2. Peptides for Bone Regeneration
Natural bone healing occurs in two different pathways: intramembranous ossification and endochondral ossification
[14][103]. During this process, a variety of cytokines are released
[15][104]. Among them, bone morphogenetic proteins (BMPs, especially BMP-2, -4, and -7) and transforming growth factor-β (TGF-β, especially the β2 and β3 subtypes) play crucial roles in stem cell differentiation by causing hypertrophy and mineralization, while vascular endothelial growth factor (VEGF) and angiopoietins promote neoangiogenesis
[16][17][105,106]. BMP-2, -3, -4, -7, TNF-α, interferon-γ (IFN-γ), and certain hormones regulate the remodeling phase
[18][107]. Since bioactive peptides follow similar cell-signaling pathways with these proteins and may be beneficial for both in vitro and in vivo bone formation, they are grouped into three categories based on their role in osteo-induction, biomineralization, and angiogenesis.
2.1. Osteo-Inducers
2.1.1. Collagen-Mimetic/Derived Peptides
Peptides derived from integrin-binding motifs of collagen type I, which is the most significant ECM protein, are GFOGER, P15, KOD, DGEA, and BCSP1, which can promote osteogenic activity and in vivo bone formation.
Collagen-mimetic GFOGER peptide, which is derived from the collagen α1 chain, selectively promotes α2β1 integrin binding required for osteoblastic differentiation
[19][14]. Besides improving cell attachment, GFOGER successfully induces in vitro osteogenic differentiation and in vivo bone healing
[19][20][21][22][23][14,67,108,109,110]. GFOGER coating on synthetic PCL scaffolds remarkably enhanced bone formation in critically sized segmental defects in rats by stimulating osteoblast adhesion and differentiation
[21][108]. P15, which is a 15-mer peptide derived from the collagen type I α1 chain, has a strong affinity for the cell surface α2β1 integrin receptors. By releasing growth factors and cytokines, the peptide dramatically enhanced the osteogenic differentiation of MSCs
[24][15]. The commercially available P15 formulations significantly enhanced the regeneration of alveolar bone and tibial defects in osteoporotic dogs
[25][26][111,112]. The collagen-mimetic KOD peptide, which is made of three units, namely, ((PKG)
4-(POG)
4-(DOG)
4), forms a hydrogel through self-assembly inducing platelet activation and blood clotting associated with hematoma formation
[27][113]. POG-based poly-amphiphilic hydrogels allowed for faster recovery (within two weeks) of intervertebral disc defects in rabbits due to a significant increase in ECM deposition
[28][16]. DGEA derived from collagen type I adhesive motif serves as a crucial ligand for osteoblast differentiation
[29][114]. DGEA-containing PA hydrogels seeded with hMSCs substantially upregulated osteogenic markers (OCN, RUNX2, and ALP)
[30][17].
Using a bone- and cartilage-stimulating peptide (BCSP™-1 or NGLPGPIGP) present in human collagen type-I, the proliferation of rat bone-marrow-derived osteoblasts and human or bovine chondrocytes was drastically improved with enhanced the bone mineral density (BMD) and bone mineral content (BMC) in male Wistar rats
[31][18]. Three highly osteogenic peptides (GPAGPHGPVG, APDPFRMY, and TPERYY) derived from tilapia scale collagen hydrolysate notably increased the MC3T3-E1 cell proliferation and mineralization activity (ALP synthesis, osteogenic-related gene expression) at concentrations of 50 μg/mL
[32][19].
2.1.2. BMP-Mimetic/Derived Peptides
As members of the TGF-β superfamily, BMPs are primarily produced by endothelial cells (ECs), osteoblasts, and hypertrophic chondrocytes and can recruit MSCs to the site of injury and differentiate into osteoblasts while inducing ectopic bone formation.
The KIPKASSVPTELSAISTLYL peptide, which is derived from the knuckle epitope of BMP2, increased the ALP activity of osteoprogenitor cells
[33][115]. P24 is a BMP-2 mimetic peptide with a 24-mer peptide bearing the knuckle epitope of the protein that facilitates binding with BMP receptors. P24 successfully induced ectopic bone formation in rodents
[34][35][36][22,23,24]. The PEP7 peptide (CKIPKPSSVP-TELSAISMLYL) derived from BMP-2 promoted adhesion, proliferation, and differentiation of MG-63 cells, as well as new bone formation in a supra-alveolar peri-implant defect model in a micropig mandible
[37][25]. The BMP peptide (KIPKASSVPTELSAISTLYL) derived from BMP2 increased the ALP activity, which is an early marker for bone formation, in murine osteoprogenitor cells
[38][20] and other cell types
[39][40][41][42][43][116,117,118,119,120], as well as the dose-dependent healing of rabbit radial bone defects
[44][21]. The other osteo-inductive or osteogenic peptides derived from BMP-2 (NSVNSKIPKACCVPTELSAI, KIPKASSVPTELSAISTLYL, DWIVA) produced differential effects on in vitro osteogenic differentiation, as well as ectopic or orthotopic bone formation in vivo
[11]. The bone-forming peptide (BFP-2) with a VEHDKEFFHPRYHH sequence, which was isolated from the immature BMP-7 precursor, triggered osteogenic differentiation of BMSCs and induced ectopic bone formation after subcutaneous implantation of BFP-2-treated BMSCs in mice
[45][27]. Similarly, the effects of various osteo-inductive peptides derived from BMP-4 (RKKNPNCRRH), BMP-7 (TVPKPSSAPTQLNAISTLYF, GQGFSYPYKAVFSTQ, ETLDGQSINPKLAGL), and BMP-9 (KVGKACCVPTKLSPISVLY) were reviewed
[46][26]. The casein kinase 2 (CK2)-related peptide has a great influence on cell proliferation and apoptosis, and it facilitates in vivo bone formation by interacting with BMP receptor type Ia (BMPRIa)
[47][121]. Three BMP-2 mimetic peptides, namely, CK2.1, CK2.2, and CK2.3, triggered the BMP signaling pathways by inhibiting CK2 binding to BMPRIa
[48][28]. C2C12 cells treated with CK2.3 peptide resulted in osteogenesis, while CK2.2 led to both osteogenesis and adipogenesis
[47][49][121,122].
2.1.3. Hormone-Derived Peptides
Parathyroid hormone (PTH) is a major regulator of mineral homeostasis. Parathyroid hormone (PTH)-related peptides called Teriparatide, which are 1–34 peptide domains of PTH (PTH1–34), stimulated osteoblast activity and increased bone density at the fracture site, leading to the healing of non-unions
[50][51][52][53][54][123,124,125,126,127]. On the other hand, endogenous PTH-related protein (PTHrP) analogs, namely, PTHrP1–34, PTHrP1–36, and PTHrP107–111, increased osteoblast activity and local bone formation
[55][56][57][29,30,31]. Calcitonin gene-related peptide (CGRP) is a 37-mer neuropeptide with two isoforms: α- and β-CGRP. They were found to stimulate the proliferation and differentiation of osteoprogenitor cells
[58][59][60][61][32,33,34,35], production of osteogenic molecules like insulin-like growth factor (IGF, especially IGF-1 and -2), BMP-2
[62][63][128,129], and reparative bone formation
[64][130].
2.1.4. Circulating Peptides
Osteogenic growth peptide (OGP), which is a 14-mer peptide occurring in mammalian blood, increases bone formation through anabolic effects on bone cells
[65][66][131,132] and differentiation of osteoprogenitor cells, leading to upregulated osteogenic markers, including mineralization
[67][68][69][36,37,38]. Thrombin peptide 508 (TP508) or Chrysalin is a 23-amino acid peptide and receptor binding domain of thrombin, which enhanced the proliferation, differentiation, and chemotaxis of human osteoblasts
[70][71][39,40] and VEGF-stimulated angiogenesis
[72][133]. TP508 injected into the fracture gap promoted fracture healing and increased blood vessel formation
[73][74][75][61,134,135].
2.1.5. Other ECM-Derived Peptides
Signaling domains on ECM protein chains are capable of interacting with cell membrane receptors. Various peptides (e.g., FN III9-10/12-14) derived from fibronectin (FN) were shown to promote osteoblast activity and mineralization
[76][41], rabbit calvarial defects healing
[77][136], and augmented BMP-2 and platelet-derived growth factor (PDGF) activities for bone regeneration in vivo
[78][137].
Collagen-binding motif (CBM) is a cleavage product of osteopontin (OPN) that can specifically bind to collagen
[79][138] and promote migration, osteogenic differentiation
[80][139], and bone formation in a rabbit calvarial defect model
[81][42]. The SVVYGLR peptide adjacent to the RGD sequence in OPN significantly enhanced the adhesion and proliferation of MSCs, neovascularization, upregulation of osteogenesis, and angiogenesis when delivered through a collagen sponge
[82][83][84][43,44,45]. FHRRIKA, which is a cell-binding and heparin-binding domain of bone sialoprotein (BSP) exerts a favorable effect on osteoblast adhesion, spreading, and mineralization
[85][46]. Higher cell proliferation and viability were observed on rat calvarial osteoblasts that seeded scaffolds containing the RGD and FHRRIKA sequences
[86][140].
2.2. Biomineralizing Peptides
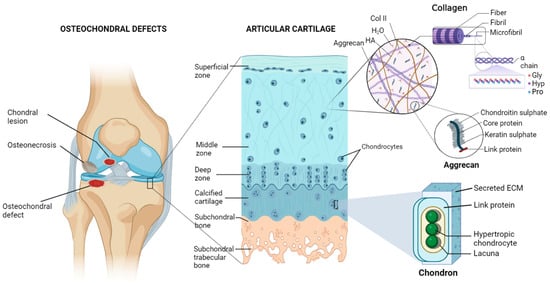
Non-collagenous proteins (NCPs), such as dentin sialophosphoprotein, dentin matrix protein 1 (DMP1), and dentin phosphoprotein (DPP), play a significant role in biomineralization. The negatively charged domains (carboxylic acid and phosphate groups) in NCPs serve as preferential sites for the nucleation of hydroxyapatites (HAPs) while stabilizing them into the self-assembled collagen fibrils that act as a template for crystal growth. Peptides derived from such proteins significantly enhance bone formation.
The Asp–Ser–Ser (DSS) repeating motifs present in DPP have a remarkably strong binding affinity toward calcium ions and HAP
[87][141]. 8DSS, which is a DPP peptide with eight repetitive units of DSS, was the most promising for promoting the mineralization and remineralization of acid-etched enamel
[88][89][47,48]. Like DSS, 3NSS with three repetitive units of asparagine–serine–serine (aspartic acid in DSS is substituted with asparagine) could remineralize the acid-etched enamel
[90][49]. On the other hand, the DSESSEEDR sequence in dentin matrix protein 1 (DMP1) could bind to demineralized dentin and promote remineralization
[91][50]. The other phosphoprotein-derived peptides, such as SN15, SNA15, DpSpSEEKC, DDDEEK, and DDDEEKC, exhibited high affinity toward HAP
[92][142].
Amelogenin, which is found at the dentin–enamel interface, interacts with collagen to control the formation of HAP crystals and their structural alignment
[93][143]. In addition to remineralizing enamel caries, amelogenin-inspired peptides, such as shADP5, QP5, P26, and P32, helped to restore demineralized dentin
[94][95][51,52]. Since leucine-rich amelogenin peptide (LRAP) is more hydrophilic than amelogenin, the demineralized enamel treated with CS-LRAP hydrogel exhibited quicker nucleation and development of HAP crystals than amelogenin-containing chitosan hydrogel (CS-AMEL)
[96][53]. A non-amelogenin protein called tuftelin is present in tooth enamel and has a role in the mineralization of dental enamel. Tuftelin-derived peptide (TDP) encouraged the remineralization of early carious lesions by attracting calcium and phosphate ions
[97][54]. Self-assembling amphiphilic oligopeptide derived from cementum protein 1 (CEMP1), which is a regulator of cementum-matrix mineralization, induced intrafibrillar mineralization of collagen fibrils in the presence of calcium ions
[98][55]. P11-4, which is another self-assembling peptide, acted as a scaffold to enhance HAP nucleation de novo
[99][56].
2.3. Angiogenic Peptides
Vascularization is a crucial process during natural bone formation. Many peptides are derived from angiogenic growth factors (e.g., VEGF, fibroblast growth factor-2 (FGF-2), and PDGF), ECM (e.g., OPN, ON), and other proteins that have crucial roles in blood vessel formation
[100][144].
VEGF-mimicking QK or KLT peptide (KLTWQELQLKYKGIGGG), which is derived from the VEGF receptors binding domain 17–35, not only induces EC migration and proliferation but also triggers other complex processes, like chemotaxis and capillary sprouting and organization similar to VEGF
[101][57]. PDGF-BB-derived PBA2-1c peptide interacts with α- and β-PDGF receptors. Though its in vivo proangiogenic activity is still unclear, it functions similarly to PDGF in establishing mature blood vessels that are created by VEGF
[102][58]. Exendin-4, which is a glucagon-like peptide 1 (Glp-1) analog, stimulates human umbilical vein endothelial cells’ (HUVECs’) motility, sprouting, and tube formation in vitro, in addition to in vivo sprout outgrowth
[103][59]. While OPN is widely distributed in the bone matrix to help with bone metabolism, OPN-derived peptide (OPD) does not induce EC proliferation in vitro. However, like VEGF, it facilitated EC migration and tube formation using 3D collagen gels
[104][145], suppressed osteoclastogenesis
[82][43], and promoted the adhesion and proliferation of MSCs, as well as neovascularization in a rat tibial defect model
[105][146]. SPARC113 and SPARC118, which are two OPN-derived peptides that exhibit potent angiogenic activity
[106][147], stimulated in vivo angiogenesis when delivered through MMPs degradable hydrogel
[107][60]. TP508 enhanced neoangiogenesis in femoral defects produced in rats
[73][61] and mice
[74][134] following one hour of local administration. The synthetic 12-mer peptides, known as RoY peptides, which were created via the phage-display technology, may also promote in vitro EC proliferation, tube formation, and sprouting, as well as induce in vivo angiogenesis via a distinct mechanism from VEGF
[108][62].
3. Peptides for Cartilage Regeneration
3.1. Chondroinductive/Chondrogenic Peptides
Numerous peptides were identified to imitate the functions of ECM components, cell–cell junction molecules, and chondroinductive/chondrogenic ligands triggering specific cell-signaling pathways. Motif-derived fibronectins, like RGD, decorin, collagen, and MMPs, display chondrogenic properties. These peptides are often used to functionalize scaffolds that encourage chondrocyte adhesion, migration, and proliferation, in addition to MSC differentiation into the chondrogenic lineage.
3.1.1. TGF-β Mimetic Peptides
TGF-β improves cell differentiation, collagen synthesis, and matrix deposition in cartilage tissue engineering
[109][148]. Therefore, peptides mimicking TGF-β activity were used for cartilage tissue regeneration. TGF-β mimetic peptides, i.e., cytomodulins (CMs), are oligopeptides containing 4–6 amino acids
[110][149]. CMs immobilized on a solid surface can potentially induce chondrogenic differentiation better compared with its soluble form
[111][112][63,64].
3.1.2. BMP2-Derived/Mimetic Peptides
BMP-2, which is a member of the TGF-β super-family, is one of the main chondrogenic growth factors that induce in vitro chondrogenic differentiation and cartilage regeneration in vivo. Human MSCs (hMSCs) cultured with ≥100 µg/mL of the BMP peptide (KIPKASSVPTELSAISTLYL) resulted in glycosaminoglycan (GAGs) production and increased levels of collagen production and matrix accumulation without extensive upregulation of hypertrophic markers
[111][112][113][114][115][63,64,150,151,152]. The injection of BMP-2 mimetic CK2.1 peptide into a mouse’s tail vein enhanced chondrogenesis and articular cartilage formation without any effects on osteogenesis or BMD
[116][65]. BMP peptide stimulated chondrogenic differentiation of hMSCs without additional growth factors. At a 100 μg/mL concentration, BMP peptide enhanced proteoglycan production and chondrogenic gene expression without causing hypertrophy, as occurs with BMP-2
[117][153].