Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kausik Kapat | -- | 2418 | 2024-03-15 10:58:02 | | | |
| 2 | Wendy Huang | Meta information modification | 2418 | 2024-03-19 08:07:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kapat, K.; Kumbhakarn, S.; Sable, R.; Gondane, P.; Takle, S.; Maity, P. Peptide for Bone and Cartilage Regeneration. Encyclopedia. Available online: https://encyclopedia.pub/entry/56337 (accessed on 28 February 2026).
Kapat K, Kumbhakarn S, Sable R, Gondane P, Takle S, Maity P. Peptide for Bone and Cartilage Regeneration. Encyclopedia. Available at: https://encyclopedia.pub/entry/56337. Accessed February 28, 2026.
Kapat, Kausik, Sakshi Kumbhakarn, Rahul Sable, Prashil Gondane, Shruti Takle, Pritiprasanna Maity. "Peptide for Bone and Cartilage Regeneration" Encyclopedia, https://encyclopedia.pub/entry/56337 (accessed February 28, 2026).
Kapat, K., Kumbhakarn, S., Sable, R., Gondane, P., Takle, S., & Maity, P. (2024, March 15). Peptide for Bone and Cartilage Regeneration. In Encyclopedia. https://encyclopedia.pub/entry/56337
Kapat, Kausik, et al. "Peptide for Bone and Cartilage Regeneration." Encyclopedia. Web. 15 March, 2024.
Copy Citation
The healing of osteochondral defects (OCDs) that result from injury, osteochondritis, or osteoarthritis and bear lesions in the cartilage and bone, pain, and loss of joint function in middle- and old-age individuals presents challenges to clinical practitioners because of non-regenerative cartilage and the limitations of current therapies. Bioactive peptide-based osteochondral (OC) tissue regeneration is becoming more popular because it does not have the immunogenicity, misfolding, or denaturation problems associated with original proteins.
osteochondral
osteogenic
chondrogenic
cartilage
peptide
regeneration
1. Introduction
An articulating joint’s osteochondral (OC) unit comprises the vascularized and mineralized subchondral bone and the acellular and avascular hyaline cartilage connected by a seamless interface [1]. The structural heterogenicity of the OC tissue arises from its diverse organic (cells, aggrecan, collagen) and inorganic (hydroxyapatite) components, along with their spatial orientation, forming gradients from the superficial cartilage to the subchondral bone via middle, deeper, and calcified layers (Figure 1). Cartilage exerts cushioning effects on joint bones and prevents damage from the physiological load; however, an injured cartilage cannot spontaneously regenerate. OCDs are created via a variety of biological (aging, osteochondritis, osteoarthritis) or mechanical (accidental trauma, sports injury, prolonged wear) factors and are characterized by cartilage and bone lesions, severe joint pain, and loss of joint function. They are more common in the middle-aged and older population. The World Health Organization estimates that 595 million people worldwide, or 7.6 percent of the world’s population, suffered from osteoarthritis alone in 2020. This number has increased by 132.2 percent since 1990 [2].
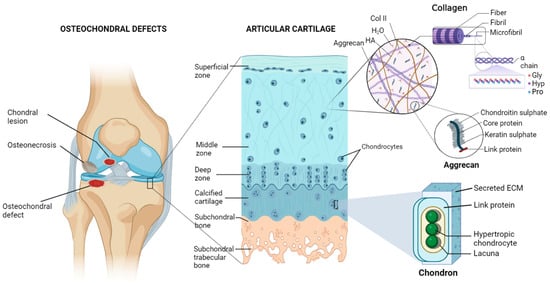
Figure 1. Diagram of the osteochondral unit showing the structural hierarchy, zonal arrangements, and extracellular matrix composition of the articular cartilage. The cartilage matrix is mainly composed of collagen type II (Col II), hyaluronic acid (HA), aggrecan, and a large amount of water (H2O). Alignments of chondrocytes are flattened, spherical, and columnar in superficial, middle, and deep zones, respectively. On the other hand, chondrocytes are hypertrophic in the calcified matrix, primarily made of nanohydroxyapatite and collagen type I, and found inside lacunae. Pro—proline, Hyp—hydroxyproline, Gly—glycine. Figure generated via biorender.com.
Unfortunately, none of the existing procedures can fully repair an OCD or form hyaline cartilage instead of fibrocartilage [3][4][5][6][7]. OC tissue engineering (TE) seems promising for regenerating functional OC tissue [8]. Growth factors can promote MSCs, osteoblast or chondrocyte recruitment, proliferation and differentiation, vascularization, and maintenance of cartilage homeostasis. However, regulating their target selectivity, dose selection, release kinetics, and spatial distribution is quite challenging. Bioactive peptides are short-chain amino acid sequences that mimic the signaling or binding domains of larger proteins and create a biomimetic environment for recruiting host cells while controlling their activity and differentiation [9]. The ECM or growth-factor-derived peptides that self-assemble into hydrogels were investigated for preparing peptide-enhanced bone graft substitutes or scaffolds for OC regeneration [10][11][12][13]. They are more effective, stable, scalable, and affordable than large proteins, and they do not experience issues like immunogenicity, protein folding, and denaturation [10].
2. Peptides for Bone Regeneration
Natural bone healing occurs in two different pathways: intramembranous ossification and endochondral ossification [14]. During this process, a variety of cytokines are released [15]. Among them, bone morphogenetic proteins (BMPs, especially BMP-2, -4, and -7) and transforming growth factor-β (TGF-β, especially the β2 and β3 subtypes) play crucial roles in stem cell differentiation by causing hypertrophy and mineralization, while vascular endothelial growth factor (VEGF) and angiopoietins promote neoangiogenesis [16][17]. BMP-2, -3, -4, -7, TNF-α, interferon-γ (IFN-γ), and certain hormones regulate the remodeling phase [18]. Since bioactive peptides follow similar cell-signaling pathways with these proteins and may be beneficial for both in vitro and in vivo bone formation, they are grouped into three categories based on their role in osteo-induction, biomineralization, and angiogenesis.
2.1. Osteo-Inducers
2.1.1. Collagen-Mimetic/Derived Peptides
Peptides derived from integrin-binding motifs of collagen type I, which is the most significant ECM protein, are GFOGER, P15, KOD, DGEA, and BCSP1, which can promote osteogenic activity and in vivo bone formation.
Collagen-mimetic GFOGER peptide, which is derived from the collagen α1 chain, selectively promotes α2β1 integrin binding required for osteoblastic differentiation [19]. Besides improving cell attachment, GFOGER successfully induces in vitro osteogenic differentiation and in vivo bone healing [19][20][21][22][23]. GFOGER coating on synthetic PCL scaffolds remarkably enhanced bone formation in critically sized segmental defects in rats by stimulating osteoblast adhesion and differentiation [21]. P15, which is a 15-mer peptide derived from the collagen type I α1 chain, has a strong affinity for the cell surface α2β1 integrin receptors. By releasing growth factors and cytokines, the peptide dramatically enhanced the osteogenic differentiation of MSCs [24]. The commercially available P15 formulations significantly enhanced the regeneration of alveolar bone and tibial defects in osteoporotic dogs [25][26]. The collagen-mimetic KOD peptide, which is made of three units, namely, ((PKG)4-(POG)4-(DOG)4), forms a hydrogel through self-assembly inducing platelet activation and blood clotting associated with hematoma formation [27]. POG-based poly-amphiphilic hydrogels allowed for faster recovery (within two weeks) of intervertebral disc defects in rabbits due to a significant increase in ECM deposition [28]. DGEA derived from collagen type I adhesive motif serves as a crucial ligand for osteoblast differentiation [29]. DGEA-containing PA hydrogels seeded with hMSCs substantially upregulated osteogenic markers (OCN, RUNX2, and ALP) [30].
Using a bone- and cartilage-stimulating peptide (BCSP™-1 or NGLPGPIGP) present in human collagen type-I, the proliferation of rat bone-marrow-derived osteoblasts and human or bovine chondrocytes was drastically improved with enhanced the bone mineral density (BMD) and bone mineral content (BMC) in male Wistar rats [31]. Three highly osteogenic peptides (GPAGPHGPVG, APDPFRMY, and TPERYY) derived from tilapia scale collagen hydrolysate notably increased the MC3T3-E1 cell proliferation and mineralization activity (ALP synthesis, osteogenic-related gene expression) at concentrations of 50 μg/mL [32].
2.1.2. BMP-Mimetic/Derived Peptides
As members of the TGF-β superfamily, BMPs are primarily produced by endothelial cells (ECs), osteoblasts, and hypertrophic chondrocytes and can recruit MSCs to the site of injury and differentiate into osteoblasts while inducing ectopic bone formation.
The KIPKASSVPTELSAISTLYL peptide, which is derived from the knuckle epitope of BMP2, increased the ALP activity of osteoprogenitor cells [33]. P24 is a BMP-2 mimetic peptide with a 24-mer peptide bearing the knuckle epitope of the protein that facilitates binding with BMP receptors. P24 successfully induced ectopic bone formation in rodents [34][35][36]. The PEP7 peptide (CKIPKPSSVP-TELSAISMLYL) derived from BMP-2 promoted adhesion, proliferation, and differentiation of MG-63 cells, as well as new bone formation in a supra-alveolar peri-implant defect model in a micropig mandible [37]. The BMP peptide (KIPKASSVPTELSAISTLYL) derived from BMP2 increased the ALP activity, which is an early marker for bone formation, in murine osteoprogenitor cells [38] and other cell types [39][40][41][42][43], as well as the dose-dependent healing of rabbit radial bone defects [44]. The other osteo-inductive or osteogenic peptides derived from BMP-2 (NSVNSKIPKACCVPTELSAI, KIPKASSVPTELSAISTLYL, DWIVA) produced differential effects on in vitro osteogenic differentiation, as well as ectopic or orthotopic bone formation in vivo [11]. The bone-forming peptide (BFP-2) with a VEHDKEFFHPRYHH sequence, which was isolated from the immature BMP-7 precursor, triggered osteogenic differentiation of BMSCs and induced ectopic bone formation after subcutaneous implantation of BFP-2-treated BMSCs in mice [45]. Similarly, the effects of various osteo-inductive peptides derived from BMP-4 (RKKNPNCRRH), BMP-7 (TVPKPSSAPTQLNAISTLYF, GQGFSYPYKAVFSTQ, ETLDGQSINPKLAGL), and BMP-9 (KVGKACCVPTKLSPISVLY) were reviewed [46]. The casein kinase 2 (CK2)-related peptide has a great influence on cell proliferation and apoptosis, and it facilitates in vivo bone formation by interacting with BMP receptor type Ia (BMPRIa) [47]. Three BMP-2 mimetic peptides, namely, CK2.1, CK2.2, and CK2.3, triggered the BMP signaling pathways by inhibiting CK2 binding to BMPRIa [48]. C2C12 cells treated with CK2.3 peptide resulted in osteogenesis, while CK2.2 led to both osteogenesis and adipogenesis [47][49].
2.1.3. Hormone-Derived Peptides
Parathyroid hormone (PTH) is a major regulator of mineral homeostasis. Parathyroid hormone (PTH)-related peptides called Teriparatide, which are 1–34 peptide domains of PTH (PTH1–34), stimulated osteoblast activity and increased bone density at the fracture site, leading to the healing of non-unions [50][51][52][53][54]. On the other hand, endogenous PTH-related protein (PTHrP) analogs, namely, PTHrP1–34, PTHrP1–36, and PTHrP107–111, increased osteoblast activity and local bone formation [55][56][57]. Calcitonin gene-related peptide (CGRP) is a 37-mer neuropeptide with two isoforms: α- and β-CGRP. They were found to stimulate the proliferation and differentiation of osteoprogenitor cells [58][59][60][61], production of osteogenic molecules like insulin-like growth factor (IGF, especially IGF-1 and -2), BMP-2 [62][63], and reparative bone formation [64].
2.1.4. Circulating Peptides
Osteogenic growth peptide (OGP), which is a 14-mer peptide occurring in mammalian blood, increases bone formation through anabolic effects on bone cells [65][66] and differentiation of osteoprogenitor cells, leading to upregulated osteogenic markers, including mineralization [67][68][69]. Thrombin peptide 508 (TP508) or Chrysalin is a 23-amino acid peptide and receptor binding domain of thrombin, which enhanced the proliferation, differentiation, and chemotaxis of human osteoblasts [70][71] and VEGF-stimulated angiogenesis [72]. TP508 injected into the fracture gap promoted fracture healing and increased blood vessel formation [73][74][75].
2.1.5. Other ECM-Derived Peptides
Signaling domains on ECM protein chains are capable of interacting with cell membrane receptors. Various peptides (e.g., FN III9-10/12-14) derived from fibronectin (FN) were shown to promote osteoblast activity and mineralization [76], rabbit calvarial defects healing [77], and augmented BMP-2 and platelet-derived growth factor (PDGF) activities for bone regeneration in vivo [78].
Collagen-binding motif (CBM) is a cleavage product of osteopontin (OPN) that can specifically bind to collagen [79] and promote migration, osteogenic differentiation [80], and bone formation in a rabbit calvarial defect model [81]. The SVVYGLR peptide adjacent to the RGD sequence in OPN significantly enhanced the adhesion and proliferation of MSCs, neovascularization, upregulation of osteogenesis, and angiogenesis when delivered through a collagen sponge [82][83][84]. FHRRIKA, which is a cell-binding and heparin-binding domain of bone sialoprotein (BSP) exerts a favorable effect on osteoblast adhesion, spreading, and mineralization [85]. Higher cell proliferation and viability were observed on rat calvarial osteoblasts that seeded scaffolds containing the RGD and FHRRIKA sequences [86].
2.2. Biomineralizing Peptides
Non-collagenous proteins (NCPs), such as dentin sialophosphoprotein, dentin matrix protein 1 (DMP1), and dentin phosphoprotein (DPP), play a significant role in biomineralization. The negatively charged domains (carboxylic acid and phosphate groups) in NCPs serve as preferential sites for the nucleation of hydroxyapatites (HAPs) while stabilizing them into the self-assembled collagen fibrils that act as a template for crystal growth. Peptides derived from such proteins significantly enhance bone formation.
The Asp–Ser–Ser (DSS) repeating motifs present in DPP have a remarkably strong binding affinity toward calcium ions and HAP [87]. 8DSS, which is a DPP peptide with eight repetitive units of DSS, was the most promising for promoting the mineralization and remineralization of acid-etched enamel [88][89]. Like DSS, 3NSS with three repetitive units of asparagine–serine–serine (aspartic acid in DSS is substituted with asparagine) could remineralize the acid-etched enamel [90]. On the other hand, the DSESSEEDR sequence in dentin matrix protein 1 (DMP1) could bind to demineralized dentin and promote remineralization [91]. The other phosphoprotein-derived peptides, such as SN15, SNA15, DpSpSEEKC, DDDEEK, and DDDEEKC, exhibited high affinity toward HAP [92].
Amelogenin, which is found at the dentin–enamel interface, interacts with collagen to control the formation of HAP crystals and their structural alignment [93]. In addition to remineralizing enamel caries, amelogenin-inspired peptides, such as shADP5, QP5, P26, and P32, helped to restore demineralized dentin [94][95]. Since leucine-rich amelogenin peptide (LRAP) is more hydrophilic than amelogenin, the demineralized enamel treated with CS-LRAP hydrogel exhibited quicker nucleation and development of HAP crystals than amelogenin-containing chitosan hydrogel (CS-AMEL) [96]. A non-amelogenin protein called tuftelin is present in tooth enamel and has a role in the mineralization of dental enamel. Tuftelin-derived peptide (TDP) encouraged the remineralization of early carious lesions by attracting calcium and phosphate ions [97]. Self-assembling amphiphilic oligopeptide derived from cementum protein 1 (CEMP1), which is a regulator of cementum-matrix mineralization, induced intrafibrillar mineralization of collagen fibrils in the presence of calcium ions [98]. P11-4, which is another self-assembling peptide, acted as a scaffold to enhance HAP nucleation de novo [99].
2.3. Angiogenic Peptides
Vascularization is a crucial process during natural bone formation. Many peptides are derived from angiogenic growth factors (e.g., VEGF, fibroblast growth factor-2 (FGF-2), and PDGF), ECM (e.g., OPN, ON), and other proteins that have crucial roles in blood vessel formation [100].
VEGF-mimicking QK or KLT peptide (KLTWQELQLKYKGIGGG), which is derived from the VEGF receptors binding domain 17–35, not only induces EC migration and proliferation but also triggers other complex processes, like chemotaxis and capillary sprouting and organization similar to VEGF [101]. PDGF-BB-derived PBA2-1c peptide interacts with α- and β-PDGF receptors. Though its in vivo proangiogenic activity is still unclear, it functions similarly to PDGF in establishing mature blood vessels that are created by VEGF [102]. Exendin-4, which is a glucagon-like peptide 1 (Glp-1) analog, stimulates human umbilical vein endothelial cells’ (HUVECs’) motility, sprouting, and tube formation in vitro, in addition to in vivo sprout outgrowth [103]. While OPN is widely distributed in the bone matrix to help with bone metabolism, OPN-derived peptide (OPD) does not induce EC proliferation in vitro. However, like VEGF, it facilitated EC migration and tube formation using 3D collagen gels [104], suppressed osteoclastogenesis [82], and promoted the adhesion and proliferation of MSCs, as well as neovascularization in a rat tibial defect model [105]. SPARC113 and SPARC118, which are two OPN-derived peptides that exhibit potent angiogenic activity [106], stimulated in vivo angiogenesis when delivered through MMPs degradable hydrogel [107]. TP508 enhanced neoangiogenesis in femoral defects produced in rats [73] and mice [74] following one hour of local administration. The synthetic 12-mer peptides, known as RoY peptides, which were created via the phage-display technology, may also promote in vitro EC proliferation, tube formation, and sprouting, as well as induce in vivo angiogenesis via a distinct mechanism from VEGF [108].
3. Peptides for Cartilage Regeneration
3.1. Chondroinductive/Chondrogenic Peptides
Numerous peptides were identified to imitate the functions of ECM components, cell–cell junction molecules, and chondroinductive/chondrogenic ligands triggering specific cell-signaling pathways. Motif-derived fibronectins, like RGD, decorin, collagen, and MMPs, display chondrogenic properties. These peptides are often used to functionalize scaffolds that encourage chondrocyte adhesion, migration, and proliferation, in addition to MSC differentiation into the chondrogenic lineage.
3.1.1. TGF-β Mimetic Peptides
TGF-β improves cell differentiation, collagen synthesis, and matrix deposition in cartilage tissue engineering [109]. Therefore, peptides mimicking TGF-β activity were used for cartilage tissue regeneration. TGF-β mimetic peptides, i.e., cytomodulins (CMs), are oligopeptides containing 4–6 amino acids [110]. CMs immobilized on a solid surface can potentially induce chondrogenic differentiation better compared with its soluble form [111][112].
3.1.2. BMP2-Derived/Mimetic Peptides
BMP-2, which is a member of the TGF-β super-family, is one of the main chondrogenic growth factors that induce in vitro chondrogenic differentiation and cartilage regeneration in vivo. Human MSCs (hMSCs) cultured with ≥100 µg/mL of the BMP peptide (KIPKASSVPTELSAISTLYL) resulted in glycosaminoglycan (GAGs) production and increased levels of collagen production and matrix accumulation without extensive upregulation of hypertrophic markers [111][112][113][114][115]. The injection of BMP-2 mimetic CK2.1 peptide into a mouse’s tail vein enhanced chondrogenesis and articular cartilage formation without any effects on osteogenesis or BMD [116]. BMP peptide stimulated chondrogenic differentiation of hMSCs without additional growth factors. At a 100 μg/mL concentration, BMP peptide enhanced proteoglycan production and chondrogenic gene expression without causing hypertrophy, as occurs with BMP-2 [117].
References
- Yildirim, N.; Amanzhanova, A.; Kulzhanova, G.; Mukasheva, F.; Erisken, C. Osteochondral interface: Regenerative engineering and challenges. ACS Biomater. Sci. Eng. 2023, 9, 1205–1223.
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522.
- Howell, M.; Liao, Q.; Gee, C.W. Surgical management of osteochondral defects of the knee: An educational review. Curr. Rev. Musculoskelet. Med. 2021, 14, 60–66.
- Zhu, M.; Zhong, W.; Cao, W.; Zhang, Q.; Wu, G. Chondroinductive/chondroconductive peptides and their-functionalized biomaterials for cartilage tissue engineering. Bioact. Mater. 2022, 9, 221–238.
- Madry, H. Surgical therapy in osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1019–1034.
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855.
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34.
- Chen, L.; Wei, L.; Su, X.; Qin, L.; Xu, Z.; Huang, X.; Chen, H.; Hu, N. Preparation and Characterization of Biomimetic Functional Scaffold with Gradient Structure for Osteochondral Defect Repair. Bioengineering 2023, 10, 213.
- Rizzo, M.G.; Palermo, N.; D’Amora, U.; Oddo, S.; Guglielmino, S.P.P.; Conoci, S.; Szychlinska, M.A.; Calabrese, G. Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 7388.
- Bullock, G.; Atkinson, J.; Gentile, P.; Hatton, P.; Miller, C. Osteogenic peptides and attachment methods determine tissue regeneration in modified bone graft substitutes. J. Funct. Biomater. 2021, 12, 22.
- Visser, R.; Rico-Llanos, G.A.; Pulkkinen, H.; Becerra, J. Peptides for bone tissue engineering. J. Control. Release 2016, 244, 122–135.
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: A systematic review. BMC Med. 2016, 14, 103.
- Ai, C.; Lee, Y.H.D.; Tan, X.H.; Tan, S.H.S.; Hui, J.H.P.; Goh, J.C.-H. Osteochondral tissue engineering: Perspectives for clinical application and preclinical development. J. Orthop. Transl. 2021, 30, 93–102.
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54.
- Pozos-Guillén, A.; Molina, G.; Soviero, V.; Arthur, R.A.; Chavarria-Bolaños, D.; Acevedo, A.M. Management of dental caries lesions in Latin American and Caribbean countries. Braz. Oral Res. 2021, 35, e055.
- Mehta, M.; Schmidt-Bleek, K.; Duda, G.N.; Mooney, D.J. Biomaterial delivery of morphogens to mimic the natural healing cascade in bone. Adv. Drug Deliv. Rev. 2012, 64, 1257–1276.
- Gerber, H.-P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628.
- Calori, G.; Albisetti, W.; Agus, A.; Iori, S.; Tagliabue, L. Risk factors contributing to fracture non-unions. Injury 2007, 38, S11–S18.
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; García, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235.
- Connelly, J.; Petrie, T.; García, A.; Levenston, M. Fibronectin-and Collagen-Mimetic Ligands Regulate BMSC Chondrogenesis in 3D Hydrogels. Eur. Cells Mater. 2011, 22, 168.
- Wojtowicz, A.M.; Shekaran, A.; Oest, M.E.; Dupont, K.M.; Templeman, K.L.; Hutmacher, D.W.; Guldberg, R.E.; García, A.J. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 2010, 31, 2574–2582.
- Shekaran, A.; García, J.R.; Clark, A.Y.; Kavanaugh, T.E.; Lin, A.S.; Guldberg, R.E.; García, A.J. Bone regeneration using an alpha 2 beta 1 integrin-specific hydrogel as a BMP-2 delivery vehicle. Biomaterials 2014, 35, 5453–5461.
- Kolambkar, Y.M.; Bajin, M.; Wojtowicz, A.; Hutmacher, D.W.; García, A.J.; Guldberg, R.E. Nanofiber orientation and surface functionalization modulate human mesenchymal stem cell behavior in vitro. Tissue Eng. Part A 2014, 20, 398–409.
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of biomimetic habitats for tissue engineering with P-15, a synthetic peptide analogue of collagen. Tissue Eng. 1999, 5, 53–65.
- Hestehave Pedersen, R.; Rasmussen, M.; Overgaard, S.; Ding, M. Effects of P-15 peptide coated hydroxyapatite on Tibial defect repair in vivo in Normal and osteoporotic rats. BioMed Res. Int. 2015, 2015, 253858.
- Schmitt, C.M.; Koepple, M.; Moest, T.; Neumann, K.; Weisel, T.; Schlegel, K.A. In vivo evaluation of biofunctionalized implant surfaces with a synthetic peptide (P-15) and its impact on osseointegration. A preclinical animal study. Clin. Oral Implant. Res. 2016, 27, 1339–1348.
- Kumar, V.A.; Taylor, N.L.; Jalan, A.A.; Hwang, L.K.; Wang, B.K.; Hartgerink, J.D. A nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules 2014, 15, 1484–1490.
- Uysal, O.; Arslan, E.; Gulseren, G.; Kilinc, M.C.; Dogan, I.; Ozalp, H.; Caglar, Y.S.; Guler, M.O.; Tekinay, A.B. Collagen peptide presenting nanofibrous scaffold for intervertebral disc regeneration. ACS Appl. Bio Mater. 2019, 2, 1686–1695.
- Harbers, G.M.; Healy, K.E. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 75, 855–869.
- Anderson, J.M.; Vines, J.B.; Patterson, J.L.; Chen, H.; Javed, A.; Jun, H.-W. Osteogenic differentiation of human mesenchymal stem cells synergistically enhanced by biomimetic peptide amphiphiles combined with conditioned medium. Acta Biomater. 2011, 7, 675–682.
- Sindrey, D.; Pugh, S.; Smith, T. Connective Tissue Stimulating Peptides. U.S. Patent US20050288229A1, 29 December 2005.
- Huang, W.; Yu, K.; Kang, M.; Wang, Q.; Liao, W.; Liang, P.; Liu, G.; Cao, Y.; Miao, J. Identification and functional analysis of three novel osteogenic peptides isolated from tilapia scale collagen hydrolysate. Food Res. Int. 2022, 162, 111993.
- Lam, H.; Li, S.; Lou, N.; Chu, J.; Bhatnagar, R. Synthetic peptides cytomodulin-1 (CM-1) and cytomodulin-2 (CM-2) promote collagen synthesis and wound healing in vitro. In Proceedings of the the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004; pp. 5028–5030.
- Lin, Z.-Y.; Duan, Z.-X.; Guo, X.-D.; Li, J.-F.; Lu, H.-W.; Zheng, Q.-X.; Quan, D.-P.; Yang, S.-H. Bone induction by biomimetic PLGA-(PEG-ASP) n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J. Control. Release 2010, 144, 190–195.
- Duan, Z.; Zheng, Q.; Guo, X.; Yuan, Q.; Chen, S. Experimental research on ectopic osteogenesis of BMP2-derived peptide P24 combined with PLGA copolymers. J. Huazhong Univ. Sci. Technol. 2007, 27, 179–182.
- Saito, A.; Suzuki, Y.; Ogata, S.I.; Ohtsuki, C.; Tanihara, M. Prolonged ectopic calcification induced by BMP-2–derived synthetic peptide. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 70, 115–121.
- Kang, E.-J.; Kim, S.-K.; Eom, T.-G.; Choi, K.-O.; Lee, T.-H. Evaluation of the osteogenic activity of the BMP-2 mimetic peptide, PEP7, in vitro and in vivo. Int. J. Oral Maxillofac. Implant. 2013, 28, 749–756.
- Saito, A.; Suzuki, Y.; Ogata, S.-i.; Ohtsuki, C.; Tanihara, M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2003, 1651, 60–67.
- Moore, N.M.; Lin, N.J.; Gallant, N.D.; Becker, M.L. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta Biomater. 2011, 7, 2091–2100.
- He, X.; Ma, J.; Jabbari, E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir 2008, 24, 12508–12516.
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 2009, 134, 111–117.
- Cho, Y.-J.; Yeo, S.-I.; Park, J.-W.; Shin, H.-I.; Bae, Y.-C.; Suh, J.-Y. The effects of synthetic peptide derived from hBMP-2 on bone formation in rabbit calvarial defect. Tissue Eng. Regen. Med. 2008, 5, 488–497.
- Lee, J.S.; Lee, J.S.; Murphy, W.L. Modular peptides promote human mesenchymal stem cell differentiation on biomaterial surfaces. Acta Biomater. 2010, 6, 21–28.
- Saito, A.; Suzuki, Y.; Kitamura, M.; Ogata, S.I.; Yoshihara, Y.; Masuda, S.; Ohtsuki, C.; Tanihara, M. Repair of 20-mm long rabbit radial bone defects using BMP-derived peptide combined with an α-tricalcium phosphate scaffold. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2006, 77, 700–706.
- Kim, H.K.; Lee, J.S.; Kim, J.H.; Seon, J.K.; Park, K.S.; Jeong, M.H.; Yoon, T.R. Bone-forming peptide-2 derived from BMP-7 enhances osteoblast differentiation from multipotent bone marrow stromal cells and bone formation. Exp. Mol. Med. 2017, 49, e328.
- Patterson, J. Peptide-functionalized Biomaterials with Osteoinductive or Anti-biofilm Activity. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Springer: Morgantown, WV, USA, 2020; pp. 129–168.
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; Gurski, L.; Bonor, J.; Price, C.; Wang, L.; Beamer, W.G.; Nohe, A. Casein kinase 2 regulates in vivo bone formation through its interaction with bone morphogenetic protein receptor type Ia. Bone 2011, 49, 944–954.
- Akkiraju, H.; Bonor, J.; Nohe, A. CK2. 1, a novel peptide, induces articular cartilage formation in vivo. J. Orthop. Res. 2017, 35, 876–885.
- Akkiraju, H.; Bonor, J.; Olli, K.; Bowen, C.; Bragdon, B.; Coombs, H.; Donahue, L.R.; Duncan, R.; Nohe, A. Systemic injection of CK2. 3, a novel peptide acting downstream of bone morphogenetic protein receptor BMPRIa, leads to increased trabecular bone mass. J. Orthop. Res. 2015, 33, 208–215.
- Alkhiary, Y.M.; Gerstenfeld, L.C.; Krall, E.; Westmore, M.; Sato, M.; Mitlak, B.H.; Einhorn, T.A. Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1–34). JBJS 2005, 87, 731–741.
- Komrakova, M.; Stuermer, E.K.; Werner, C.; Wicke, M.; Kolios, L.; Sehmisch, S.; Tezval, M.; Daub, F.; Martens, T.; Witzenhausen, P. Effect of human parathyroid hormone hPTH (1–34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone 2010, 47, 480–492.
- Mognetti, B.; Marino, S.; Barberis, A.; Bravo Martin, A.-S.; Bala, Y.; Di Carlo, F.; Boivin, G.; Portigliatti Barbos, M. Experimental stimulation of bone healing with teriparatide: Histomorphometric and microhardness analysis in a mouse model of closed fracture. Calcif. Tissue Int. 2011, 89, 163–171.
- Chintamaneni, S.; Finzel, K.; Gruber, B. Successful treatment of sternal fracture nonunion with teriparatide. Osteoporos. Int. 2010, 21, 1059–1063.
- Whitfield, J.F.; Motley, P.; Willick, G.E. Parathyroid hormone, its fragments and their analogs for the treatment of osteoporosis. Treat. Endocrinol. 2002, 1, 175–190.
- Peggion, E.; Mammi, S.; Schievano, E.; Silvestri, L.; Schiebler, L.; Bisello, A.; Rosenblatt, M.; Chorev, M. Structure−Function Studies of Analogues of Parathyroid Hormone (PTH)-1–34 Containing β-Amino Acid Residues in Positions 11–13. Biochemistry 2002, 41, 8162–8175.
- de Castro, L.F.; Lozano, D.; Portal-Núñez, S.; Maycas, M.; De la Fuente, M.; Caeiro, J.R.; Esbrit, P. Comparison of the skeletal effects induced by daily administration of PTHrP (1–36) and PTHrP (107–139) to ovariectomized mice. J. Cell. Physiol. 2012, 227, 1752–1760.
- Trejo, C.G.; Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Dapía, S.; Gómez-Barrena, E.; Vallet-Regí, M.; García-Honduvilla, N.; Buján, J. The osteoinductive properties of mesoporous silicate coated with osteostatin in a rabbit femur cavity defect model. Biomaterials 2010, 31, 8564–8573.
- Mrak, E.; Guidobono, F.; Moro, G.; Fraschini, G.; Rubinacci, A.; Villa, I. Calcitonin gene-related peptide (CGRP) inhibits apoptosis in human osteoblasts by β-catenin stabilization. J. Cell. Physiol. 2010, 225, 701–708.
- Villa, I.; Melzi, R.; Pagani, F.; Ravasi, F.; Rubinacci, A.; Guidobono, F. Effects of calcitonin gene-related peptide and amylin on human osteoblast-like cells proliferation. Eur. J. Pharmacol. 2000, 409, 273–278.
- Wang, L.; Shi, X.; Zhao, R.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-κB activation, osteoclastogenesis and bone resorption. Bone 2010, 46, 1369–1379.
- Xu, G.; Jiang, D. The role and mechanism of exogenous calcitonin gene-related peptide on mesenchymal stem cell proliferation and osteogenetic formation. Cell Biochem. Biophys. 2014, 69, 369–378.
- Calland, J.W.; Harris, S.E.; Carnes, D.L., Jr. Human pulp cells respond to calcitonin gene-related peptide in vitro. J. Endod. 1997, 23, 485–489.
- Vignery, A.; McCarthy, T. The neuropeptide calcitonin gene-related peptide stimulates insulin-like growth factor I production by primary fetal rat osteoblasts. Bone 1996, 18, 331–335.
- Ballica, R.; Valentijn, K.; Khachatryan, A.; Guerder, S.; Kapadia, S.; Gundberg, C.; Gilligan, J.; Flavell, R.A.; Vignery, A. Targeted expression of calcitonin gene-related peptide to osteoblasts increases bone density in mice. J. Bone Miner. Res. 1999, 14, 1067–1074.
- Bab, I.; Gazit, D.; Chorev, M.; Muhlrad, A.; Shteyer, A.; Greenberg, Z.; Namdar, M.; Kahn, A. Histone H4-related osteogenic growth peptide (OGP): A novel circulating stimulator of osteoblastic activity. EMBO J. 1992, 11, 1867–1873.
- Gabet, Y.; Müller, R.; Regev, E.; Sela, J.; Shteyer, A.; Salisbury, K.; Chorev, M.; Bab, I. Osteogenic growth peptide modulates fracture callus structural and mechanical properties. Bone 2004, 35, 65–73.
- Brager, M.A.; Patterson, M.J.; Connolly, J.F.; Nevo, Z. Osteogenic growth peptide normally stimulated by blood loss and marrow ablation has local and systemic effects on fracture healing in rats. J. Orthop. Res. 2000, 18, 133–139.
- Fei, Q.; Guo, C.; Xu, X.; Gao, J.; Zhang, J.; Chen, T.; Cui, D. Osteogenic growth peptide enhances the proliferation of bone marrow mesenchymal stem cells from osteoprotegerin-deficient mice by CDK2/cyclin A. Acta Biochim. Biophys. Sin. 2010, 42, 801–806.
- An, G.; Xue, Z.; Zhang, B.; Deng, Q.; Wang, Y.; Lv, S. Expressing osteogenic growth peptide in the rabbit bone mesenchymal stem cells increased alkaline phosphatase activity and enhanced the collagen accumulation. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1618–1624.
- Li, G.; Cui, Y.; McILmurray, L.; Allen, W.E.; Wang, H. rhBMP-2, rhVEGF165, rhPTN and thrombin-related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. J. Orthop. Res. 2005, 23, 680–685.
- Vordemvenne, T.; Paletta, J.R.; Hartensuer, R.; Pap, T.; Raschke, M.J.; Ochman, S. Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC Musculoskelet. Disord. 2011, 12, 263.
- Olszewska-Pazdrak, B.; Carney, D.H. Systemic administration of thrombin peptide TP508 enhances VEGF-stimulated angiogenesis and attenuates effects of chronic hypoxia. J. Vasc. Res. 2013, 50, 186–196.
- Wang, H.; Li, X.; Tomin, E.; Doty, S.B.; Lane, J.M.; Carney, D.H.; Ryaby, J.T. Thrombin peptide (TP508) promotes fracture repair by up-regulating inflammatory mediators, early growth factors, and increasing angiogenesis. J. Orthop. Res. 2005, 23, 671–679.
- Hanratty, B.M.; Ryaby, J.T.; Pan, X.-H.; Li, G. Thrombin related peptide TP508 promoted fracture repair in a mouse high energy fracture model. J. Orthop. Surg. Res. 2009, 4, 1.
- Li, X.; Wang, H.; Touma, E.; Qi, Y.; Rousseau, E.; Quigg, R.J.; Ryaby, J.T. TP508 accelerates fracture repair by promoting cell growth over cell death. Biochem. Biophys. Res. Commun. 2007, 364, 187–193.
- Kim, Y.-J.; Park, Y.-J.; Lee, Y.-M.; Rhyu, I.-C.; Ku, Y. The biological effects of fibrin-binding synthetic oligopeptides derived from fibronectin on osteoblast-like cells. J. Periodontal Implant. Sci. 2012, 42, 113–118.
- Lee, J.-A.; Ku, Y.; Rhyu, I.-C.; Chung, C.-P.; Park, Y.-J. Effects of fibrin-binding oligopeptide on osteopromotion in rabbit calvarial defects. J. Periodontal Implant. Sci. 2010, 40, 211–219.
- Martino, M.M.; Tortelli, F.; Mochizuki, M.; Traub, S.; Ben-David, D.; Kuhn, G.A.; Müller, R.; Livne, E.; Eming, S.A.; Hubbell, J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med. 2011, 3, 100ra189.
- Lee, J.-Y.; Choo, J.-E.; Park, H.-J.; Park, J.-B.; Lee, S.-C.; Jo, I.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. Injectable gel with synthetic collagen-binding peptide for enhanced osteogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2007, 357, 68–74.
- Shin, M.K.; Kim, M.-K.; Bae, Y.-S.; Jo, I.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cell. Signal. 2008, 20, 613–624.
- Lee, J.-Y.; Choo, J.-E.; Choi, Y.-S.; Park, J.-B.; Min, D.-S.; Lee, S.-J.; Rhyu, H.K.; Jo, I.-H.; Chung, C.-P.; Park, Y.-J. Assembly of collagen-binding peptide with collagen as a bioactive scaffold for osteogenesis in vitro and in vivo. Biomaterials 2007, 28, 4257–4267.
- Egusa, H.; Kaneda, Y.; Akashi, Y.; Hamada, Y.; Matsumoto, T.; Saeki, M.; Thakor, D.K.; Tabata, Y.; Matsuura, N.; Yatani, H. Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials 2009, 30, 4676–4686.
- Hamada, Y.; Yuki, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Hashida, M.K.; Harutsugu, K.; Nokihara, K.; Daito, M.; Matsuura, N. Osteopontin-derived peptide SVVYGLR induces angiogenesis in vivo. Dent. Mater. J. 2004, 23, 650–655.
- Park, K.M.; Lee, Y.; Son, J.Y.; Bae, J.W.; Park, K.D. In situ SVVYGLR peptide conjugation into injectable gelatin-poly (ethylene glycol)-tyramine hydrogel via enzyme-mediated reaction for enhancement of endothelial cell activity and neo-vascularization. Bioconjugate Chem. 2012, 23, 2042–2050.
- Rezania, A.; Healy, K.E. Biomimetic peptide surfaces that regulate adhesion, spreading, cytoskeletal organization, and mineralization of the matrix deposited by osteoblast-like cells. Biotechnol. Prog. 1999, 15, 19–32.
- Stile, R.A.; Healy, K.E. Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromolecules 2001, 2, 185–194.
- Yarbrough, D.K.; Hagerman, E.; Eckert, R.; He, J.; Choi, H.; Cao, N.; Le, K.; Hedger, J.; Qi, F.; Anderson, M. Specific binding and mineralization of calcified surfaces by small peptides. Calcif. Tissue Int. 2010, 86, 58–66.
- El Gezawi, M.; Wölfle, U.C.; Haridy, R.; Fliefel, R.; Kaisarly, D. Remineralization, regeneration, and repair of natural tooth structure: Influences on the future of restorative dentistry practice. ACS Biomater. Sci. Eng. 2019, 5, 4899–4919.
- Hsu, C.; Chung, H.; Yang, J.-M.; Shi, W.; Wu, B. Influence of 8DSS peptide on nano-mechanical behavior of human enamel. J. Dent. Res. 2011, 90, 88–92.
- Chung, H.-Y.; Li, C.C. Microstructure and nanomechanical properties of enamel remineralized with asparagine–serine–serine peptide. Mater. Sci. Eng. C 2013, 33, 969–973.
- Padovano, J.; Ravindran, S.; Snee, P.; Ramachandran, A.; Bedran-Russo, A.; George, A. DMP1-derived peptides promote remineralization of human dentin. J. Dent. Res. 2015, 94, 608–614.
- Tang, S.; Dong, Z.; Ke, X.; Luo, J.; Li, J. Advances in biomineralization-inspired materials for hard tissue repair. Int. J. Oral Sci. 2021, 13, 42.
- Deshpande, A.S.; Fang, P.-A.; Simmer, J.P.; Margolis, H.C.; Beniash, E. Amelogenin-collagen interactions regulate calcium phosphate mineralization in vitro. J. Biol. Chem. 2010, 285, 19277–19287.
- Mukherjee, K.; Visakan, G.; Phark, J.-H.; Moradian-Oldak, J. Enhancing collagen mineralization with amelogenin peptide: Toward the restoration of dentin. ACS Biomater. Sci. Eng. 2020, 6, 2251–2262.
- Lv, X.; Yang, Y.; Han, S.; Li, D.; Tu, H.; Li, W.; Zhou, X.; Zhang, L. Potential of an amelogenin based peptide in promoting reminerlization of initial enamel caries. Arch. Oral Biol. 2015, 60, 1482–1487.
- Mukherjee, K.; Ruan, Q.; Liberman, D.; White, S.N.; Moradian-Oldak, J. Repairing human tooth enamel with leucine-rich amelogenin peptide–chitosan hydrogel. J. Mater. Res. 2016, 31, 556–563.
- Ding, L.; Han, S.; Peng, X.; Wang, K.; Zheng, S.; Li, H.; Niu, Y.; Li, W.; Zhang, L. Tuftelin-derived peptide facilitates remineralization of initial enamel caries in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 3261–3269.
- Wang, Q.-Q.; Miao, L.; Zhang, H.; Wang, S.Q.; Li, Q.; Sun, W. A novel amphiphilic oligopeptide induced the intrafibrillar mineralisation via interacting with collagen and minerals. J. Mater. Chem. B 2020, 8, 2350–2362.
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Müller, B.; Pieles, U. Biomimetic remineralization of carious lesions by self-assembling peptide. J. Dent. Res. 2017, 96, 790–797.
- Van Hove, A.H.; Benoit, D.S. Depot-based delivery systems for pro-angiogenic peptides: A review. Front. Bioeng. Biotechnol. 2015, 3, 102.
- Finetti, F.; Basile, A.; Capasso, D.; Di Gaetano, S.; Di Stasi, R.; Pascale, M.; Turco, C.M.; Ziche, M.; Morbidelli, L.; D’Andrea, L.D. Functional and pharmacological characterization of a VEGF mimetic peptide on reparative angiogenesis. Biochem. Pharmacol. 2012, 84, 303–311.
- Chen, R.R.; Silva, E.A.; Yuen, W.W.; Mooney, D.J. Spatio–temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm. Res. 2007, 24, 258–264.
- Kang, H.-M.; Kang, Y.; Chun, H.J.; Jeong, J.-W.; Park, C. Evaluation of the in vitro and in vivo angiogenic effects of exendin-4. Biochem. Biophys. Res. Commun. 2013, 434, 150–154.
- Hamada, Y.; Nokihara, K.; Okazaki, M.; Fujitani, W.; Matsumoto, T.; Matsuo, M.; Umakoshi, Y.; Takahashi, J.; Matsuura, N. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochem. Biophys. Res. Commun. 2003, 310, 153–157.
- Hamada, Y.; Egusa, H.; Kaneda, Y.; Hirata, I.; Kawaguchi, N.; Hirao, T.; Matsumoto, T.; Yao, M.; Daito, K.; Suzuki, M. Synthetic osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dent. Mater. J. 2007, 26, 487–492.
- Lane, T.F.; Iruela-Arispe, M.L.; Johnson, R.S.; Sage, E.H. SPARC is a source of copper-binding peptides that stimulate angiogenesis. J. Cell Biol. 1994, 125, 929–943.
- Van Hove, A.H.; Burke, K.; Antonienko, E.; Brown, E., III; Benoit, D.S. Enzymatically-responsive pro-angiogenic peptide-releasing poly (ethylene glycol) hydrogels promote vascularization in vivo. J. Control. Release 2015, 217, 191–201.
- Hardy, B.; Raiter, A.; Weiss, C.; Kaplan, B.; Tenenbaum, A.; Battler, A. Angiogenesis induced by novel peptides selected from a phage display library by screening human vascular endothelial cells under different physiological conditions. Peptides 2007, 28, 691–701.
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 37–51.
- Bhatnagar, R.S.; Qian, J.J. Peptide Compositions with Growth Factor-like Activity. U.S. Patent 5,661,127, 26 August 1997.
- Renner, J.N.; Liu, J.C. Investigating the effect of peptide agonists on the chondrogenic differentiation of human mesenchymal stem cells using design of experiments. Biotechnol. Prog. 2013, 29, 1550–1557.
- Zhang, Z.; Gupte, M.J.; Jin, X.; Ma, P.X. Injectable peptide decorated functional nanofibrous hollow microspheres to direct stem cell differentiation and tissue regeneration. Adv. Funct. Mater. 2015, 25, 350–360.
- Mittal, A.; Kumar, R.; Parsad, D.; Kumar, N. Cytomodulin-functionalized porous PLGA particulate scaffolds respond better to cell migration, actin production and wound healing in rodent model. J. Tissue Eng. Regen. Med. 2014, 8, 351–363.
- Zhao, B.; Yang, D.; Wong, J.H.; Wang, J.; Yin, C.; Zhu, Y.; Fan, S.; Ng, T.B.; Xia, J.; Li, Z. A Thioether-Stabilized d-Proline–l-Proline-Induced β-Hairpin Peptide of Defensin Segment Increases Its Anti-Candida albicans Ability. ChemBioChem 2016, 17, 1416–1420.
- Ruczynski, J.; Wierzbicki, P.M.; Kogut-Wierzbicka, M.; Mucha, P.; Siedlecka-Kroplewska, K.; Rekowski, P. Cell-penetrating peptides as a promising tool for delivery of various molecules into the cells. Folia Histochem. Cytobiol. 2014, 52, 257–269.
- Akkiraju, H.; Srinivasan, P.P.; Xu, X.; Jia, X.; Safran, C.B.K.; Nohe, A. CK2. 1, a bone morphogenetic protein receptor type Ia mimetic peptide, repairs cartilage in mice with destabilized medial meniscus. Stem Cell Res. Ther. 2017, 8, 82.
- Renner, J.N.; Kim, Y.; Liu, J.C. Bone morphogenetic protein-derived peptide promotes chondrogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A 2012, 18, 2581–2589.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.0K
Entry Collection:
Peptides for Health Benefits
Revisions:
2 times
(View History)
Update Date:
19 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





