Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria de los Angeles Garavagno | -- | 2397 | 2024-03-26 15:52:31 | | | |
| 2 | Camila Xu | Meta information modification | 2397 | 2024-03-27 03:42:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Garavagno, M.D.L.A.; Holland, R.; Khan, M.A.H.; Orr-Ewing, A.J.; Shallcross, D.E. Toxicity and Physicochemical Properties of Trifluoroacetic Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/56491 (accessed on 08 February 2026).
Garavagno MDLA, Holland R, Khan MAH, Orr-Ewing AJ, Shallcross DE. Toxicity and Physicochemical Properties of Trifluoroacetic Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/56491. Accessed February 08, 2026.
Garavagno, Maria De Los Angeles, Rayne Holland, Md Anwar Hossain Khan, Andrew J. Orr-Ewing, Dudley E. Shallcross. "Toxicity and Physicochemical Properties of Trifluoroacetic Acid" Encyclopedia, https://encyclopedia.pub/entry/56491 (accessed February 08, 2026).
Garavagno, M.D.L.A., Holland, R., Khan, M.A.H., Orr-Ewing, A.J., & Shallcross, D.E. (2024, March 26). Toxicity and Physicochemical Properties of Trifluoroacetic Acid. In Encyclopedia. https://encyclopedia.pub/entry/56491
Garavagno, Maria De Los Angeles, et al. "Toxicity and Physicochemical Properties of Trifluoroacetic Acid." Encyclopedia. Web. 26 March, 2024.
Copy Citation
Trifluoroacetic acid (TFA) is a known and persistent pollutant in the environment. Although several direct anthropogenic sources exist, production from the atmospheric degradation of fluorocarbons such as some hydrofluorocarbons (HFCs) has been a known source for some time. The current transition from HFCs to HFOs (hydrofluoroolefins) is beneficial from a global warming viewpoint because HFOs are much shorter-lived and pose a much smaller threat in terms of warming, but the fraction of HFOs converted into TFA is higher than seen for the corresponding HFCs and the region in which TFA is produced is close to the source. Therefore, it is timely to review the role of TFA in the Earth’s environment.
TFA
HFO
sources and sinks
lifetime
toxicity
1. Introduction
Trifluoroacetic acid (TFA, CF3COOH) is the shortest-chain species of perfluorinated carboxylic acid (PFCA) and the broader family of perfluorinated carboxylates. It is one of thousands of compounds categorized as a per- and polyfluoroalkyl substance (PFAS). PFCAs include all substances that have a carboxylic group and at least one perfluoroalkyl moiety, represented by CnF2n+1 (where n ≥ 1) [1]. Many long-chain PFASs have been found to potentially cause adverse effects on human and animal health in several in vitro and in vivo studies [2][3][4][5][6][7][8][9], to be ubiquitous and persistent in the environment, and to bio-magnify along food chains [2][10][11][12][13][14][15]. Over 9000 PFASs have been identified to date and many face regulations that restrict their use because of their toxicity to humans and animals [16][17][18][19][20][21]. In view of these regulations, studies have recently shifted their focus towards shorter-chain PFASs, which are set to replace those long-chain versions most used [22][23]. In fact, many of the short-chain PFASs that are replacing more traditional long-chain PFASs are already beginning to see widespread environmental occurrence and may present similar challenges [24][25].
Unlike many other PFASs, which are non-polar and largely insoluble in water, short-chain PFASs such as TFA are highly mobile in the environment and accumulate in environmental aqueous phases due to their high solubility [26]. Current research suggests that most short-chain PFASs, including TFA, do not bioaccumulate in food chains and are rapidly excreted from humans, although some reports state that TFA has the potential to bioaccumulate in plant material [27]. A 2012 study by Russell et al. [25], sponsored by DuPont, collected measurement data for TFA in aquatic systems across the world and then carried out a detailed modelling analysis of TFA levels in aquatic systems across the USA. Comparing measured and modelled data with ecotoxicity data for freshwater algae, marine algae, aquatic plants, crustacea, and fish, they concluded that, even with rising levels of TFA predicted from the use of HFOs, aquatic life would be ‘unaffected’ by these levels of TFA [28]. Most tests on microorganisms have found that high TFA concentrations cause no harm, although one species of algae did show inhibited growth when exposed to extremely high concentrations [27]. Biological and medical research is encouraging in its unanimous findings that TFA does not bioaccumulate and exhibits low-to-moderate toxicity in a range of organisms, even in instances of very high exposure [29][30]. Furthermore, anthropogenically generated TFA is not expected to contribute significantly to acid rain or further terrestrial acidification [31]. But caution must be taken not to underestimate the impacts of TFA as its anthropogenic sources increase.
Even in the absence of evidence that TFA presents a significant risk to humans or the environment, its persistence due to its environmental stability [29][32][33], combined with constant or increasing emissions, will likely result in significant accumulation in the environment. TFA contamination would then be widespread, long-lasting, and difficult to remove; this would present a huge challenge if future research were to discover any detrimental effects of TFA. Indeed, TFA is already being observed in significant and increasing quantities in surface water, rain, fog, sewage treatment plants, snow, the atmosphere, and sediments [30][34][35][36][37][38][39][40][41][42][43][44]. TFA rapidly dissociates into its deprotonated form, trifluoroacetate, in an aquatic environment, particularly in aqueous phases [30]. Therefore, in the context of environmental contamination, TFA salts are the most important compounds to consider. As a result, TFA and subsequent trifluoroacetate contamination have been considered in a range of international regulatory assessments conducted by the United Nations Environmental Program (UNEP) since 1998 [30][41]. Depending on their ecological or toxicological properties, persistent substances can pose a threat to the environment as they are irrecoverable and lead to environmental pollution lasting decades to centuries, and eventually longer.
2. Physicochemical Properties
TFA (CAS: 76-05-1, MW = 114.02 g mol−1) is a colorless, volatile liquid with a distinctive odor and a density of about 1.49 g cm−3 at 25 °C. It has a melting point of approximately −15.4 °C and a boiling point between 72 and 74 °C [30][45]. It is soluble in water and various organic solvents such as methanol, ethanol, acetone, and chloroform [45]. The solubility of TFA in water has been shown to decrease with decreasing pH, as would be expected for an acidic species [46][47]. The physicochemical properties of TFA have been listed in Table 1.
Table 1. Physicochemical properties of TFA.
TFA is strongly acidic due to the inductive effect of the highly electronegative fluorine atoms, which draw electron density away from the carboxylate group and stabilize the anion. Similarly, the resonance of the C-F bond stabilizes the anion as electron density is distributed more evenly. The acid dissociation constant of TFA, the pKa, is contested in the literature, but is generally accepted to be between 0.2 and 0.5 [30][45][46][48][49][50][51]. This value is over 34,000 times more acidic than acetic acid. As a result, TFA is highly soluble in water. This rapid dissociation in aqueous solutions produces the TFA anion, known as trifluoroacetate, Figure 1. Environmentally, this means that TFA is expected to partition entirely into aqueous phases.
Figure 1. The equilibrium between trifluoroacetic acid and its conjugate base.
The trifluoroacetate anion persists in soil and environmental aqueous phases due to the high chemical and biological stability of the C-F bond [30]. TFA and its salts are highly soluble. As such, they are found in rain, fog, and water bodies, with large reservoirs present in the ocean. TFA has no known degradation pathways in environmental aqueous phases [53].
Henry’s Law describes the proportionality between the amount of dissolved gas in a liquid and the partial pressure above said liquid; this is a metric commonly used in atmospheric chemistry. The Henry’s Law constant of TFA was originally reported to be (9 ± 2) × 103 mol kg−1 atm−1 at 298 K [49]. More recently, Kutsuna et al. [46] proposed a Henry’s law constant 0.63 times smaller than that of Bowden et al. [49] when assuming pKa = 0.47, and equal to that determined by Bowden et al. [49] when assuming pKa = 0.2 [46]. The uncertainty in the pKa of TFA, arising as a result of significant variability in values reported depending on the measurement technique used, is suggested to be the main cause of discrepancies between calculated Henry’s Law constants [46]. Additionally, the solvation energy used is crucial in the calculation of pKa and reported values for this property vary significantly [46][49].
The behavior of TFA with respect to gas-to-aerosol partitioning around 298 K is likely to be similar to that discussed by Bowden et al. [49]. These authors reported that TFA in the atmosphere will partition entirely into fog and cloud water, but that partitioning into smaller amounts of liquid water, e.g., aerosol, is more complex. Here, TFA may partition into the liquid phase in alkaline aerosol, but less so as the aerosol becomes more acidic, and this idea has support in the wider literature [49][54][55]. Interestingly, Kazil et al. suggested that, upon cloud evaporation, dissolved TFA is released into the gas phase [54]. While the participation of TFA in aerosol formation and its ability to partition into existing aerosols is complicated, with dependencies on pH, temperature, and aerosol composition [49], research supports the contribution of TFA to aerosol formation [56]. However, a recent study demonstrated that quantifying the gas-particle-phase partitioning of TFA is particularly sensitive to predicted physical properties under atmospherically relevant conditions [55].
Using previous global model integrations as a basis for research [57] (see Supplementary Materials for more details about the integration carried out) and utilizing the global 3D chemical transport model STOCHEM-CRI, integrations have been carried out to assess the sensitivity of the global TFA burden to the choice of Henry’s Law constant. Specifically, the TFA Henry’s Law constant presented in Kutsana et al. [46] 5.7 × 101 mol m−3 Pa−1 has been used to determine the upper limits for dynamic and convective scavenging coefficients (2.2 cm−1 and 4.3 cm−1, respectively) that represent wet deposition [57]. The model used has been described elsewhere, and previously published work provides a STOCHEM-base scenario (which involves HFO-CI-HETFA, using dynamic and convective scavenging coefficients of 1.9 cm–1 and 3.8 cm–1 derived from the average of the Henry’s Law constants reported by Sander et al. [58]) for comparison [57].
The updated simulation with altered scavenging coefficients, referred to as STOCHEM-USC, shows a small increase in the wet deposition flux of TFA by ~0.5% compared to the base scenario, with a corresponding decrease in TFA global burden of ~4.5%. Figure 2 depicts the absolute and percentage variations between the STOCHEM-base and STOCHEM-USC simulations. While a small shift is seen globally, the most significant absolute changes are found in a small section of the upper latitudes.
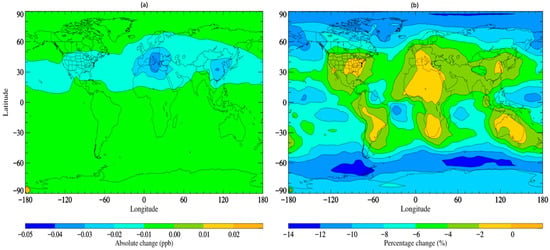
Figure 2. Surface distribution plot depicting the (a) absolute and (b) percentage changes in. TFA concentrations from STOCHEM-base to STOCHEM-USC simulation.
Areas of reduced atmospheric TFA concentrations represent areas of greater deposition and therefore higher environmental contamination.
Wet deposition is the dominant loss process for atmospheric TFA (being responsible for approximately 80% of total atmospheric TFA loss according to results published previously) [57]. Therefore, it is also the primary route to TFA environmental contamination. Given its high solubility, major concerns with TFA contamination center around environmental aqueous phases, e.g., rivers, lakes, and surface waters. As a result of pervasive TFA contamination, there have been some attempts to quantify the toxicity of TFA and its salts, particularly for species whose habitats are especially impacted.
3. Toxicity
The accumulation of TFA, or rather its deprotonated form trifluoroacetate, in environmental aqueous phases is well established; as a result, it is becoming increasingly important to consider the degree of its potential toxicity. To date, the toxicological and ecotoxicological properties of short-chain PFASs, including TFA, have arguably been only sparsely investigated [41].
Given that TFA is likely to partition in the environment into its deprotonated form, toxicity assessments are generally conducted with the anion, trifluoroacetate. Overall, the reported toxicity is low, with only one aquatic alga presenting a concerning sensitivity to TFA [59]. More generally, the resistance of aquatic plants to the effects of TFA contamination is demonstrated in several reports [60][61], with Berends et al. reporting a ‘safe’ TFA level of 0.10 mg L−1 for aquatic life [59].
While aquatic plant species are expected to be exposed to elevated TFA concentrations given its accumulation in water, terrestrial plants associated with these same water systems may also experience significant exposure [62][63]. For example, Zhang et al. reported the efficient absorption and uptake of TFA by wheat roots, with no tendency to reach a steady state [64]. Additionally, a number of studies reported that direct uptake from the atmosphere can contribute to high levels of TFA in terrestrial plant leaves [65][66][67]. Some terrestrial plants have been reported to bioaccumulate TFA [68], with bioconcentration factors reported to span from 4.9 to 1439 [29][65][69][70]. As a result, the toxicity they experience may be higher.
The unreactive nature of TFA in the environment appears to translate into an inertness in organisms, thereby limiting its toxic effects [63]. TFA has been detected in a wide range of organisms [59][61][65][70][71][72][73][74], but a primary concern relates to its potential toxicity in humans and other mammals. A full review of the question of mammalian toxicity can be found in Dekant et al. [74], but can be summarized with the following: at the time of writing, the potential of TFA to induce toxicity in living organisms is considered to be very low. Additionally, TFA is reported to be easily excreted by mammals and so is unlikely to bioaccumulate [74][75][76]. Despite this, some bioaccumulation of TFA has been reported. For example, Lan et al. demonstrated the ability of TFA to bioaccumulate in a range of organisms and suggested the use of locusts as a biomonitoring tool for ecological risk assessment [70].
Additionally, a recent study reported a significant detection frequency of TFA (>90%) in the serum of 252 subjects in China [77]. The authors obtained a median concentration of 8.46 ng mL−1 across subjects and saw that TFA concentrations were correlated with age, which could suggest accumulation. However, another report suggested that the TFA concentrations measured by Duan et al. [77] might result from its metabolic generation from longer-chain PFASs, or from elevated local exposure [78]. Both the detection of metabolically generated TFA in mammals and elevated TFA exposure from certain occupational sources have been reported [79][80][81]. If this is the case, concentrations reported by Duan et al. [73] might not be representative of the general population. On the other hand, Zheng et al. [82] also detected TFA in human serum that was in good agreement with the values reported by Duan et al. [77].
Kim et al. have recently reported what they believe to be the first study detecting TFA in urine samples taken from the general population [83]. Later, Zheng et al. [82] reported the detection of TFA in 31% of urine samples taken, with some of the samples showing high concentrations. However, it has also been suggested that urine may not be a suitable matrix for long-term screening [83]. Overall, there is a clear need for development of a quantitative biomonitoring strategy for emerging contaminants such as TFA and further studies are urgently required.
Much of the research on potential toxicity of TFA contamination has been conducted via modelling studies. Russell et al. [28] evaluated potential aquatic risk related to TFA accumulation in several modelling assessments, and predicted that after 50 years of precursor emissions, trifluoroacetate concentrations would reach 1–15 mg L−1 over most of the continental United States. Such values remain well below the nominal ecotoxicological endpoint [28]. They also modelled a ‘worst-case’ scenario of 50 years of continuous upper-bound emissions with no TFA loss in low-rainfall regions; in this instance, concentrations range between 50 and 200 mg L−1, resulting in reduced risk quotients of 5–20 [28]. This was conducted using toxicological data from Berends et al. [59] and Hanson et al. [61][73].
Overall, Russell et al. reported that it is unlikely that increased TFA contamination will impair aquatic systems given the relative insensitivity of relevant organisms to TFA [28]. Similar results emerged from additional studies relating to rainwater and surface waters [37][39][84][85]; in all examples, modelled TFA proved to be below the ‘no observed effect concentration’ reported by Berends et al. [55].
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541.
- Herzke, D.; Nikiforov, V.; Yeung, L.W.Y.; Moe, B.; Routti, H.; Nygård, T.; Gabrielsen, G.W.; Hanssen, L. Targeted PFAS analyses and extractable organofluorine—Enhancing our understanding of the presence of unknown PFAS in Norwegian wildlife. Environ. Int. 2023, 171, 107640.
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J. Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 2007, 99, 366–394.
- Letcher, R.J.; Bustnes, J.O.; Dietz, R.; Jenssen, B.M.; Jorgensen, E.H.; Sonne, C.; Verreault, J.; Vijayan, M.M.; Gabrielsen, G.W. Exposure and effects assessment of persistent organohalogen contaminants in arctic wildlife and fish. Sci. Total Environ. 2010, 408, 2995–3043.
- Blevin, P.; Tartu, S.; Ellis, H.I.; Chastel, O.; Bustamante, P.; Parenteau, C.; Herzke, D.; Angelier, F.; Gabrielsen, G.W. Contaminants and energy expenditure in an Arctic seabird: Organochlorine pesticides and perfluoroalkyl substances are associated with metabolic rate in a contrasted manner. Environ. Res. 2017, 157, 118–126.
- Briels, N.; Ciesielski, T.M.; Herzke, D.; Jaspers, V.L.B. Developmental Toxicity of Perfluorooctanesulfonate (PFOS) and Its Chlorinated Polyfluoroalkyl Ether Sulfonate Alternative F-53B in the Domestic Chicken. Environ. Sci. Technol. 2018, 52, 12859–12867.
- Routti, H.; Berg, M.K.; Lille-Langoy, R.; Oygarden, L.; Harju, M.; Dietz, R.; Sonne, C.; Goksoyr, A. Environmental contaminants modulate the transcriptional activity of polar bear (Ursus maritimus) and human peroxisome proliferator-activated receptor alpha (PPARA). Sci. Rep. 2019, 9, 6918.
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150.
- Tomy, G.T.; Budakowski, W.; Halldorson, T.; Helm, P.A.; Stern, G.A.; Friesen, K.; Pepper, K.; Tittlemier, S.A.; Fisk, A.T. Fluorinated organic compounds in an eastern Arctic marine food web. Environ. Sci. Technol. 2004, 38, 6475–6481.
- Conder, J.M.; Hoke, R.A.; De Wolf, W.; Russell, M.H.; Buck, R.C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003.
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342.
- Haukas, M.; Berger, U.; Hop, H.; Gulliksen, B.; Gabrielsen, G.W. Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Sea food web. Environ. Pollut. 2007, 148, 360–371.
- Kannan, K.; Koistinen, J.; Beckmen, K.; Evans, T.; Gorzelany, J.F.; Hansen, K.J.; Jones, P.D.; Helle, E.; Nyman, M.; Giesy, J.P. Accumulation of perfluorooctane sulfonate in marine mammals. Environ. Sci. Technol. 2001, 35, 1593–1598.
- Kelly, B.C.; Ikonomou, M.G.; Blair, J.D.; Surridge, B.; Hoover, D.; Grace, R.; Gobas, F.A.P.C. Perfluoroalkyl Contaminants in an Arctic Marine Food Web: Trophic Magnification and Wildlife Exposure. Environ. Sci. Technol. 2009, 43, 4037–4043.
- Verreault, J.; Houde, M.; Gabrielsen, G.W.; Berger, U.; Haukas, M.; Letcher, R.J.; Muir, D.C.G. Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian Arctic. Environ. Sci. Technol. 2005, 39, 7439–7445.
- US EPA. PFAS Master List of PFAS Substances (Version 2); U.S. Environmental Protection Agency: Washington, DC, USA, 2021.
- OECD Global PFAS List. Toward a New Comprehensive Global Database of Per-and Polyfluoroalkyl Substances (PFASs); OECD: Paris, France, 2018.
- Land, M.; De Wit, C.A.; Bignert, A.; Cousins, I.T.; Herzke, D.; Johansson, J.H.; Martin, J.W. What is the effect of phasing out long-chain per-and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environ. Evid. 2018, 7, 4.
- Tyrrell, N.D. A Proposal That Would Ban Manufacture, Supply, and Use of All Fluoropolymers and Most Fluorinated Reagents within the Entire EU. Org. Process Res. Dev. 2023, 27, 1422–1426.
- Madronich, S.; Sulzberger, B.; Longstreth, J.D.; Schikowski, T.; Andersen, M.P.S.; Solomon, K.R.; Wilson, S.R. Changes in tropospheric air quality related to the protection of stratospheric ozone in a changing climate. Photoch. Photobio. Sci. 2023, 22, 1129–1176.
- Wallington, T.J.; Andersen, M.P.S.; Nielsen, O.J. The case for a more precise definition of regulated PFAS. Environ. Sci.-Proc. Imp. 2021, 23, 1834–1838.
- Harris, K.J.; Munoz, G.; Woo, V.; Sauve, S.; Rand, A.A. Targeted and Suspect Screening of Per- and Polyfluoroalkyl Substances in Cosmetics and Personal Care Products. Environ. Sci. Technol. 2022, 56, 14594–14604.
- Gomis, M.I.; Vestergren, R.; Borg, D.; Cousins, I.T. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ. Int. 2018, 113, 1–9.
- Bjornsdotter, M.K.; Yeung, L.W.Y.; Karrman, A.; Jogsten, I.E. Ultra-Short-Chain Perfluoroalkyl Acids Including Trifluoromethane Sulfonic Acid in Water Connected to Known and Suspected Point Sources in Sweden. Environ. Sci. Technol. 2019, 53, 11093–11101.
- Jiao, E.M.; Zhu, Z.L.; Yin, D.Q.; Qiu, Y.L.; Karrman, A.; Yeung, L.W.Y. A pilot study on extractable organofluorine and per- and polyfluoroalkyl substances (PFAS) in water from drinking water treatment plants around Taihu Lake, China: What is missed by target PFAS analysis? Environ. Sci. Process. Impacts 2022, 24, 1060–1070.
- Cousins, I.T.; Vestergren, R.; Wang, Z.Y.; Scheringer, M.; McLachlan, M.S. The precautionary principle and chemicals management: The example of perfluoroalkyl acids in groundwater. Environ. Int. 2016, 94, 331–340.
- European Fluorocarbons Technical Comittee (EFCTC). Special Review on … Understanding TFA. Available online: https://www.fluorocarbons.org/wp-content/uploads/2016/09/EFCTC-Special-Review-on-TFA-2017_07_11F.pdf (accessed on 23 August 2023).
- Russell, M.H.; Hoogeweg, G.; Webster, E.M.; Ellis, D.A.; Waterland, R.L.; Hoke, R.A. TFA from HFO-1234yf: Accumulation and aquatic risk in terminal water bodies. Environ. Toxicol. Chem. 2012, 31, 1957–1965.
- Boutonnet, J.C.; Bingham, P.; Calamari, D.; de Rooij, C.; Franklin, J.; Kawano, T.; Libre, J.M.; McCulloch, A.; Malinverno, G.; Odom, J.M.; et al. Environmental risk assessment of trifluoroacetic acid. Hum. Ecol. Risk Assess. 1999, 5, 59–124.
- Solomon, K.R.; Velders, G.J.M.; Wilson, S.R.; Madronich, S.; Longstreth, J.; Aucamp, P.J.; Bornman, J.F. Sources, fates, toxicity, and risks of trifluoroacetic acid and its salts: Relevance to substances regulated under the Montreal and Kyoto Protocols. J. Toxicol. Env. Heal. B 2016, 19, 289–304.
- Lindley, A.; McCulloch, A.; Vink, T. Contribution of hydrofluorocarbons (HFCs) and hydrofluoro-olefins (HFOs) atmospheric breakdown products to acidification (“Acid Rain”) in the EU at present and in the future. Open J. Air Pollut. 2019, 8, 81–95.
- Key, B.D.; Howell, R.D.; Criddle, C.S. Fluorinated organics in the biosphere. Environ. Sci. Technol. 1997, 31, 2445–2454.
- Ellis, D.A.; Hanson, M.L.; Sibley, P.K.; Shahid, T.; Fineberg, N.A.; Solomon, K.R.; Muir, D.C.G.; Mabury, S.A. The fate and persistence of trifluoroacetic and chloroacetic acids in pond waters. Chemosphere 2001, 42, 309–318.
- Scott, B.F.; Mactavish, D.; Spencer, C.; Strachan, W.M.J.; Muir, D.C.G. Haloacetic acids in Canadian lake waters and precipitation. Environ. Sci. Technol. 2000, 34, 4266–4272.
- Rompp, A.; Klemm, O.; Fricke, W.; Frank, H. Haloacetates in fog and rain. Environ. Sci. Technol. 2001, 35, 1294–1298.
- Wujcik, C.E.; Cahill, T.M.; Seiber, J.N. Determination of trifluoroacetic acid in 1996–1997 precipitation and surface waters in California and Nevada. Environ. Sci. Technol. 1999, 33, 1747–1751.
- Scott, B.F.; Spencer, C.; Marvin, C.H.; MacTavish, D.C.; Muir, D.C.G. Distribution of haloacetic acids in the water columns of the Laurentian Great Lakes and Lake Malawi. Environ. Sci. Technol. 2002, 36, 1893–1898.
- Frank, H.; Christoph, E.H.; Holm-Hansen, O.; Bullister, J.L. Trifluoroacetate in ocean waters. Environ. Sci. Technol. 2002, 36, 12–15.
- Berg, M.; Muller, S.R.; Muhlemann, J.; Wiedmer, A.; Schwarzenbach, R.P. Concentrations and mass fluxes of chloroacetic acids and trifluoroacetic acid in rain and natural waters in Switzerland. Environ. Sci. Technol. 2000, 34, 2675–2683.
- Von Sydow, L.M.; Grimvall, A.B.; Boren, H.B.; Laniewski, K.; Nielsen, A.T. Natural background levels of trifluoroacetate in rain and snow. Environ. Sci. Technol. 2000, 34, 3115–3118.
- Brunn, H.; Arnold, G.; Korner, W.; Rippen, G.; Steinhauser, K.G.; Valentin, I. PFAS: Forever chemicals-persistent, bioaccumulative and mobile. Reviewing the status and the need for their phase out and remediation of contaminated sites. Environ. Sci. Eur. 2023, 35, 20.
- Freeling, F.; Bjornsdotter, M.K. Assessing the environmental occurrence of the anthropogenic contaminant trifluoroacetic acid (TFA). Curr. Opin. Green Sustain. Chem. 2023, 41, 100807.
- Pickard, H.M.; Criscitiello, A.S.; Persaud, D.; Spencer, C.; Muir, D.C.G.; Lehnherr, I.; Sharp, M.J.; De Silva, A.O.; Young, C.J. Ice Core Record of Persistent Short-Chain Fluorinated Alkyl Acids: Evidence of the Impact From Global Environmental Regulations. Geophys. Res. Lett. 2020, 47, e2020GL087535.
- Freeling, F.; Scheurer, M.; Koschorreck, J.; Hoffmann, G.; Ternes, T.A.; Nodler, K. Levels and Temporal Trends of Trifluoroacetate (TFA) in Archived Plants: Evidence for Increasing Emissions of Gaseous TFA Precursors over the Last Decades. Environ. Sci. Tech. Let. 2022, 9, 400–405.
- O’Neil, M.J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; RSC Publishing: Cambridge, UK, 2013.
- Kutsuna, S.; Hori, H. Experimental determination of Henry’s law constants of trifluoroacetic acid at 278–298 K. Atmos. Environ. 2008, 42, 1399–1412.
- Andersen, M.P.S.; Axson, J.L.; Michelsen, R.R.H.; Nielsen, O.J.; Iraci, L.T. Solubility of Acetic Acid and Trifluoroacetic Acid in Low-Temperature (207–245 K) Sulfuric Acid Solutions: Implications for the Upper Troposphere and Lower Stratosphere. J. Phys. Chem. A 2011, 115, 4388–4396.
- Buckingham, J. Dictionary of Organic Compounds; CRC Press: Boca Raton, FL, USA, 1996; Volume 1.
- Bowden, D.J.; Clegg, S.L.; Brimblecombe, P. The Henry’s law constant of trifluoroacetic acid and its partitioning into liquid water in the atmosphere. Chemosphere 1996, 32, 405–420.
- Namazian, M.; Zakery, M.; Noorbala, M.R.; Coote, M.L. Accurate calculation of the pK(a) of trifluoroacetic acid using high-level ab initio calculations. Chem. Phys. Lett. 2008, 451, 163–168.
- Rayne, S.; Forest, K. Theoretical studies on the pK(a) values of perfluoroalkyl carboxylic acids. J. Mol. Struc.-Theochem. 2010, 949, 60–69.
- Ellis, D.A.; Mabury, S.A.; Martin, J.W.; Muir, D.C. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 2001, 412, 321–324.
- Luecken, D.J.; Waterland, R.L.; Papasavva, S.; Taddonio, K.N.; Hutzell, W.T.; Rugh, J.P.; Andersen, S.O. Ozone and TFA Impacts in North America from Degradation of 2,3,3,3-Tetrafluoropropene (HFO-1234yf), A Potential Greenhouse Gas Replacement. Environ. Sci. Technol. 2010, 44, 343–348.
- Kazil, J.; McKeen, S.; Kim, S.W.; Ahmadov, R.; Grell, G.; Talukdar, R.; Ravishankara, A. Deposition and rainwater concentrations of trifluoroacetic acid in the United States from the use of HFO-1234yf. J. Geophys. Res. Atmos. 2014, 119, 14059–14079.
- Tao, Y.; VandenBoer, T.C.; Ye, R.X.; Young, C.J. Exploring controls on perfluorocarboxylic acid (PFCA) gas-particle partitioning using a model with observational constraints. Environ. Sci. Process. Impacts 2023, 25, 264–276.
- Lu, Y.; Liu, L.; Ning, A.; Yang, G.; Liu, Y.; Kurtén, T.; Vehkamäki, H.; Zhang, X.; Wang, L. Atmospheric Sulfuric Acid-Dimethylamine Nucleation Enhanced by Trifluoroacetic Acid. Geophys. Res. Lett. 2020, 47, e2019GL085627.
- Holland, R.; Khan, M.A.H.; Driscoll, I.; Chhantyal-Pun, R.; Derwent, R.G.; Taatjes, C.A.; Orr-Ewing, A.J.; Percival, C.J.; Shallcross, D.E. Investigation of the Production of Trifluoroacetic Acid from Two Halocarbons, HFC-134a and HFO-1234yf and Its Fates Using a Global Three-Dimensional Chemical Transport Model. ACS Earth Space Chem. 2021, 5, 849–857.
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981.
- Berends, A.G.; Boutonnet, J.C.; de Rooij, C.G.; Thompson, R.S. Toxicity of trifluoroacetate to aquatic organisms. Environ. Toxicol. Chem. 1999, 18, 1053–1059.
- Hanson, M.L.; Sibley, P.K.; Ellis, D.A.; Fineberg, N.A.; Mabury, S.A.; Solomon, K.R.; Muir, D.C. Trichloroacetic acid fate and toxicity to the macrophytes Myriophyllum spicatum and Myriophyllum sibiricum under field conditions. Aquat. Toxicol. 2002, 56, 241–255.
- Hanson, M.L.; Solomon, K.R. Haloacetic acids in the aquatic environment. Part I: Macrophyte toxicity. Environ. Pollut. 2004, 130, 371–383.
- Wang, Z.Y.; Wang, Y.H.; Li, J.F.; Henne, S.; Zhang, B.Y.; Hu, J.X.; Zhang, J.B. Impacts of the Degradation of 2,3,3,3-Tetrafluoropropene into Trifluoroacetic Acid from Its Application in Automobile Air Conditioners in China, the United States, and Europe. Environ. Sci. Technol. 2018, 52, 2819–2826.
- Seiber, J.N.; Cahill, T.M. Trifluoroacetic Acid from CFC Replacements: An Atmopsheric Toxicant Becomes a Terrestrial Problem. In Pesticides, Organic Contaminants, and Pathogens in Air: Chemodynamics, Health Effects, Sampling, and Analysis; Taylor & Francis: Abingdon-on-Thames, UK, 2022.
- Zhang, L.; Sun, H.W.; Wang, Q.; Chen, H.; Yao, Y.M.; Zhao, Z.; Alder, A.C. Uptake mechanisms of perfluoroalkyl acids with different carbon chain lengths (C2–C8) by wheat (Triticum acstivnm L.). Sci. Total Environ. 2019, 654, 19–27.
- Benesch, J.A.; Gustin, M.S. Uptake of trifluoroacetate by Pinus ponderosa via atmospheric pathway. Atmos. Environ. 2002, 36, 1233–1235.
- Chen, H.; Yao, Y.M.; Zhao, Z.; Wang, Y.; Wang, Q.; Ren, C.; Wang, B.; Sun, H.W.; Alder, A.C.; Kannan, K. Multimedia Distribution and Transfer of Per- and Polyfluoroalkyl Substances (PFASs) Surrounding Two Fluorochemical Manufacturing Facilities in Fuxin, China. Environ. Sci. Technol. 2018, 52, 8263–8271.
- Tian, Y.; Yao, Y.M.; Chang, S.; Zhao, Z.; Zhao, Y.Y.; Yuan, X.J.; Wu, F.C.; Sun, H.W. Occurrence and Phase Distribution of Neutral and Ionizable Per- and Polyfluoroalkyl Substances (PFASs) in the Atmosphere and Plant Leaves around Landfills: A Case Study in Tianjin, China. Environ. Sci. Technol. 2018, 52, 1301–1310.
- Rollins, A.; Barber, J.; Elliott, R.; Wood, B. Xenobiotic monitoring in plants by 19F and 1H nuclear magnetic resonance imaging and spectroscopy: Uptake of trifluoroacetic acid in Lycopersicon esculentum. Plant Physiol. 1989, 91, 1243–1246.
- Cahill, T.M.; Thomas, C.M.; Schwarzbach, S.E.; Seiber, J.N. Accumulation of trifluoroacetate in seasonal wetlands in California. Environ. Sci. Technol. 2001, 35, 820–825.
- Lan, Z.H.; Yao, Y.M.; Xu, J.Y.; Chen, H.; Ren, C.; Fang, X.G.; Zhang, K.; Jin, L.T.; Hua, X.; Alder, A.C.; et al. Novel and legacy per- and polyfluoroalkyl substances (PFASs) in a farmland environment: Soil distribution and biomonitoring with plant leaves and locusts. Environ. Pollut. 2020, 263, 114487.
- Blake, D.; Barry, J.; Cascorbi, H. A note on the effect of trifluoroacetate on the growth of rat liver. Naunyn Schmiedebergs Arch. Pharmacol. 1969, 265, 474–475.
- Fraser, J.M.; Kaminsky, L.S. 2, 2, 2-Trifluoroethanol intestinal and bone marrow toxicity: The role of its metabolism to 2, 2, 2-trifluoroacetaldehyde and trifluoroacetic acid. Toxicol. Appl. Pharmacol. 1988, 94, 84–92.
- Hanson, M.L.; Solomon, K.R. Haloacetic acids in the aquatic environment. Part II: Ecological risk assessment. Environ. Pollut. 2004, 130, 385–401.
- Dekant, W.; Dekant, R. Mammalian toxicity of trifluoroacetate and assessment of human health risks due to environmental exposures. Arch. Toxicol. 2023, 97, 1069–1077.
- Wark, H.; Earl, J.; Chau, D.D.; Overton, J. Halothane Metabolism in Children. Br. J. Anaesth. 1990, 64, 474–481.
- Sutton, T.S.; Koblin, D.D.; Gruenke, L.D.; Weiskopf, R.B.; Rampil, I.J.; Waskell, L.; Eger, E.I. Fluoride Metabolites after Prolonged Exposure of Volunteers and Patients to Desflurane. Anesth. Analg. 1991, 73, 180–185.
- Duan, Y.S.; Sun, H.W.; Yao, Y.M.; Meng, Y.; Li, Y.C. Distribution of novel and legacy per-/polyfluoroalkyl substances in serum and its associations with two glycemic biomarkers among Chinese adult men and women with normal blood glucose levels. Environ. Int. 2020, 134, 105295.
- Neale, R.E.; Barnes, P.W.; Robson, T.M.; Neale, P.J.; Williamson, C.E.; Zepp, R.G.; Wilson, S.R.; Madronich, S.; Andrady, A.L.; Heikkila, A.M.; et al. Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP Environmental Effects Assessment Panel, Update 2020. Photochem. Photobiol. Sci. 2021, 20, 1–67.
- Ghantous, H.; Parnerud, I.; Danielsson, B.R.G.; Dencker, L. Distribution of Halothane and the Metabolites Trifluoroacetic-Acid and Bromide in the Conceptus after Halothane Inhalation by Pregnant Mice. Acta Pharmacol. Toxicol. 1986, 59, 370–376.
- Cappon, G.D.; Keller, D.A.; Brock, W.J.; Slauter, R.W.; Hurtt, M.E. Effects of HCFC-123 exposure to maternal and infant rhesus monkeys on hepatic biochemistry, lactational parameters and postnatal growth. Drug Chem. Toxicol. 2002, 25, 481–496.
- Zhao, L.C.; Cheng, Z.P.; Zhu, H.K.; Chen, H.; Yao, Y.M.; Baqar, M.; Yu, H.; Qiao, B.T.; Sun, H.W. Electronic-waste-associated pollution of per- and polyfluoroalkyl substances: Environmental occurrence and human exposure. J. Hazard. Mater. 2023, 451, 131204.
- Zheng, G.M.; Eick, S.M.; Salamova, A. Elevated Levels of Ultrashort- and Short-Chain Perfluoroalkyl Acids in US Homes and People. Environ. Sci. Technol. 2023, 57, 15782–15793.
- Kim, D.H.; Jeong, Y.; Belova, L.; Roggeman, M.; Fernandez, S.F.; Poma, G.; Remy, S.; Verheyen, V.J.; Schoeters, G.; van Nuijs, A.L.N.; et al. Comprehensive investigation of persistent and mobile chemicals and per-and polyfluoroalkyl substances in urine of flemish adolescents using a suspect screening approach. Environ. Pollut. 2022, 312, 119972.
- Henne, S.; Shallcross, D.E.; Reimann, S.; Xiao, P.; Boulos, S.; Gerecke, A.C.; Brunner, D. Environmental Impacts of HFO-1234yf and Other HFOs. In Ashrae/Nist Refrigerants Conference; ASHRAE: Peachtree Corners, GA, USA, 2012; pp. 182–194.
- David, L.M.; Barth, M.; Hoglund-Isaksson, L.; Purohit, P.; Velders, G.J.M.; Glaser, S.; Ravishankara, A.R. Trifluoroacetic acid deposition from emissions of HFO-1234yf in India, China, and the Middle East. Atmos. Chem. Phys. 2021, 21, 14833–14849.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
27 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




