Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Charles E. Norton and Version 2 by Camila Xu.
Pulmonary fibrosis (PF) is a disease in which the lungs become scarred over time. It can result from occupational exposure, genetic defects, acute lung injury, or idiopathic causes. Sensory nerves are responsible for detecting harmful airborne stimuli and provide input to a variety of cells within the lungs, including airways and blood vessels. They play a critical role in regulating cardiopulmonary functions and maintaining homeostasis in healthy lungs. This review discusses the various effects of sensory nerve signaling in the setting of pulmonary fibrosis.
- pulmonary fibrosis
- pulmonary hypertension
- sensory nerves
- cough
1. Introduction
Pulmonary fibrosis (PF) is a disease in which the lungs become scarred over time. It can result from occupational exposure, genetic defects, acute lung injury, or idiopathic causes. PF may also result from a secondary effect of other diseases, including autoimmune disorders and infections. This debilitating condition is associated with dyspnea, cough, and fatigue [1][2][1,2] resulting from impaired gas exchange caused by the excessive deposition of extracellular matrix components [3]. This is characterized by fibroproliferation and mononuclear inflammation. The incidence of PF has increased over the last several decades [4], which may be related to increased smoke and particle inhalation, as well as mineral and dust exposure associated with modern, urban lifestyles. The average life expectancy for an individual after being diagnosed with PF is 3 to 5 years [5]. There is currently no cure for the disease and limited therapy options. Thus, identifying treatments that prevent or slow the progression of this disease is vital to improving human health.
Sensory nerves are responsible for detecting harmful airborne stimuli and provide input to a variety of cells within the lungs, including airways and blood vessels. They play a critical role in regulating cardiopulmonary functions and maintaining homeostasis in healthy lungs. Alterations in the phenotype and sensitivity of these fibers are a hallmark of lung diseases, including asthma, viral infections, chronic obstructive pulmonary disease, and pulmonary fibrosis [6][7][6,7]. Despite such changes in function, sensory nerve signaling can downregulate PF [8][9][8,9], but the mechanisms through which sensory nerves are modified by and contribute to this disease are just beginning to be explored.
2. Sensory Nerves in the Lungs
Within the lungs, innervation is most dense in extrapulmonary and hilar arteries [10][11][12][10,11,12]. The penetration of sympathetic and parasympathetic fibers varies between species but frequently stops shortly after the lung hilus [13][14][13,14]. Sensory/peptidergic fibers, in contrast, are found sparingly in vessels throughout the pulmonary vascular tree, airways, and alveoli [15][16][17][18][19][15,16,17,18,19]. Sensory nerves confer information about the local environment to the central nervous system, with their cell bodies located within the dorsal root ganglia [20]. Within the lungs, sensory (peptidergic) fibers play a vital role in regulating cardiopulmonary function under both healthy and disease conditions. These nerves are not homogenous in nature, having different anatomical and physiological phenotypes reflecting their location and purpose, and sensory nerve pattern and density vary with age, tissue, and vascular bed [21][22][21,22]. Each of these subtypes provides input to the central nervous system and is capable of driving cardiorespiratory reflexes. Unlike sympathetic nerves, sensory nerves can signal both antidromically and orthodromically, thus facilitating their participation in local axon reflexes independent of efferent signaling from the cell body [23]. Ergo, local stimuli experienced in the tissue, such as mechanical or chemical responses, can lead to neurotransmitter release and signaling independent of the central nervous system. Bronchopulmonary sensory nerves are highly varied based on properties including location in the lungs, ganglionic origin, activation profile, conduction velocity, and responses that nerve activation elicits. Key classes of sensory fibers include nociceptors and mechanosensors. Stretch-sensitive mechanosensors are a group of afferent nerve fibers that respond to the nonharmful distension of the lungs that occurs during respiration [24]. The activation of these fibers is dependent on the rate and depth of breathing (i.e., tidal volume), and they can be grouped into rapidly adapting fibers located in the mucosal layer and slow-adapting fibers located in proximity to SMCs [25]. Nociceptors, in contrast, respond to lung injury and are classified into touch-sensitive cough fibers and bronchopulmonary C fibers [24]. Regardless of fiber type, pulmonary sensory nerves produce biologically active peptides, including substance P, neurokinin A, and calcitonin gene-related peptide (CGRP) [26], and immunostaining for these transmitters is utilized to identify sensory nerves (Figure 1) [19][27][19,27].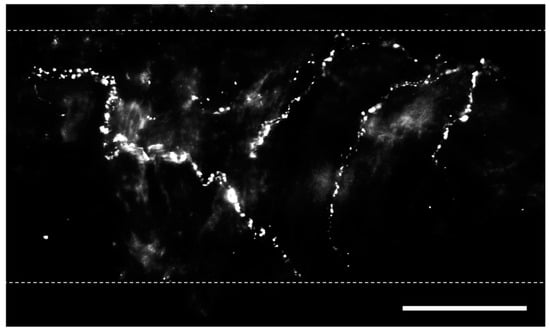
Figure 1. Perivascular sensory nerves on mouse pulmonary arteries. Representative calcitonin gene-related peptide (CGRP) staining (maximum z projections) on the surface of a ~100 µm pulmonary artery. Dotted lines indicate approximate vessel edge. Scale bar = 50 µm.
