
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yogeswaran Lokanathan | -- | 2517 | 2024-03-04 10:17:11 | | | |
| 2 | Lindsay Dong | Meta information modification | 2517 | 2024-03-05 01:49:27 | | |
Video Upload Options
Systemic lupus erythematosus (SLE) is a multisystemic autoimmune disease that affects nearly 3.41 million people globally, with 90% of the cases affecting women of childbearing age. Extracellular vesicles (EVs) can reduce the pro-inflammatory cytokines and increase the anti-inflammatory cytokines. Moreover, EVs can increase the levels of regulatory T cells, thus reducing inflammation. EVs also have the potential to regulate B cells to alleviate SLE and reduce its adverse effects.
1. Introduction
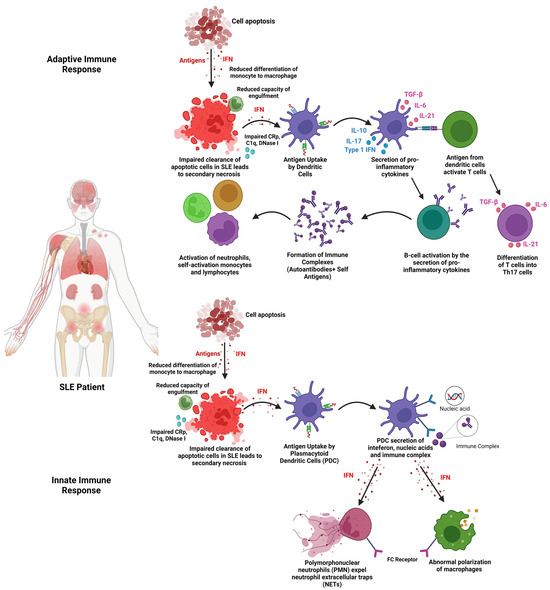
2. Extracellular Vesicles (EVs)
3. Stem Cell-Derived Extracellular Vesicles in Systemic Lupus Erythematosus
3.1. Isolation of EVs
The isolation method of EVs is crucial to obtain the possible highest purity of EVs to further enhance the specific mechanism of action required [22]. In all the studies, EVs were isolated from the supernatant only after reaching 80–90% confluency of MSCs. The supernatant derived from the conditioned media undergoes centrifugation between 10,000× g and 125,000× g and further undergoes ultracentrifugation at 140,000× g to isolate the EVs based on the size from the precipitate obtained [37]. Apart from that, some studies have included EVs that are also isolated from supernatants using super high-speed centrifugation of 175,000× g. There are other studies that have stated the use of only ultracentrifugation between 125,000× g and 140,000× g for the isolation of EVs [1][38][39][40][41].
3.2. Characterisation of EVs
3.3. Range of Dose of EVs Administered
3.4. Mechanism of Action (EVs)
3.4.1. Effects of EVs on Pro-Inflammatory Cytokines
3.4.2. Effects of EVs on Anti-Inflammatory Cytokines
3.4.3. Effects of EVs on T Cell Lineage
3.4.4. Effects of EVs on B Cells
3.4.5. Effects of EVs on Lupus Nephritis (c-Complements)
3.5. Role of miRNAs and tsRNAs in Extracellular Vesicles in Ameliorating the Disease Progression of SLE
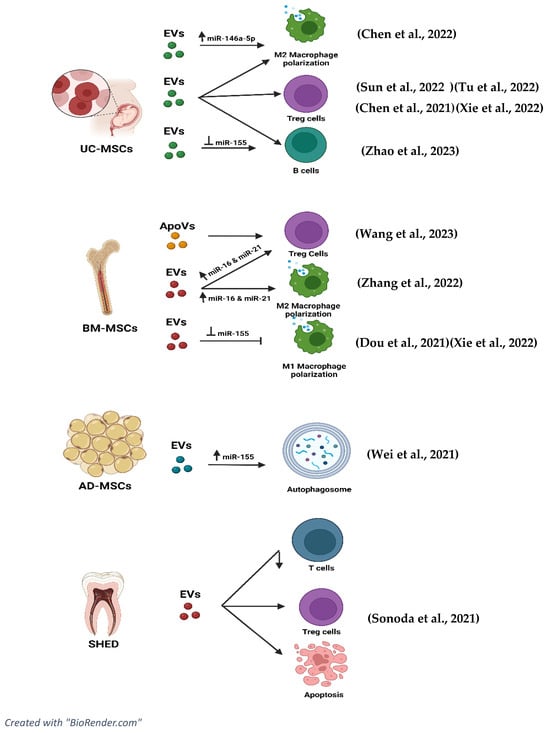
3.6. EVs and Signalling Pathways
References
- Xie, M.; Li, C.; She, Z.; Wu, F.; Mao, J.; Hun, M.; Luo, S.; Wan, W.; Tian, J.; Wen, C. Human umbilical cord mesenchymal stem cells derived extracellular vesicles regulate acquired immune response of lupus mouse in vitro. Sci. Rep. 2022, 12, 13101.
- Justiz Vaillant, A.A.; Goyal, A.; Varacallo, M. Systemic Lupus Erythematosus. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023.
- Kuhn, A.; Bonsmann, G.; Anders, H.-J.; Herzer, P.; Tenbrock, K.; Schneider, M. The Diagnosis and Treatment of Systemic Lupus Erythematosus. Dtsch. Arztebl. Int. 2015, 112, 423–432.
- Kamen, D.L. Environmental influences on systemic lupus erythematosus expression. Rheum. Dis. Clin. N. Am. 2014, 40, 401–412.
- Kernder, A.; Richter, J.G.; Fischer-Betz, R.; Winkler-Rohlfing, B.; Brinks, R.; Aringer, M.; Schneider, M.; Chehab, G. Delayed diagnosis adversely affects outcome in systemic lupus erythematosus: Cross sectional analysis of the LuLa cohort. Lupus 2021, 30, 431–438.
- Imran, S.; Thabah, M.M.; Azharudeen, M.; Ramesh, A.; Bobby, Z.; Negi, V.S. Liver Abnormalities in Systemic Lupus Erythematosus: A Prospective Observational Study. Cureus 2021, 13, e15691.
- Chen, S.-L.; Zheng, H.-J.; Zhang, L.-Y.; Xu, Q.; Lin, C.-S. Case report: Joint deformity associated with systemic lupus erythematosus. Immun. Inflamm. Dis. 2022, 10, e717.
- Sequeira, J.F.; Cesic, D.; Keser, G.; Bukelica, M.; Karanagnostis, S.; Khamashta, M.A.; Hughes, G.R. Allergic disorders in systemic lupus erythematosus. Lupus 1993, 2, 187–191.
- Wuthisiri, W.; Lai, Y.H.; Capasso, J.; Blidner, M.; Salz, D.; Kruger, E.; Levin, A.V. Autoimmune retinopathy associated with systemic lupus erythematosus: A diagnostic dilemma. Taiwan J. Ophthalmol. 2017, 7, 172–176.
- Abu Bakar, F.; Sazliyana Shaharir, S.; Mohd, R.; Mohamed Said, M.S.; Rajalingham, S.; Wei Yen, K. Burden of Systemic Lupus Erythematosus on Work Productivity and Daily Living Activity: A Cross-Sectional Study Among Malaysian Multi-Ethnic Cohort. Arch. Rheumatol. 2020, 35, 205–213.
- Mohamed, M.H.; Gopal, S.; Idris, I.B.; Aizuddin, A.N.; Miskam, H.M. Mental Health Status Among Systemic Lupus Erythematosus (SLE) Patients at Tertiary Hospital in Malaysia. Asian Soc. Work. J. 2020, 5, 30–39.
- Fava, A.; Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 2019, 96, 1–13.
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Primers 2016, 2, 16039.
- Pan, L.; Lu, M.-P.; Wang, J.-H.; Xu, M.; Yang, S.-R. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J. Pediatr. 2020, 16, 19–30.
- McKeon, K.P.; Jiang, S.H. Treatment of systemic lupus erythematosus. Aust. Prescr. 2020, 43, 85–90.
- Sakthiswary, R.; Suresh, E. Methotrexate in systemic lupus erythematosus: A systematic review of its efficacy. Lupus 2014, 23, 225–235.
- Zhang, K.; Liu, L.; Shi, K.; Zhang, K.; Zheng, C.; Jin, Y. Extracellular Vesicles for Immunomodulation in Tissue Regeneration. Tissue Eng. Part C Methods 2022, 28, 393–404.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, aau6977.
- Song, Y.; Kim, Y.; Ha, S.; Sheller-Miller, S.; Yoo, J.; Choi, C.; Park, C.H. The emerging role of exosomes as novel therapeutics: Biology, technologies, clinical applications, and the next. Am. J. Reprod. Immunol. 2021, 85, e13329.
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21.
- Huldani, H.; Jasim, S.A.; Bokov, D.O.; Abdelbasset, W.K.; Shalaby, M.N.; Thangavelu, L.; Margiana, R.; Qasim, M.T. Application of extracellular vesicles derived from mesenchymal stem cells as potential therapeutic tools in autoimmune and rheumatic diseases. Int. Immunopharmacol. 2022, 106, 108634.
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Qasim, M.; Kim, J.-H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307.
- Li, M.; Li, S.; Du, C.; Zhang, Y.; Li, Y.; Chu, L.; Han, X.; Galons, H.; Zhang, Y.; Sun, H.; et al. Exosomes from different cells: Characteristics, modifications, and therapeutic applications. Eur. J. Med. Chem. 2020, 207, 112784.
- Silva, T.A.; Smuczek, B.; Valadão, I.C.; Dzik, L.M.; Iglesia, R.P.; Cruz, M.C.; Zelanis, A.; de Siqueira, A.S.; Serrano, S.M.T.; Goldberg, G.S.; et al. AHNAK enables mammary carcinoma cells to produce extracellular vesicles that increase neighboring fibroblast cell motility. Oncotarget 2016, 7, 49998–50016.
- Ozawa, P.M.M.; Alkhilaiwi, F.; Cavalli, I.J.; Malheiros, D.; de Souza Fonseca Ribeiro, E.M.; Cavalli, L.R. Extracellular vesicles from triple-negative breast cancer cells promote proliferation and drug resistance in non-tumorigenic breast cells. Breast Cancer Res. Treat. 2018, 172, 713–723.
- Stronati, E.; Conti, R.; Cacci, E.; Cardarelli, S.; Biagioni, S.; Poiana, G. Extracellular Vesicle-Induced Differentiation of Neural Stem Progenitor Cells. Int. J. Mol. Sci. 2019, 20, 3691.
- Jiang, J.; Mei, J.; Ma, Y.; Jiang, S.; Zhang, J.; Yi, S.; Feng, C.; Liu, Y.; Liu, Y. Tumor hijacks macrophages and microbiota through extracellular vesicles. Exploration 2022, 2, 20210144.
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143.
- Liu, Y.-J.; Wang, C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal. 2023, 21, 77.
- Yekula, A.; Muralidharan, K.; Kang, K.M.; Wang, L.; Balaj, L.; Carter, B.S. From laboratory to clinic: Translation of extracellular vesicle based cancer biomarkers. Methods 2020, 177, 58–66.
- Reed, S.L.; Escayg, A. Extracellular vesicles in the treatment of neurological disorders. Neurobiol. Dis. 2021, 157, 105445.
- Akbar, N.; Azzimato, V.; Choudhury, R.P.; Aouadi, M. Extracellular vesicles in metabolic disease. Diabetologia 2019, 62, 2179–2187.
- Zhu, L.; Kalimuthu, S.; Oh, J.M.; Gangadaran, P.; Baek, S.H.; Jeong, S.Y.; Lee, S.-W.; Lee, J.; Ahn, B.-C. Enhancement of antitumor potency of extracellular vesicles derived from natural killer cells by IL-15 priming. Biomaterials 2019, 190–191, 38–50.
- Bruno, S.; Collino, F.; Deregibus, M.C.; Grange, C.; Tetta, C.; Camussi, G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013, 22, 758–771.
- Katsuda, T.; Oki, K.; Ochiya, T. Potential application of extracellular vesicles of human adipose tissue-derived mesenchymal stem cells in Alzheimer’s disease therapeutics. In Renewal and Cell-Cell Communication; Turksen, K., Ed.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1212, pp. 171–181.
- Cosenza, S.; Toupet, K.; Maumus, M.; Luz-Crawford, P.; Blanc-Brude, O.; Jorgensen, C.; Noël, D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics 2018, 8, 1399–1410.
- Chen, X.; Su, C.; Wei, Q.; Sun, H.; Xie, J.; Nong, G. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviate Diffuse Alveolar Hemorrhage Associated with Systemic Lupus Erythematosus in Mice by Promoting M2 Macrophage Polarization via the microRNA-146a-5p/NOTCH1 Axis. Immunol. Investig. 2022, 51, 1975–1993.
- Tu, J.; Zheng, N.; Mao, C.; Liu, S.; Zhang, H.; Sun, L. UC-BSCs Exosomes Regulate Th17/Treg Balance in Patients with Systemic Lupus Erythematosus via miR-19b/KLF13. Cells 2022, 11, 4123.
- Zhao, Y.; Song, W.; Yuan, Z.; Li, M.; Wang, G.; Wang, L.; Liu, Y.; Diao, B. Exosome Derived from Human Umbilical Cord Mesenchymal Cell Exerts Immunomodulatory Effects on B Cells from SLE Patients. J. Immunol. Res. 2023, 2023, 3177584.
- Chen, X.; Wei, Q.; Sun, H.M.; Zhang, X.B.; Yang, C.R.; Tao, Y.; Nong, G.M. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Regulate Macrophage Polarization to Attenuate Systemic Lupus Erythematosus-Associated Diffuse Alveolar Hemorrhage in Mice. Int. J. Stem Cells 2021, 14, 331–340.
- Sun, W.; Yan, S.; Yang, C.; Yang, J.; Wang, H.; Li, C.; Zhang, L.; Zhao, L.; Zhang, J.; Cheng, M.; et al. Mesenchymal Stem Cells-derived Exosomes Ameliorate Lupus by Inducing M2 Macrophage Polarization and Regulatory T Cell Expansion in MRL/lpr Mice. Immunol. Investig. 2022, 51, 1785–1803.
- Wei, S.S.; Zhang, Z.W.; Yan, L.; Mo, Y.J.; Qiu, X.W.; Mi, X.B.; Lai, K. miR-20a Overexpression in Adipose-Derived Mesenchymal Stem Cells Promotes Therapeutic Efficacy in Murine Lupus Nephritis by Regulating Autophagy. Stem Cells Int. 2021, 2021, 3746335.
- Dou, R.; Zhang, X.; Xu, X.; Wang, P.; Yan, B. Mesenchymal stem cell exosomal tsRNA-21109 alleviate systemic lupus erythematosus by inhibiting macrophage M1 polarization. Mol. Immunol. 2021, 139, 106–114.
- Sonoda, S.; Murata, S.; Kato, H.; Zakaria, F.; Kyumoto-Nakamura, Y.; Uehara, N.; Yamaza, H.; Kukita, T.; Yamaza, T. Targeting of Deciduous Tooth Pulp Stem Cell-Derived Extracellular Vesicles on Telomerase-Mediated Stem Cell Niche and Immune Regulation in Systemic Lupus Erythematosus. J. Immunol. 2021, 206, 3053–3063.
- Zhang, M.; Johnson-Stephenson, T.K.; Wang, W.; Wang, Y.; Li, J.; Li, L.; Zen, K.; Chen, X.; Zhu, D. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17+ regulatory T cell. Stem Cell Res. Ther. 2022, 13, 484.
- Nordin, F.; Shaharir, S.S.; Abdul Wahab, A.; Mustafar, R.; Abdul Gafor, A.H.; Mohamed Said, M.S.; Rajalingham, S.; Shah, S.A. Serum and urine interleukin-17A levels as biomarkers of disease activity in systemic lupus erythematosus. Int. J. Rheum. Dis. 2019, 22, 1419–1426.
- Wang, R.; Hao, M.; Kou, X.; Sui, B.; Sanmillan, M.L.; Zhang, X.; Liu, D.; Tian, J.; Yu, W.; Chen, C.; et al. Apoptotic vesicles ameliorate lupus and arthritis via phosphatidylserine-mediated modulation of T cell receptor signaling. Bioact. Mater. 2023, 25, 472–484.
- Shah, K.; Al-Haidari, A.; Sun, J.; Kazi, J.U. T cell receptor (TCR) signaling in health and disease. Signal Transduct. Target. Ther. 2021, 6, 412.




