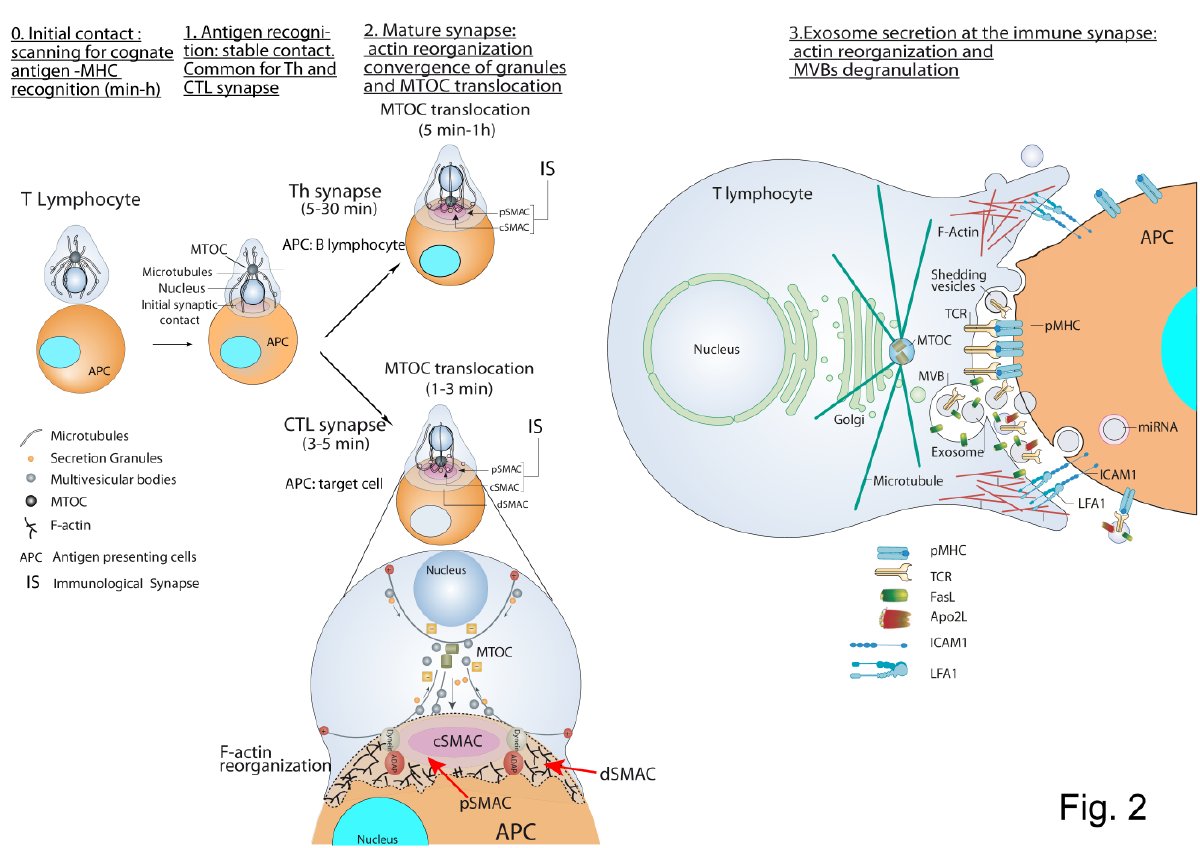
Exosomes are extracellular vesicles (EV) of endosomal origin (multivesicular bodies, MVB) constitutively released by many different eukaryotic cells by fusion of MVB to the plasma membrane. However, inducible exosome secretion controlled by cell surface receptors is restricted to very few cell types and a limited number of cell surface receptors. Among these, exosome secretion is induced in T lymphocytes and B lymphocytes when stimulated at the immune synapse (IS) via T-cell receptor (TCR) and B-cell receptor (BCR), respectively. IS formation by T and B lymphocytes constitutes a crucial event involved in antigen-specific, cellular and humoral immune responses. Upon IS formation by T and B lymphocytes with antigen-presenting cells (APC) the convergence of MVB towards the microtubule organization center (MTOC), and MTOC polarization to the IS, are involved in polarized exosome secretion at the synaptic cleft. This specialized mechanism provides the immune system with a finely-tuned strategy to increase the specificity and efficiency of crucial secretory effector functions of B and T lymphocytes. Since inducible exosome secretion by antigen-receptors is a critical and unique feature of the immune system this review considers the study of the traffic events leading to polarized exosome secretion at the IS and some of their biological consequences.
- exosomes
- T lymphocytes
- B lymphocytes
- polarized secretion
- immune synapse
- T-cell receptor
- B-cell receptor
- multivesicular bodies
- diacylglycerol
- MHC-class II compartment
T Lymphocyte–Antigen Presenting Cell Immune Synapse: Exosome Secretion by T Lymphocytes
CTL-Target Cell Immune Synapse
Th Immune Synapse
- Calvo, V.; Izquierdo, M. Imaging Polarized Secretory Traffic at the Immune Synapse in Living T Lymphocytes. Front. Immunol. 2018, 9, 684. [Google Scholar] [CrossRef] [PubMed]
- Friedl, P.; den Boer, A.T.; Gunzer, M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat. Rev. Immunol. 2005, 5, 532–545. [Google Scholar] [CrossRef] [PubMed]
- Peters, P.J.; Borst, J.; Oorschot, V.; Fukuda, M.; Krahenbuhl, O.; Tschopp, J.; Slot, J.W.; Geuze, H.J. Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 1991, 173, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Vignaux, F.; Vivier, E.; Malissen, B.; Depraetere, V.; Nagata, S.; Golstein, P. TCR/CD3 coupling to Fas-based cytotoxicity. J. Exp. Med. 1995, 181, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Bossi, G.; Griffiths, G.M. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat. Med. 1999, 5, 90–96. [Google Scholar] [CrossRef]
- Nagata, S. Apoptosis by death factor. Cell 1997, 88, 355–365. [Google Scholar] [CrossRef]
- Alonso, R.; Rodriguez, M.C.; Pindado, J.; Merino, E.; Merida, I.; Izquierdo, M. Diacylglycerol kinase alpha regulates the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. J. Biol. Chem. 2005, 280, 28439–28450. [Google Scholar] [CrossRef]
- Blanchard, N.; Lankar, D.; Faure, F.; Regnault, A.; Dumont, C.; Raposo, G.; Hivroz, C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J. Immunol. 2002, 168, 3235–3241. [Google Scholar] [CrossRef]
- Quann, E.J.; Merino, E.; Furuta, T.; Huse, M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat. Immunol. 2009, 10, 627–635. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Jones, D.R.; Izquierdo, M.; Merida, I. Role of diacylglycerol kinase alpha in the attenuation of receptor signaling. J. Cell Biol. 2001, 153, 207–220. [Google Scholar] [CrossRef]
- Chauveau, A.; Le Floc’h, A.; Bantilan, N.S.; Koretzky, G.A.; Huse, M. Diacylglycerol kinase alpha establishes T cell polarity by shaping diacylglycerol accumulation at the immunological synapse. Sci. Signal. 2014, 7, ra82. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, S.; Merida, I. Diacylglycerol, when simplicity becomes complex. Trends Biochem. Sci. 2007, 32, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.S.; Monu, N.; Shen, D.T.; Mecklenbrauker, I.; Radoja, N.; Haydar, T.F.; Leitges, M.; Frey, A.B.; Vukmanovic, S.; Radoja, S. Protein kinase Cdelta regulates antigen receptor-induced lytic granule polarization in mouse CD8+ CTL. J. Immunol. 2007, 178, 7814–7821. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.S.; Haydar, T.F.; Radoja, S. Protein kinase C delta localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J. Immunol. 2008, 181, 4716–4722. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Sattentau, Q.J. Regulated secretion from CD4+ T cells. Trends Immunol. 2007, 28, 474–481. [Google Scholar] [CrossRef]
- Huse, M.; Lillemeier, B.F.; Kuhns, M.S.; Chen, D.S.; Davis, M.M. T cells use two directionally distinct pathways for cytokine secretion. Nat. Immunol. 2006, 7, 247–255. [Google Scholar] [CrossRef]
- Huse, M.; Quann, E.J.; Davis, M.M. Shouts, whispers and the kiss of death: directional secretion in T cells. Nat. Immunol. 2008, 9, 1105–1111. [Google Scholar] [CrossRef]
- Desai, D.M.; Newton, M.E.; Kadlecek, T.; Weiss, A. Stimulation of the phosphatidylinositol pathway can induce T-cell activation. Nature 1990, 348, 66–69. [Google Scholar] [CrossRef]
- Izquierdo, M.; Ruiz-Ruiz, M.C.; Lopez-Rivas, A. Stimulation of phosphatidylinositol turnover is a key event for Fas-dependent, activation-induced apoptosis in human T lymphocytes. J. Immunol. 1996, 157, 21–28. [Google Scholar]
- Montoya, M.C.; Sancho, D.; Bonello, G.; Collette, Y.; Langlet, C.; He, H.T.; Aparicio, P.; Alcover, A.; Olive, D.; Sanchez-Madrid, F. Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nat. Immunol. 2002, 3, 159–168. [Google Scholar] [CrossRef]
- Bello-Gamboa, A.; Izquierdo, J.M.; Velasco, M.; Moreno, S.; Garrido, A.; Meyers, L.; Palomino, J.C.; Calvo, V.; Izquierdo, M. Imaging the Human Immunological Synapse. J. Vis. Exp. 2019. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Mazzeo, C.; Merida, I.; Izquierdo, M. A new role of diacylglycerol kinase alpha on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes. Biochimie 2007, 89, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Rainero, E.; Caswell, P.T.; Muller, P.A.; Grindlay, J.; McCaffrey, M.W.; Zhang, Q.; Wakelam, M.J.; Vousden, K.H.; Graziani, A.; Norman, J.C. Diacylglycerol kinase alpha controls RCP-dependent integrin trafficking to promote invasive migration. J. Cell Biol. 2012, 196, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Herranz, G.; Aguilera, P.; Davila, S.; Sanchez, A.; Stancu, B.; Gomez, J.; Fernandez-Moreno, D.; de Martin, R.; Quintanilla, M.; Fernandez, T.; et al. Protein Kinase C delta Regulates the Depletion of Actin at the Immunological Synapse Required for Polarized Exosome Secretion by T Cells. Front. Immunol. 2019, 10, 851. [Google Scholar] [CrossRef]
- Choudhuri, K.; Llodra, J.; Roth, E.W.; Tsai, J.; Gordo, S.; Wucherpfennig, K.W.; Kam, L.C.; Stokes, D.L.; Dustin, M.L. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature 2014, 507, 118–123. [Google Scholar] [CrossRef]
- Saliba, D.G.; Cespedes-Donoso, P.F.; Balint, S.; Compeer, E.B.; Korobchevskaya, K.; Valvo, S.; Mayya, V.; Kvalvaag, A.; Peng, Y.; Dong, T.; et al. Composition and structure of synaptic ectosomes exporting antigen receptor linked to functional CD40 ligand from helper T cells. Elife 2019. [Google Scholar] [CrossRef]
This entry is adapted from the peer-reviewed paper 10.3390/ijms21072631
References
- R Alonso; Carla Mazzeo; M C Rodriguez; Mark Marsh; Alberto Fraile-Ramos; V Calvo; Antonia Avila-Flores; Isabel Mérida; Manuel Izquierdo; Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death & Differentiation 2011, 18, 1161-1173, 10.1038/cdd.2010.184.
