
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Clara Malo | -- | 2232 | 2024-03-18 10:42:48 | | | |
| 2 | Lindsay Dong | Meta information modification | 2232 | 2024-03-19 02:55:39 | | |
Video Upload Options
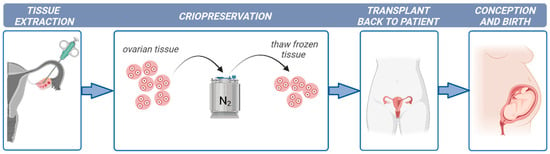
Ovarian tissue cryopreservation is gaining importance as a successful method to restore fertility to girls and young women at high risk of sterility. However, there are concerns regarding the safety of transplantation after ovarian tissue cryopreservation due to the high risk of reintroducing cancer cells and causing disease recurrence. In these cases, the development of culture systems that support oocyte development from the primordial follicle stage is required.
1. Introduction
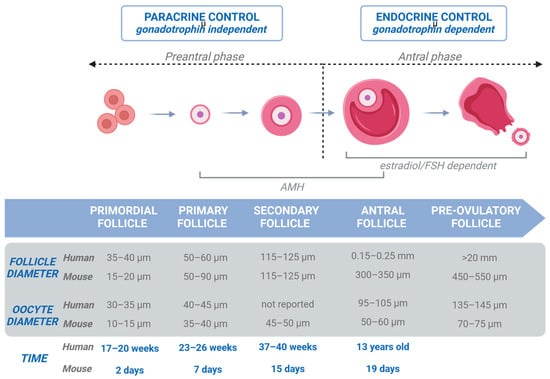
2. Folliculogenesis in Human Ovaries
3. Culture Systems
3.1. Two-Dimensional Culture Systems
3.2. Three-Dimensional Culture Systems
3.3. Critical Cell–Cell and Cell–Matrix Interactions for Improved Oocyte Survival and Growth
4. Organ-on-a-Chip Technology
Although the activation of growth of primordial follicles has been achieved, a limited number of follicles progress to secondary follicles [33]. Decent results were obtained in primates using expandable matrixes in 3D systems but the fertilization ability of the oocytes obtained was low and no blastocyst development was observed [34][35]. One of the reasons for the limited success of this technology can be found in the lack of more appropriate and physiological culture systems because it does not recapitulate the heterogeneous nature of the ECM in the ovary, with the medulla being much softer than the cortex. The ECM is believed to not only provide a 3D network to support the ovarian tissue architecture but also to regulate (together with hormones and nutrients) cell-ECM and cell–cell interactions that are important for follicle development. Choi et al. [36] revealed the crucial role of mechanical heterogeneity in the ovary in regulating follicle development by producing ovarian microtissues by encapsulating early secondary preantral follicles in microcapsules consisting of a softer, biodegradable collagen (0.5%) hydrogel core and a harder, slowly degradable alginate (2%) hydrogel shell. Folliculogenesis mainly depends upon hormones and nutrients, and their disturbance can cause abnormal follicle growth. Hence, a precise culture system that ensures the diffusion of nutrients and gases within the tissue, maximizing the retention of essential growth factors of oocyte maturation, is needed.
Considering the aforementioned aspects, a pioneering technology known as Organ-on-a-Chip (OOC) offers the means to replicate tissue architecture and emulate fluidic conditions in an in vitro setting. Broadly, these systems facilitate the miniaturization of experimental models, resulting in several advantages such as reduced working volumes, quicker reaction times, cost-effectiveness, and enhanced precision and control over experimental designs. This innovative approach holds significant promise in advancing research capabilities and improving the efficiency of experimental processes within the field.
OOC platforms, which are founded on microfluidic devices, facilitate three-dimensional organized cell culture by employing various types of ECMs. This methodology aims to emulate tissue architecture and replicate the cellular environment, mechanisms, and physiological responses of organs in an in vitro setting. The microenvironment is established through dynamic interactions among cells, fluids, and the ECM, influencing cellular processes and functions via biophysical and biochemical signals. A distinctive advantage of OOC lies in its ability to operate under flow conditions, enabling the continual replenishment of media, a process that mirrors in vivo blood supply or interstitial flow. Consequently, fluid flow ensures a consistent and adjustable supply of nutrients and oxygen, along with the supplementation of stage-specific growth factors. This dynamic environment sustains physical interactions while preventing cellular stress induced by the formation and accumulation of reactive oxygen substances [37][38].
Another pertinent aspect of this technology is the capability to tailor the devices based on design specifications including shaped microchannels, compartments, and reservoirs or the materials used to make the devices such as polydimethylsiloxane (PDMS; most prevalent), thermoplastics, or glass. Careful consideration is essential in material selection, as the inherent physicochemical properties of certain materials may impose limitations, including transparency, cost, flexibility or rigidity, and gas permeability, as well as considerations about time-consuming fabrication processes. The choice of material is contingent upon factors such as the intended application of the device, volume requirements, and production costs.
5. Conclusions
References
- Howard, S.C.; Zaidi, A.; Cao, X.; Weil, O.; Bey, P.; Patte, C.; Samudio, A.; Haddad, L.; Lam, C.G.; Moreira, C.; et al. The My Child Matters programme: Effect of public-private partnerships on paediatric cancer care in low-income and middle-income countries. Lancet Oncol. 2018, 19, e252–e266.
- Salama, M.; Woodruff, T.K. Anticancer treatments and female fertility: Clinical concerns and role of oncologists in oncofertility practice. Expert. Rev. Anticancer Ther. 2017, 17, 687–692.
- Anderson, R.A.; Wallace, W.H.; Baird, D.T. Ovarian cryopreservation for fertility preservation: Indications and outcomes. Reproduction 2008, 136, 681–689.
- Rives, N.; Courbière, B.; Almont, T.; Kassab, D.; Berger, C.; Grynberg, M.; Papaxanthos, A.; Decanter, C.; Elefant, E.; Dhedin, N.; et al. What should be done in terms of fertility preservation for patients with cancer? The French 2021 guidelines. Eur. J. Cancer 2022, 173, 146–166.
- Filatov, M.A.; Khramova, Y.V.; Kiseleva, M.V.; Malinova, I.V.; Komarova, E.V.; Semenova, M.L. Female fertility preservation strategies: Cryopreservation and ovarian tissue in vitro culture, current state of the art and future perspectives. Zygote 2016, 24, 635–653.
- Van den Hurk, R.; Abir, R.; Telfer, E.E.; Bevers, M.M. Primate and bovine immature oocytes and follicles as sources of fertilizable oocytes. Hum. Reprod. Update 2000, 6, 457–474.
- Gougeon, A. Regulation of ovarian follicular development in primates: Facts and hypotheses. Endocr. Rev. 1996, 17, 121–155.
- Fortune, J.E. The early stages of follicular development: Activation of primordial follicles and growth of preantral follicles. Anim. Reprod. Sci. 2003, 78, 135–163.
- Jiang, Y.; He, Y.; Pan, X.; Wang, P.; Yuan, X.; Ma, B. Advances in Oocyte Maturation in Vivo and in Vitro in Mammals. Int. J. Mol. Sci. 2023, 24, 9059.
- Gougeon, A. Human ovarian follicular development: From activation of resting follicles to preovulatory maturation. Ann. Endocrinol. 2010, 71, 132–143.
- Eppig, J.J.; O’Brien, M.J. Development in vitro of mouse oocytes from primordial follicles. Biol. Reprod. 1996, 54, 197–207.
- Telfer, E.E.; McLaughlin, M.; Ding, C.; Thong, K.J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Hum. Reprod. 2008, 23, 1151–1158.
- Nation, A.; Selwood, L. The production of mature oocytes from adult ovaries following primary follicle culture in a marsupial. Reproduction 2009, 138, 247–255.
- Cortvrindt, R.; Smitz, J.; Van Steirteghem, A.C. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum. Reprod. 1996, 11, 2656–2666.
- Oktem, O.; Oktay, K. The role of extracellular matrix and activin-A in in vitro growth and survival of murine preantral follicles. Reprod. Sci. 2007, 14, 358–366.
- Figueiredo, J.R.; Hulshof, S.C.; Thiry, M.; Van den Hurk, R.; Bevers, M.M.; Nusgens, B.; Beckers, J.F. Extracellular matrix proteins and basement membrane: Their identification in bovine ovaries and significance for the attachment of cultured preantral follicles. Theriogenology 1995, 43, 845–858.
- O’Brien, M.J.; Pendola, J.K.; Eppig, J.J. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol. Reprod. 2003, 68, 1682–1686.
- Picton, H.M.; Harris, S.E.; Muruvi, W.; Chambers, E.L. The in vitro growth and maturation of follicles. Reproduction 2008, 136, 703–715.
- Telfer, E.E. Future developments: In vitro growth (IVG) of human ovarian follicles. Acta Obstet. Gynecol. Scand. 2019, 98, 653–658.
- Eppig, J.J.; Pendola, F.L.; Wigglesworth, K.; Pendola, J.K. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: Amino acid transport. Biol. Reprod. 2005, 73, 351–357.
- Gougeon, A.; Testart, J. Germinal vesicle breakdown in oocytes of human atretic follicles during the menstrual cycle. J. Reprod. Fertil. 1986, 78, 389–401.
- Green, L.J.; Shikanov, A. In vitro culture methods of preantral follicles. Theriogenology 2016, 86, 229–238.
- Jones, A.S.K.; Shikanov, A. Follicle development as an orchestrated signaling network in a 3D organoid. J. Biol. Eng. 2019, 13, 2.
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. 2006, 7, 211–224.
- Scott, J.E.; Carlsson, I.B.; Bavister, B.D.; Hovatta, O. Human ovarian tissue cultures: Extracellular matrix composition, coating density and tissue dimensions. Reprod. Biomed. Online 2004, 9, 287–293.
- Higuchi, C.M.; Maeda, Y.; Horiuchi, T.; Yamazaki, Y. A Simplified Method for Three-Dimensional (3-D) Ovarian Tissue Culture Yielding Oocytes Competent to Produce Full-Term Offspring in Mice. PLoS ONE 2015, 10, e0143114.
- Jiao, Z.X.; Woodruff, T.K. Follicle microenvironment-associated alterations in gene expression in the mouse oocyte and its polar body. Fertil. Steril. 2013, 99, 1453–1459.
- Ouni, E.; Peaucelle, A.; Haas, K.T.; Van Kerk, O.; Dolmans, M.-M.; Tuuri, T.; Otala, M.; Amorim, C.A. A blueprint of the topology and mechanics of the human ovary for next-generation bioengineering and diagnosis. Nat. Commun. 2021, 12, 5603.
- West, E.R.; Xu, M.; Woodruff, T.K.; Shea, L.D. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 2007, 28, 4439–4448.
- Xu, M.; West-Farrell, E.R.; Stouffer, R.L.; Shea, L.D.; Woodruff, T.K.; Zelinski, M.B. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol. Reprod. 2009, 81, 587–594.
- Tsai, A.G.; Friesenecker, B.; Mazzoni, M.C.; Kerger, H.; Buerk, D.G.; Johnson, P.C.; Intaglietta, M. Microvascular and tissue oxygen gradients in the rat mesentery. Proc. Natl. Acad. Sci. USA 1998, 95, 6590–6595.
- Adam, A.A.; Takahashi, Y.; Katagiri, S.; Nagano, M. Effects of oxygen tension in the gas atmosphere during in vitro maturation, in vitro fertilization and in vitro culture on the efficiency of in vitro production of mouse embryos. Jpn. J. Vet. Res. 2004, 52, 77–84.
- De Vos, M.; Smitz, J.; Woodruff, T.K. Fertility preservation in women with cancer. Lancet 2015, 385, 856.
- Xu, J.; Bernuci, M.P.; Lawson, M.S.; Yeoman, R.R.; Fisher, T.E.; Zelinski, M.B.; Stouffer, R.L. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: Effects of gonadotropins and insulin. Reproduction 2010, 140, 685–697.
- Xu, J.; Xu, M.; Bernuci, M.P.; Fisher, T.E.; Shea, L.D.; Woodruff, T.K.; Zelinski, M.B.; Stouffer, R.L. Primate follicular development and oocyte maturation in vitro. Adv. Exp. Med. Biol. 2013, 761, 43–67.
- Choi, J.K.; Agarwal, P.; Huang, H.; Zhao, S.; He, X. The crucial role of mechanical heterogeneity in regulating follicle development and ovulation with engineered ovarian microtissue. Biomaterials 2014, 35, 5122–5128.
- Heo, Y.S.; Lee, H.J.; Hassell, B.A.; Irimia, D.; Toth, T.L.; Elmoazzen, H.; Toner, M. Controlled loading of cryoprotectants (CPAs) to oocyte with linear and complex CPA profiles on a microfluidic platform. Lab A Chip 2011, 11, 3530–3537.
- Lee, S.; Chung, M.; Lee, S.R.; Jeon, N.L. 3D brain angiogenesis model to reconstitute functional human blood-brain barrier in vitro. Biotechnol. Bioeng. 2020, 117, 48–762.





