Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Charles E. Norton | -- | 1355 | 2024-03-28 16:50:13 | | | |
| 2 | Camila Xu | Meta information modification | 1355 | 2024-04-01 07:55:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Norton, C.E. Role of Sensory Nerves in Pulmonary Fibrosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/56518 (accessed on 07 February 2026).
Norton CE. Role of Sensory Nerves in Pulmonary Fibrosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/56518. Accessed February 07, 2026.
Norton, Charles E.. "Role of Sensory Nerves in Pulmonary Fibrosis" Encyclopedia, https://encyclopedia.pub/entry/56518 (accessed February 07, 2026).
Norton, C.E. (2024, March 28). Role of Sensory Nerves in Pulmonary Fibrosis. In Encyclopedia. https://encyclopedia.pub/entry/56518
Norton, Charles E.. "Role of Sensory Nerves in Pulmonary Fibrosis." Encyclopedia. Web. 28 March, 2024.
Copy Citation
Pulmonary fibrosis (PF) is a disease in which the lungs become scarred over time. It can result from occupational exposure, genetic defects, acute lung injury, or idiopathic causes. Sensory nerves are responsible for detecting harmful airborne stimuli and provide input to a variety of cells within the lungs, including airways and blood vessels. They play a critical role in regulating cardiopulmonary functions and maintaining homeostasis in healthy lungs. This review discusses the various effects of sensory nerve signaling in the setting of pulmonary fibrosis.
pulmonary fibrosis
pulmonary hypertension
sensory nerves
cough
1. Introduction
Pulmonary fibrosis (PF) is a disease in which the lungs become scarred over time. It can result from occupational exposure, genetic defects, acute lung injury, or idiopathic causes. PF may also result from a secondary effect of other diseases, including autoimmune disorders and infections. This debilitating condition is associated with dyspnea, cough, and fatigue [1][2] resulting from impaired gas exchange caused by the excessive deposition of extracellular matrix components [3]. This is characterized by fibroproliferation and mononuclear inflammation. The incidence of PF has increased over the last several decades [4], which may be related to increased smoke and particle inhalation, as well as mineral and dust exposure associated with modern, urban lifestyles. The average life expectancy for an individual after being diagnosed with PF is 3 to 5 years [5]. There is currently no cure for the disease and limited therapy options. Thus, identifying treatments that prevent or slow the progression of this disease is vital to improving human health.
Sensory nerves are responsible for detecting harmful airborne stimuli and provide input to a variety of cells within the lungs, including airways and blood vessels. They play a critical role in regulating cardiopulmonary functions and maintaining homeostasis in healthy lungs. Alterations in the phenotype and sensitivity of these fibers are a hallmark of lung diseases, including asthma, viral infections, chronic obstructive pulmonary disease, and pulmonary fibrosis [6][7]. Despite such changes in function, sensory nerve signaling can downregulate PF [8][9], but the mechanisms through which sensory nerves are modified by and contribute to this disease are just beginning to be explored.
2. Sensory Nerves in the Lungs
Within the lungs, innervation is most dense in extrapulmonary and hilar arteries [10][11][12]. The penetration of sympathetic and parasympathetic fibers varies between species but frequently stops shortly after the lung hilus [13][14]. Sensory/peptidergic fibers, in contrast, are found sparingly in vessels throughout the pulmonary vascular tree, airways, and alveoli [15][16][17][18][19]. Sensory nerves confer information about the local environment to the central nervous system, with their cell bodies located within the dorsal root ganglia [20]. Within the lungs, sensory (peptidergic) fibers play a vital role in regulating cardiopulmonary function under both healthy and disease conditions. These nerves are not homogenous in nature, having different anatomical and physiological phenotypes reflecting their location and purpose, and sensory nerve pattern and density vary with age, tissue, and vascular bed [21][22]. Each of these subtypes provides input to the central nervous system and is capable of driving cardiorespiratory reflexes. Unlike sympathetic nerves, sensory nerves can signal both antidromically and orthodromically, thus facilitating their participation in local axon reflexes independent of efferent signaling from the cell body [23]. Ergo, local stimuli experienced in the tissue, such as mechanical or chemical responses, can lead to neurotransmitter release and signaling independent of the central nervous system.
Bronchopulmonary sensory nerves are highly varied based on properties including location in the lungs, ganglionic origin, activation profile, conduction velocity, and responses that nerve activation elicits. Key classes of sensory fibers include nociceptors and mechanosensors. Stretch-sensitive mechanosensors are a group of afferent nerve fibers that respond to the nonharmful distension of the lungs that occurs during respiration [24]. The activation of these fibers is dependent on the rate and depth of breathing (i.e., tidal volume), and they can be grouped into rapidly adapting fibers located in the mucosal layer and slow-adapting fibers located in proximity to SMCs [25]. Nociceptors, in contrast, respond to lung injury and are classified into touch-sensitive cough fibers and bronchopulmonary C fibers [24]. Regardless of fiber type, pulmonary sensory nerves produce biologically active peptides, including substance P, neurokinin A, and calcitonin gene-related peptide (CGRP) [26], and immunostaining for these transmitters is utilized to identify sensory nerves (Figure 1) [19][27].
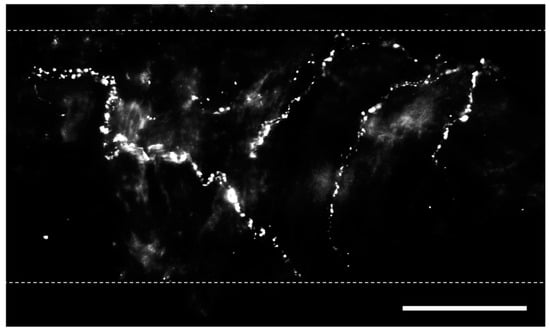
Figure 1. Perivascular sensory nerves on mouse pulmonary arteries. Representative calcitonin gene-related peptide (CGRP) staining (maximum z projections) on the surface of a ~100 µm pulmonary artery. Dotted lines indicate approximate vessel edge. Scale bar = 50 µm.
2.1. Calcitonin Gene-Related Peptide
CGRP is a 37-amino acid peptide that serves as the primary neurotransmitter released from sensory nerves [28] and is a potent vasodilator [29]. CGRP is synthesized in both central and peripheral sensory neurons and transported along axons in vesicles, where it is released [30]. Subsequently, CGRP can activate G-protein receptor-coupled CGRP receptors in airways and blood vessels [31][32]. These receptors are composed of calcitonin receptor-like receptor (CRLR), receptor activity-modifying protein 1 (RAMP1, the site of ligand binding and specificity), and receptor component protein [33]. CGRP signaling is terminated solely by degradation, as it does not undergo reuptake [28]. Although CGRP signaling can directly stimulate vasodilation in SMCs [31][34][35], an endothelium-dependent pathway that is mediated through nitric oxide signaling can also modulate this response [19][36].
Adenosine triphosphate (ATP) is released as a co-transmitter with CGRP [37][38]. ATP can activate two types of purinergic receptors, P2X and P2Y, which are located in airway and vascular cells [39][40]. Given the variety of receptor subtypes to which ATP can bind, purinergic signaling can lead to both contractile and dilatory effects on the target tissue [41]. Purinergic receptor expression also varies with vessel size [21]. Unlike CGRP, ATP, which is rapidly broken down into adenosine, can be reuptaken by the cell [42]. However, given that ATP can be released from sympathetic neurons erythrocytes, and other non-neuronal cell types in addition to sensory nerves, [43][44], it can be challenging to identify the source of ATP responsible for paracrine signaling.
2.2. Substance P
Substance P is synthesized in cell bodies within the dorsal root ganglia and transported in vesicles, along with CGRP and ATP, via axons to sensory nerve terminals [28][45]. Following its release, substance P elicits its effect by binding to neurokinin-1 receptors (NK1Rs) coupled to G-protein signaling in airways [46] and in vascular ECs [28][47]. Substance P does not undergo reuptake and, therefore, continues exerting its effects until undergoing enzymatic degradation [45]. Its vascular effects include hyperpolarization and increases in [Ca2+]i in ECs, which activate endothelial nitric oxide synthase (eNOS) [47][48]. This causes hyperpolarization and vasorelaxation in SMCs. The dependency of this response on endothelial nitric oxide signaling is demonstrated by the loss of SMC hyperpolarization when the endothelium is disrupted or when eNOS is inhibited in isolated mesenteric arteries [47].
The physiological role of substance P in the vasculature remains controversial. Given that substance P has minimal effects on adjacent SMCs, the levels that reach endothelial cells are not sufficient to alter vessel diameter and permeability in all vascular beds [49]. The exogenous application of substance P has minimal effect on vessel diameter in mesenteric and hepatic vessels [50][51]; however, it can regulate the diameter of pulmonary arteries [52].
2.3. Neurokinin A
Neurokinin A is a 10-amino acid peptide that belongs to the same family of tachykinins as substance P. Neurokinin A has the highest affinity for neurokinin-2 receptors (NK2Rs) [53], although it is also capable of NK1R activation [54]. NK2Rs are present in airways [55], vascular SMCs [56], and ECs [57]. Neurokinin A is a potent bronchoconstrictor [58] but produces a modest pressor response in the vasculature [59]. Neurokinin A is proinflammatory and can also activate macrophages [54].
2.4. Interaction with Sympathetic Nerves
In addition to their direct effects on signaling, sensory perivascular nerves can interact through negative feedback to regulate sympathetic neurotransmission. The peptidergic transmitters CGRP and substance P can reduce the amplitude of sympathetically evoked vasoconstrictions [21]. These effects are mediated by the prejunctional inhibition of sympathetic nerve terminals without affecting downstream signaling pathways [60]. In a reciprocal manner, sympathetic perivascular nerves can inhibit the activity of sensory nerves [61]. In rat mesenteric arteries, norepinephrine (NE) acts on prejunctional α2 adrenoreceptors of sensory nerve terminals to reduce the release of CGRP [62]. ATP released by sympathetic nerves can also bind to P2Y receptors on peptidergic fibers to prevent CGRP signaling [63]. As the presence of sympathetic nerves in the lungs is relatively modest in many organisms, such effects likely vary across species.
References
- Mann, J.; Goh, N.S.L.; Holland, A.E.; Khor, Y.H. Cough in Idiopathic Pulmonary Fibrosis. Front. Rehabil. Sci. 2021, 2, 751798.
- van Manen, M.J.G.; Birring, S.S.; Vancheri, C.; Cottin, V.; Renzoni, E.A.; Russel, A.; Wijsenbeek, M.S. Cough in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2016, 25, 278–286.
- Behr, J.; Ryu, J.H. Pulmonary hypertension in interstitial lung disease. Eur. Respir. J. 2008, 31, 1357–1367.
- Marshall, D.C.; Salciccioli, J.D.; Shea, B.S.; Akuthota, P. Trends in mortality from idiopathic pulmonary fibrosis in the European Union: An observational study of the WHO mortality database from 2001–2013. Eur. Respir. J. 2018, 51, 1701603.
- Ryu, J.H.; Colby, T.V.; Hartman, T.E. Idiopathic pulmonary fibrosis: Current concepts. Mayo Clin. Proc. 1998, 73, 1085–1101.
- Mazzone, S.B.; Undem, B.J. Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 2016, 96, 975–1024.
- Yegen, C.H.; Marchant, D.; Bernaudin, J.F.; Planes, C.; Boncoeur, E.; Voituron, N. Chronic pulmonary fibrosis alters the functioning of the respiratory neural network. Front. Physiol. 2023, 14, 1205924.
- Hartopo, A.B.; Emoto, N.; Vignon-Zellweger, N.; Suzuki, Y.; Yagi, K.; Nakayama, K.; Hirata, K. Endothelin-converting enzyme-1 gene ablation attenuates pulmonary fibrosis via CGRP-cAMP/EPAC1 pathway. Am. J. Respir. Cell Mol. Biol. 2013, 48, 465–476.
- Li, X.W.; Li, X.H.; Du, J.; Li, D.; Li, Y.J.; Hu, C.P. Calcitonin gene-related peptide down-regulates bleomycin-induced pulmonary fibrosis. Can. J. Physiol. Pharmacol. 2016, 94, 1315–1324.
- El-Bermani, A.W.; Bloomquist, E.I.; Montvilo, J.A. Distribution of pulmonary cholinergic nerves in the rabbit. Thorax 1982, 37, 703–710.
- Fisher, A.W.F. The intrinsic innervation of the pulmonary vessels. Acta Anat. 1965, 60, 481–496.
- Haberberger, R.; Schemann, R.; Sann, M.; Kummer, W. Innervation pattern of guinea pig pulmonary vasculature depends of vascular diameter. J. Appl. Physiol. 1997, 82, 426–434.
- Cech, S. Cholinesterase-containing nerve fibres on blood vessels in lungs of some laboratory mammals. Z. Zellforsch. Mikrosk. Anat. 1973, 140, 91–100.
- Su, Y.; Barr, J.; Jaquish, A.; Xu, J.; Verheyden, J.M.; Sun, X. Identification of lung innervating sensory neurons and their target specificity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 322, L50–L63.
- Cadieux, A.; Springall, D.R.; Mulderry, P.K.; Rodrigo, J.; Ghatei, M.A.; Terenghi, G.; Bloom, S.R.; Polak, J.M. Occurrence, distribution and ontogeny of CGRP immunoreactivity in the rat lower respiratory tract: Effect of capsaicin treatment and surgical denervations. Neuroscience 1986, 19, 605–627.
- Komatsu, T.; Yamamoto, M.; Shimokata, K.; Nagura, H. Distribution of substance P-immunoreactive and calcitonin gene-related peptide-immunoreactive nerve in normal human lungs. Int. Arch. Allergy Immunol. 1991, 95, 23–28.
- Kummer, W.A.; Fischer, A.; Kurkowski, R.; Heym, C. The sensory and sympathetic innervation of guinea-pig lung and trachea as studied by retrograde neuronal tracing and double-labelling immunohistochemistry. Neuroscience 1992, 49, 715–737.
- Martling, C.R.; Matran, R.; Alving, K.; Hökfelt; Lundberg, J.M. Innervation of lower airways and neuropeptide effects on bronchial and vascular tone in the pig. Cell Tissue Res. 1990, 260, 223–233.
- Norton, C.E.; Segal, S.S. Calcitonin gene-related peptide hyperpolarizes mouse pulmonary artery endothelial tubes through KATP activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 315, L212–L226.
- Hobara, N.; Nakamura, A.; Ohtsuka, A.; Narasaki, M.; Shibata, K.; Bomoita, Y.; Kawasaki, H. Distribution of adrenomedullin-containing perivascular nerves in the rat mesenteric artery. Peptided 2004, 25, 589–599.
- Westcott, E.B.; Segal, S.S. Perivascular innervation: A multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation 2013, 20, 217–238.
- Boerman, E.M.; Segal, S.S. Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age. J. Physiol. 2016, 594, 2323–2338.
- Yabrak, M. The axon reflex. Neuroanatomy 2008, 7, 17–19.
- Undem, B.J.; Kollarik, M. The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005, 2, 355–360.
- Sant’Ambrogio, G.; Widdicombe, J.G. Reflexes from airway rapidly adapting receptors. Respir. Physiol. 2001, 125, 33–45.
- Kummer, W.A. Pulmonary vascular innervation and its role in responses to hypoxia: Size matters! Proc. Am. Thorac. Soc. 2011, 8, 471–476.
- Grasby, D.J.; Morris, J.L.; Segal, S.S. Heterogeneity of vascular innervation in hamster cheek pouch and retractor muscle. J. Vasc. Res. 1999, 36, 465–476.
- Brain, S.D.; Grant, A.D. Vascular action of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004, 84, 903–934.
- Brain, S.D.; Williams, T.J.; Tippins, J.R.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985, 313, 54–56.
- Kashihara, Y.; Sakaguchi, M.; Kuno, M. Axonal transport and distribution of endogenous calcitonin gene-related peptide in rat peripheral nerve. J. Neurosci. 1989, 9, 3796–3802.
- Bell, D.; McDermott, B.J. Calcitonin gene-related peptide in the cardiovascular system: Characterizations of receptor populations and their (patho)physiological signaificance. Am. J. Physiol. Lung Cell. Mol. Physiol. 1996, 290, L661–L673.
- Dakhama, A.; Larsen, G.L.; Gelfand, E.W. Calcitonin gene-related peptide: Role in airway homeostasis. Curr. Opin. Pharmacol. 2004, 4, 215–220.
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339.
- Nelson, M.T.; Huang, Y.; Brayden, J.E.; Herscheler, J.; Standen, N.B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature 1990, 344, 770–773.
- Wellman, G.C.; Quayle, J.M.; Standen, N.B. ATP-sensitive K+ channel activation by calcitonin gene related peptide and protein kinase A in pig coronary arterial smooth muscle. J. Physiol. 1998, 507, 117–129.
- Wisskirchen, F.M.; Burt, R.P.; Marshall, I. Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br. J. Pharmacol. 1998, 123, 1673–1683.
- Kawasaki, H.; Takasaki, S.; Saito, A.; Goto, K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature 1988, 335, 164–167.
- Holton, P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J. Physiol. 1959, 145, 494–504.
- Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007, 87, 659–797.
- Thompson, R.J.; Sayers, I.; Kuokkanen, K.; Hall, I.P. Purinergic receptors in the airways: Potential therapeutic targets for asthma? Front. Allergy 2021, 2, 677677.
- Burnstock, G. Purinergic regulation of vascular tone and remodelling. Auton. Autacoid Pharmacol. 2009, 29, 63–72.
- Le, T.T.T.; Berg, N.K.; Harting, M.T.; Li, X. Purinergic signaling in pulmonary inflammation. Front. Immunol. 2019, 10, 1633.
- Kennedy, C.; Saville, V.L.; Burnstock, G. The contributions of noradrenaline and ATP to the responses of the rabbit central ear artery to sympathetic nerve stimulation depend on the parameters of stimulation. Eur. J. Pharmacol. 1986, 122, 291–300.
- Lohman, A.W.; Billaud, M.; Isakson, B.E. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc. Res. 2012, 95, 269–280.
- White, J.D.; Stewart, K.D.; Krause, J.E.; McKelvy, J.F. Biochemistry of peptide-secreting neurons. Physiol. Rev. 1985, 65, 553–606.
- Sponchiado, M.; Liao, Y.S.; Atanasova, K.R.; Collins, E.N.; Schurmann, V.; Bravo, L.; Reznikov, L.R. Overexpression of Substance P in pig airways increases MUC5AC through an NF-kβ pathway. Physiol. Rep. 2021, 9, e14749.
- Norton, C.E.; Boerman, E.M.; Segal, S.S. Differential hyperpolarization to substance P and calcitonin gene related peptide in smooth muscle versus endothelium of mouse mesenteric artery. Microcirculation 2021, 28, e12733.
- Brain, S.D.; Cox, H.M. Neuropeptides and their receptors: Innovative science providing novel therapeutic targets. Br. J. Pharmacol. 2006, 147, S202–S211.
- Brain, S.D. Sensory neuropeptides: Their role in inflammation and wound healing. Immunopharmacology 1997, 37, 133–152.
- Li, Y.J.; Duckles, S.P. Effect of endothelium on the actions of sympathetic and sensory nerves in the perfused rat mesentery. Eur. J. Pharmacol. 1992, 210, 23–30.
- Phillips, J.K.; Hickey, H.; Hill, C.E. Heterogeneity in mechanisms underlying vasodilatory responses in small arteries of the rat hepatic mesentery. Auton. Neurosci. 2000, 83, 159–170.
- Archer, S.L.; Kulik, T.J.; Chesler, E.; Weir, E.K. The effects of substance P on the preconstricted pulmonary vasculature of the anesthetized dog. Proc. Soc. Exp. Biol. Med. 1986, 183, 19–27.
- Regoli, D.; Drapeau, G.; Dorleans-Just, P. Pharmacological receptors for substance P and neurokinins. Life Sci. 1987, 40, 109–117.
- Sung, J.; Ramnath, D.; Tamizhselvi, R.; Bhatia, M. Neurokinin A engages neurokinin-1 receptor to induce NF-κB-dependent gene expression in murine macrophages: Implications of ERK1/2 and PI 3-kinase/Akt pathways. Am. J. Physiol. Cell Physiol. 2008, 295, C679–C691.
- Hernandez, J.M.; Cox, G.; Janssen, L.J. Involvement of the neurokinin-2 receptor in airway smooth muscle stretch-activated contractions assessed in perfused intact bovine bronchial segments. J. Pharmacol. Exp. Ther. 2008, 327, 503–510.
- Dion, S.; Rouissi, N.; Nantel, F.; Jukic, D.; Rhaleb, N.E.; Tousignant, C.; Telemaque, S.; Drapeau, G.; Regoli, D.; Naline, E.; et al. Structure-activity study of neurokinins: Antagonists for the neurokinin-2 receptor. Pharmacology 1990, 41, 184–194.
- Gallicchio, M.; Rosa, A.C.; Benetti, E.; Collino, M.; Dianzani, C.; Fantozzi, R. Substance P-induced cyclooxygenase-2 expression in human umbilical vein endothelial cells. Br. J. Pharmacol. 2006, 147, 681–689.
- Haley, K.J.; Sunday, M.E.; Osathanondh, R.; Du, J.; Vathanaprida, C.; Karpitsky, V.V.; Krause, J.E.; Lilly, C.M. Developmental expression of neurokinin A and functional neurokinin-2 receptors in lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L1348–L1358.
- Couture, R.; Laneuville, O.; Guimond, C.; Drapeau, G.; Regoli, D. Characterization of the peripheral action of neurokinins and neurokinin receptor selective agonists on the rat cardiovascular system. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1989, 340, 547–557.
- Kotecha, N.; Neild, T.O. Actions of vasodilator nerves on arteriolar smooth muscle and neurotransmitter release from sympathetic nerves in the guinea-pig small intestine. J. Physiol. 1995, 489, 849–855.
- Kawasaki, H. Regulation of vascular function by perivascular calcitonin gene-related peptide-containing nerves. Jpn. J. Pharmacol. 2002, 88, 39–43.
- Kawasaki, H.; Nuki, C.; Saito, A.; Takasaki, K. Adrenergic modulation of calcitonin gene-related peptide (CGRP)-containing nerve-mediated vasodilation in the rat mesenteric resistance vessel. Brain Res. 1990, 506, 287–290.
- Donoso, M.V.; Hermosilla, D.; Navarrete, C.; Alzvarez, P.; Lillo, J.G.; Huidobro-Toro, J.P. Reciprocal sympatho-sensory control: Functional role of nucleotides and calcitonin gene-related peptide in a peripheral neuroeffector junction. Neuroscience 2012, 203, 216–229.
More
Information
Subjects:
Physiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
453
Revisions:
2 times
(View History)
Update Date:
01 Apr 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




