in CTL (described above), DAG and DGKα can be considered as general regulators of polarized traffic . DAG-activated PKCδ is needed for cortical actin reorganization at the IS, MTOC and MVB polarization to the IS and exosome secretion in this IS model [39]. Overall, this leads us to hypothesize that an altered actin reorganization at the IS may underlie the deficient MVB polarization occurring in PKCδ-interfered T cell clones [39]. In this model, DAG-activated PKD1/2 regulates MVB maturation and polarization leading to exosome secretion [40], suggesting that several regulatory points in exosome secretion are controlled by DAG.
Microvesicles or ectosomes budding from the Th cell plasma membrane and accumulating at the IS have been described [41] (Figure 1, right side panel). These shedding vesicles were enriched in TCR and capable to trigger B-lymphocyte signaling via pMHC-II stimulation [41][42]. Thus, it appears that distinct types of EV from Th lymphocytes are secreted at the IS. Further research will be necessary to establish whether these subtypes of EV trigger different Th effector responses or, on the contrary, redundantly or synergistically trigger the same responses.
References
- Calvo, V.; Izquierdo, M; Imaging Polarized Secretory Traffic at the Immune Synapse in Living T Lymphocytes. Front. Immunol. 2018, 9, 684.
- Peter Friedl; Annemieke Th. Den Boer; Matthias Gunzer; Tuning immune responses: diversity and adaptation of the immunological synapse. Nature Reviews Immunology 2005, 5, 532-545, 10.1038/nri1647.
- P. J. Peters; J Borst; V Oorschot; M Fukuda; O Krähenbühl; J Tschopp; J W Slot; H J Geuze; Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. Journal of Experimental Medicine 1991, 173, 1099-1109, 10.1084/jem.173.5.1099.
- Geneviève De Saint Basile; Gaël Menasche; Alain Fischer; Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nature Reviews Immunology 2010, 10, 568-579, 10.1038/nri2803.
- F. Vignaux; E Vivier; B Malissen; V Depraetere; S Nagata; P Golstein; TCR/CD3 coupling to Fas-based cytotoxicity. Journal of Experimental Medicine 1995, 181, 781-786, 10.1084/jem.181.2.781.
- G. Bossi; Gillian M. Griffiths; Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nature Medicine 1999, 5, 90-96, 10.1038/4779.
- Shigekazu Nagata; Apoptosis by Death Factor. Cell 1997, 88, 355-365, 10.1016/s0092-8674(00)81874-7.
- Peter J. Peters; Hans J. Geuze; Hans A. Der Van Donk; Jan W. Slot; Janice M. Griffith; Nico J. Stam; Hans Clevers; Jannie Borst; Molecules relevant for T cell-target cell interaction are present in cytolytic granules of human T lymphocytes. European Journal of Immunology 1989, 19, 1469-1475, 10.1002/eji.1830190819.
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J; Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. Journal of Cell Science 2000, 113, 3365–3374.
- Martinez-Lorenzo, M.J.; Anel, A.; Gamen, S.; Monle n, I.; Lasierra, P.; Larrad, L.; Pineiro, A.; Alava, M.A.; Naval, J; Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. The Journal of Immunology 1999, 163, 1274–1281.
- Inmaculada Monleón; María José Martínez-Lorenzo; Luis Monteagudo; Pilar Lasierra; Marta Taulés; María Iturralde; Andrés Piñeiro; Luis Larrad; María Angeles Alava; Javier Naval; et al.Alberto Anel Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. The Journal of Immunology 2001, 167, 6736-6744, 10.4049/jimmunol.167.12.6736.
- Roberto Alonso; M. Carmen Rodríguez; Jose Pindado; Ernesto Merino; Isabel Mérida; Manuel Izquierdo; Diacylglycerol Kinase α Regulates the Secretion of Lethal Exosomes Bearing Fas Ligand during Activation-induced Cell Death of T Lymphocytes. Journal of Biological Chemistry 2005, 280, 28439-28450, 10.1074/jbc.m501112200.
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C; TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. The Journal of Immunology 2002, 168, 3235–3241.
- Emily J Quann; Ernesto Merino; Toshiaki Furuta; Morgan Huse; Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nature Immunology 2009, 10, 627-635, 10.1038/ni.1734.
- Sanjuan, M.A.; Jones, D.R.; Izquierdo, M.; Merida, I; Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J. Cell Biol. 2001, 153, 207–220.
- Chauveau, A.; Le Floc’h, A.; Bantilan, N.S.; Koretzky, G.A.; Huse, M; Diacylglycerol kinase alpha establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci. Signal. 2014, 7, ra82.
- Silvia Carrasco; Isabel Mérida; Diacylglycerol, when simplicity becomes complex. Trends in Biochemical Sciences 2007, 32, 27-36, 10.1016/j.tibs.2006.11.004.
- Ma, J.S.; Monu, N.; Shen, D.T.; Mecklenbrauker, I.; Radoja, N.; Haydar, T.F.; Leitges, M.; Frey, A.B.; Vukmanovic, S.; Radoja, S; et al. Protein kinase Cdelta regulates antigen receptor-induced lytic granule polarization in mouse CD8+ CTL. J. Immunol. 2007, 178, 7814–7821.
- Ma, J.S.; Haydar, T.F.; Radoja, S; Protein kinase C delta localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J. Immunol. 2008, 181, 4716–4722.
- Jolly, C.; Sattentau, Q.J; Regulated secretion from CD4+ T cells. Trends Immunol. 2007, 28, 474–481.
- Griffiths, G.M.; Tsun, A.; Stinchcombe, J.C; The immunological synapse: a focal point for endocytosis and exocytosis. J. Cell Biol. 2010, 189, 399–406.
- Huse, M; Microtubule-organizing center polarity and the immunological synapse: protein kinase C and beyond. Front. Immunol. 2012, 3, 235.
- Karine Chemin; Armelle Bohineust; Stephanie Dogniaux; Marie Tourret; S. Guégan; Francesc Miró-Mur; Claire Hivroz; Cytokine Secretion by CD4+ T Cells at the Immunological Synapse Requires Cdc42-Dependent Local Actin Remodeling but Not Microtubule Organizing Center Polarity. The Journal of Immunology 2012, 189, 2159-2168, 10.4049/jimmunol.1200156.
- Morgan Huse; Microtubule-organizing center polarity and the immunological synapse: protein kinase C and beyond. Frontiers in Immunology 2012, 3, 235, 10.3389/fimmu.2012.00235.
- Ueda, H.; Zhou, J.; Xie, J.; Davis, M.M; Distinct Roles of Cytoskeletal Components in Immunological Synapse Formation and Directed Secretion. J. Immunol. 2015, 195, 4117–4125.
- Morgan Huse; Björn F Lillemeier; Michael S. Kuhns; Daniel S Chen; Mark M Davis; T cells use two directionally distinct pathways for cytokine secretion. Nature Immunology 2006, 7, 247-255, 10.1038/ni1304.
- Trends Immunol.; Regulated secretion from CD4+ T cells. Jolly, C.; Sattentau, Q.J 2007, 28, 474–481.
- Morgan Huse; Emily J Quann; Mark M Davis; Shouts, whispers and the kiss of death: directional secretion in T cells. Nature Immunology 2008, 9, 1105-1111, 10.1038/ni.f.215.
- Dev M. Desai; Marianne E. Newton; Theresa Kadlecek; Arthur Weiss; Stimulation of the phosphatidyl-inositol pathway can induce T-cell activation. Nature 1990, 348, 66-69, 10.1038/348066a0.
- Izquierdo, M.; Ruiz-Ruiz, M.C.; Lopez-Rivas, A; Stimulation of phosphatidylinositol turnover is a key event for Fas-dependent, activation-induced apoptosis in human T lymphocytes.. J. Immunol. 1996, 157, 21–28.
- Calvo, V.; Izquierdo, M; Imaging Polarized Secretory Traffic at the Immune Synapse in Living T Lymphocytes. Front. Immunol. 2018, 9, 684, 10.3389/fimmu.2018.00684.
- María Montoya; David Sancho; Grégory Bonello; Yves Collette; Claire Langlet; Hai-Tao He; Pedro Aparicio; Andrés Alcover; Daniel Olive; Francisco Sánchez‐Madrid; David Sancho; Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nature Immunology 2002, 3, 159-168, 10.1038/ni753.
- Bello-Gamboa, A.; Izquierdo, J.M.; Velasco, M.; Moreno, S.; Garrido, A.; Meyers, L.; Palomino, J.C.; Calvo, V.; Izquierdo, M. Imaging the Human Immunological Synapse. J. Vis. Exp. 2019.
- Alonso, R.; Mazzeo, C.; Rodriguez, M.C.; Marsh, M.; Fraile-Ramos, A.; Calvo, V.; Avila-Flores, A.; Merida, I.; Izquierdo, M; Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011, 18, 1161–1173, 10.3791/60312.
- Mittelbrunn, M.; Gutierrez-Vazquez, C.; Villarroya-Beltri, C.; Gonzalez, S.; Sanchez-Cabo, F.; Gonzalez, M.A.; Bernad, A.; Sanchez-Madrid, F; Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282.
- Alonso, R.; Rodriguez, M.C.; Pindado, J.; Merino, E.; Merida, I.; Izquierdo, M; Diacylglycerol kinase alpha regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. J. Biol. Chem. 2005, 280, 28439–28450.
- Alonso, R.; Mazzeo, C.; Merida, I.; Izquierdo, M; A new role of diacylglycerol kinase alpha on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. Biochimie 2007, 89, 213–221, 10.3791/60312.
- Rainero, E.; Caswell, P.T.; Muller, P.A.; Grindlay, J.; McCaffrey, M.W.; Zhang, Q.; Wakelam, M.J.; Vousden, K.H.; Graziani, A.; Norman, J.C; et al. Diacylglycerol kinase alpha controls RCP-dependent integrin trafficking to promote invasive migration. J. Cell Biol. 2012, 196, 277–295, 10.3791/60312.
- Herranz, G.; Aguilera, P.; Davila, S.; Sanchez, A.; Stancu, B.; Gomez, J.; Fernandez-Moreno, D.; de Martin, R.; Quintanilla, M.; Fernandez, T; et al. Protein Kinase C delta Regulates the Depletion of Actin at the Immunological Synapse Required for Polarized Exosome Secretion by T Cells. Front. Immunol. 2019, 10, 851.
- Mazzeo, C.; Calvo, V.; Alonso, R.; Merida, I.; Izquierdo, M; Protein kinase D1/2 is involved in the maturation of multivesicular bodies and secretion of exosomes in T and B lymphocytes. Cell Death Differ. 2016, 23, 99–109.
- Choudhuri, K.; Llodra, J.; Roth, E.W.; Tsai, J.; Gordo, S.; Wucherpfennig, K.W.; Kam, L.C.; Stokes, D.L.; Dustin, M.L; Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature 2014, 507, 118–123.
- Saliba, D.G.; Cespedes-Donoso, P.F.; Balint, S.; Compeer, E.B.; Korobchevskaya, K.; Valvo, S.; Mayya, V.; Kvalvaag, A.; Peng, Y.; Dong, T.; et al. Composition and structure of synaptic ectosomes exporting antigen receptor linked to functional CD40 ligand from helper T cells. Elife 2019.