
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carlson John | -- | 5062 | 2024-02-18 17:48:33 | | | |
| 2 | Peter Tang | Meta information modification | 5062 | 2024-02-19 03:03:06 | | |
Video Upload Options
Quercus alba L., also known as white oak, eastern white oak, or American white oak, is a quintessential North American species within the white oak section (Quercus) of the genus Quercus, subgenus Quercus. This species plays a vital role in local and regional economies and as a keystone species in eastern North American forests. As a long-lived woody perennial covering an extensive natural range, Q. alba’s biology is shaped by a myriad of adaptations accumulated throughout its natural history. Populations of Q. alba are crucial repositories of genetic, genomic, and evolutionary insights, capturing the essence of successful historical adaptations and ongoing responses to contemporary environmental challenges in the Anthropocene. This intersection offers an exceptional opportunity to integrate genomic knowledge with the discovery of climate-relevant traits, advancing tree improvement, forest ecology, and forest management strategies.
1. Introduction
1.1. Quercus alba L.
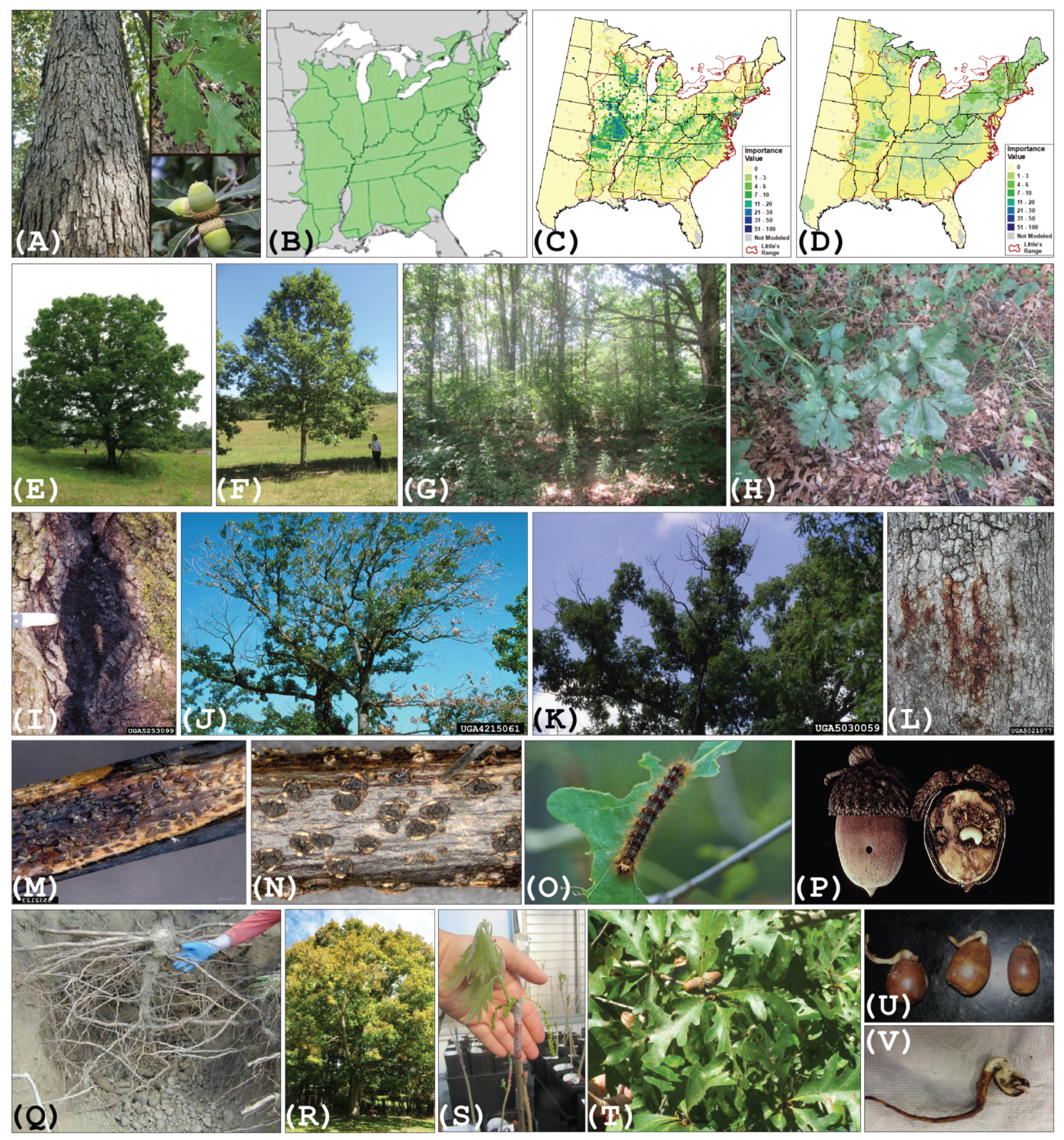
1.2. Challenges for Oaks in the Anthropocene
2. Climate Change and Quercus alba Biology: Direct Impacts
2.1. Water Relations: Control of Root Growth in Oaks
2.2. Reproduction
2.3. Pollination
2.4. Flowering
2.5. Masting
2.6. Seed Germination
2.7. Seedling Growth Control
3. Climate Change and Quercus alba Biology: Indirect Impacts
|
Disease |
Pathogen/Pest |
Relevant Classification |
Key Features |
Q. alba Resistance? |
References |
|---|---|---|---|---|---|
|
Oak wilt |
Bretziella fagacearum (Bretz) (Microascales: Certocystidaceae) (formerly Ceratocystis fagacearum) |
Ascomycete fungus, Necrotrophic |
Vascular wilt, vectored through root grafts and sap-feeding beetles Scolytidae and Nitidulidae. |
Somewhat resistant, exhibiting slower fungal growth. |
[123][124][125][126][127][128][129] [130][131][132][133][134][135] [136][137][138][139] |
|
Oak decline |
Agrilus bilineatus Weber and Armillaria mellea [Vahl. Ex Fr.] and Phytophthora cinnamomi |
Coleptylebran beetle and oomycete hemibiotroph interaction. |
Caused by the interaction between severely stressed trees, secondary pests, such as the two-lined chestnut borer, and root diseases like armillaria root rot and ink disease. |
Less susceptible than other North American oak species and less severe in young (less than 70 years) and heterogenous stands. |
|
|
Hypoxylon cankers |
Hypoxylon atropunctatum (Schw. ex Fr.) Cke |
Ascomycete fungus, Necrotrophic |
Less pathogenic fungal species that frequently accompanies dieback. |
Live healthy unstressed trees less susceptible. |
[144] |
|
Root and crown rot |
Phytophthora cinnamomi |
Oomycete hemibiotrophic |
Extirpated American chestnut and a component of oak decline. |
White oaks less effected by this pathogen? |
|
|
Anthracnose |
Dendrostoma leiphaemia Senan. and K.D. Hyde (formerly Discula quercina (Westend.) Arx Anamorph of Ascomycete Apiognomonia quercina. |
Ascomycete fungus, hemibiotrophic |
One of the most damaging leaf and twig diseases, impacting reproduction and masting; widespread across North America. |
Leaves are less susceptible as they age due to thicker protective cuticle; large range of the pathogen across a variety of climates suggests adaptation to distinct climates |
|
|
Twig, branch and rots cankers |
Botryosphaeria spp. (including B. rhodina [Berk. and Curt.] von Arx, B. dothidea [Moug. ex Fr.] Ces. and de Not., B. obtusa [Schw.] Shoemaker, and B. quercum [Sch.: Fr.] Saccardo) and Botryodiplodia gallae (Schw.) Petrak and Sydow |
Ascomycete fungus, necrotrophic/hemi-biotrophic |
Can play role in oak decline syndrome. |
Most susceptible under drought or cold stress. |
[148] |
|
Spongy moth (formally gypsy moth) |
Lymantia dispar |
Lepidopteran insect |
A significant insect pest of Q. alba forests, usually in low numbers but occasionally surging to severe outbreaks. |
Q. alba is preferred over the hundreds of tree species spongy moth caterpillars feed on. |
|
|
Acorn weevil |
Curculio and Conotrachelus spp. |
Coleopteran insect |
The major oak seed predator and a factor in the reduced regeneration in the eastern United States. |
Unknown. |
References
- Hacket-Pain, A.; Bogdziewicz, M. Climate Change and Plant Reproduction: Trends and Drivers of Mast Seeding Change. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20200379.
- Fralish, J.S. The Keystone Role of Oak and Hickory in the Central Hardwood Forest; Gen. Tech. Rep. SRS-73; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2004; pp. 78–87.
- Binder, S.; Haight, R.G.; Polasky, S.; Warziniack, T.; Mockrin, M.H.; Deal, R.L.; Arthaud, G. Assessment and Valuation of Forest Ecosystem Services: State of the Science Review; Gen. Tech. Rep. NRS-170; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2017; pp. 1–47.
- Grattapaglia, D.; Plomion, C.; Kirst, M.; Sederoff, R.R. Genomics of Growth Traits in Forest Trees. Curr. Opin. Plant Biol. 2009, 12, 148–156.
- Saleh, D.; Chen, J.; Leplé, J.-C.; Leroy, T.; Truffaut, L.; Dencausse, B.; Lalanne, C.; Labadie, K.; Lesur, I.; Bert, D.; et al. Genome-Wide Evolutionary Response of European Oaks during the Anthropocene. Evol. Lett. 2022, 6, 4–20.
- Gollihue, J.; Pook, V.G.; DeBolt, S. Sources of Variation in Bourbon Whiskey Barrels: A Review. J. Inst. Brew. 2021, 127, 210–223.
- Mosedale, J.R.; Feuillat, F.; Baumes, R.; Dupouey, J.-L.; Puech, J.-L. Variability of Wood Extractives among Quercus robur and Quercus petraea Trees from Mixed Stands and Their Relation to Wood Anatomy and Leaf Morphology. Can. J. For. Res. 1998, 28, 994–1006.
- Abrams, M.D. Where Has All the White Oak Gone? BioScience 2003, 53, 927.
- Steiner, K. Genetic Improvement of Oaks in North America. Ann. Des Sci. For. 1993, 50 (Suppl. S1), 359s–367s.
- Stringer, J.; Morris, D. Landowners Guide to: Understanding the Importance of White Oak; FOR-147; Cooperative Extension Service, University of Kentucky, Department of Forestry and Natural Resources: Lexington, KY, USA, 2022; p. 3.
- Abrams, M.D. History of eastern oak forests. In Managing Oak Forests in the Eastern United States; Keyser, P.D., Fearer, T., Harper, C.A., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 7–17.
- Abrams, M.D.; Nowacki, G.J.; Hanberry, B.B. Oak forests and woodlands as Indigenous landscapes in the Eastern United States. J. Torrey Bot. Soc. 2021, 149, 101–121.
- Whitney, G.G. From Coastal Wilderness to Fruited Plain: A History of Environmental Change in Temperate North America from 1500 to the Present; Cambridge University Press: Cambridge, UK, 1996.
- Bogdziewicz, M.; Hacket-Pain, A.; Kelly, D.; Thomas, P.A.; Lageard, J.; Tanentzap, A.J. Climate warming causes mast seeding to break down by reducing sensitivity to weather cues. Glob. Change Biol. 2021, 27, 1952–1961.
- Pesendorfer, M.B.; Ascoli, D.; Bogdziewicz, M.; Hacket-Pain, A.; Pearse, I.S.; Vacchiano, G. The ecology and evolution of synchronized reproduction in long-lived plants. Philos. Trans. R. Soc. B 2021, 376, 20200369.
- Bogdziewicz, M.; Kelly, D.; Thomas, P.A.; Lageard, J.G.A.; Hacket-Pain, A. Climate Warming Disrupts Mast Seeding and Its Fitness Benefits in European Beech. Nat. Plants 2020, 6, 88–94.
- Nussbaumer, A.; Waldner, P.; Apuhtin, V.; Aytar, F.; Benham, S.; Bussotti, F.; Eichhorn, J.; Eickenscheidt, N.; Fabianek, P.; Falkenried, L.; et al. Impact of Weather Cues and Resource Dynamics on Mast Occurrence in the Main Forest Tree Species in Europe. For. Ecol. Manag. 2018, 429, 336–350.
- Fraga, H.; Moriondo, M.; Leolini, L.; Santos, J.A. Mediterranean Olive Orchards under Climate Change: A Review of Future Impacts and Adaptation Strategies. Agronomy 2021, 11, 56.
- Leisner, C.P. Review: Climate Change Impacts on Food Security-Focus on Perennial Cropping Systems and Nutritional Value. Plant Sci. 2020, 293, 110412.
- Tuskan, G.A.; Groover, A.T.; Schmutz, J.; DiFazio, S.P.; Myburg, A.; Grattapaglia, D.; Smart, L.B.; Yin, T.; Aury, J.M.; Kremer, A.; et al. Hardwood Tree Genomics: Unlocking Woody Plant Biology. Front. Plant Sci. 2018, 9, 1799.
- Abbott, A.G.; Georgi, L.L.; Yvergniaux, D.; Wang, Y.; Blenda, A.V.; Reighard, G.L.; Martinez Inigo, M.J.; Sosinski, B. Peach: The Model Genome for Rosaceae. Acta Hortic. 2002, 575, 145–155.
- Jansson, S.; Douglas, C.J. Populus: A Model System for Plant Biology. Annu. Rev. Plant Biol. 2007, 58, 435–458.
- Jansson, S.; Bhalerao, R.; Groover, A. Springerlink, Online Service. In Genetics and Genomics of Populus; Springer: New York, NY, USA, 2010; Volume 8.
- Arús, P.; Verde, I.; Sosinski, B.; Zhebentyayeva, T.; Abbott, A.G. The Peach Genome. Tree Genet. Genomes 2012, 8, 531–547.
- Friedman, J.; Rubin, M.J. All in good time: Understanding annual and perennial strategies in plants. Am. J. Bot. 2015, 102, 497–499.
- Hatfield, J.L.; Dold, C. Water-Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 103.
- Soh, W.K.; Yiotis, C.; Murray, M.; Parnell, A.; Wright, I.J.; Spicer, R.A.; Lawson, T.; Caballero, R.; McElwain, J.C. Rising CO2 drives divergence in water use efficiency of evergreen and deciduous plants. Sci. Adv. 2019, 5, 7906.
- Durand, M.; Brendel, O.; Buré, C.; Le Thiec, D. Altered Stomatal Dynamics Induced by Changes in Irradiance and Vapour-Pressure Deficit under Drought: Impacts on the Whole-Plant Transpiration Efficiency of Poplar Genotypes. New Phytol. 2019, 222, 1789–1802.
- Torres-Ruiz, J.M.; Kremer, A.; Carins Murphy, M.R.; Brodribb, T.; Lamarque, L.J.; Truffaut, L.; Bonne, F.; Ducousso, A.; Delzon, S. Genetic Differentiation in Functional Traits among European Sessile Oak Populations. Tree Physiol. 2019, 39, 1736–1749.
- Homolka, A.; Schueler, S.; Burg, K.; Fluch, S.; Kremer, A. Insights into Drought Adaptation of Two European Oak Species Revealed by Nucleotide Diversity of Candidate Genes. Tree Genet. Genomes 2013, 9, 1179–1192.
- Arend, M.; Kuster, T.; Gunthardt-Goerg, M.S.; Dobbertin, M. Provenance-Specific Growth Responses to Drought and Air Warming in Three European Oak Species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiol. 2011, 31, 287–297.
- Spieß, N.; Oufir, M.; Matušíková, I.; Stierschneider, M.; Kopecky, D.; Homolka, A.; Burg, K.; Fluch, S.; Hausman, J.-F.; Wilhelm, E. Ecophysiological and Transcriptomic Responses of Oak (Quercus Robur) to Long-Term Drought Exposure and Rewatering. Environ. Exp. Bot. 2012, 77, 117–126.
- Magalhães, A.P.; Verde, N.; Reis, F.; Martins, I.; Costa, D.; Lino-Neto, T.; Castro, P.H.; Tavares, R.M.; Azevedo, H. RNA-Seq and Gene Network Analysis Uncover Activation of an ABA-Dependent Signalosome during the Cork Oak Root Response to Drought. Front. Plant Sci. 2016, 6, 1195.
- Reed, W.P.; Kaye, M.W. Bedrock Type Drives Forest Carbon Storage and Uptake across the Mid-Atlantic Appalachian Ridge and Valley, U.S.A. For. Ecol. Manag. 2020, 460, 117881.
- Tirmenstein, D.A. Quercus alba. In Fire Effects Information System, ; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer): Fort Collins, CO, USA, 1991. Available online: https://www.fs.usda.gov/database/feis/plants/tree/quealb/all.html (accessed on 30 November 2023).
- Abrams, M.D. Adaptations and Responses to Drought in Quercus Species of North America. Tree Physiol. 1990, 7, 227–238.
- Abrams, M. Distribution, Historical Development and Ecophysiological Attributes of Oak Species in the Eastern United States. Ann. Sci. For. 1996, 53, 487–512.
- De Micco, V.; Balzano, A.; Wheeler, E.A.; Baas, P. Tyloses and gums: A review of structure, function and occurrence of vessel occlusions. IAWA J. 2016, 37, 186–205.
- Allen, M. Managing Oak Woodlands in a Dynamic World. In Proceedings of the 7th California Oak Symposium, Visalia, CA, USA, 3–6 November 2014; General Technical Report PSW-GTR-251 FS. U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Berkeley, CA, USA, 2015.
- Cavender-Bares, J. Diversification, adaptation, and community assembly of the American oaks (Quercus), a model clade for integrating ecology and evolution. New Phytol. 2019, 221, 669–692.
- Rogers, R. Quercus alba L. White oak. In Silvics of North America: Hardwoods; Burns, R.M., Ed.; USDA Forest Service: Washington, DC, USA, 1990; pp. 605–613.
- Kaproth, M.A.; Hahn, M.; Manos, P.S.; Hipp, L.; González-Rodríguez, A.; Cavender-Bares, J. Functional Leaf and Stem Traits of the Oaks of the Americas. Retrieved from the Data Repository for the University of Minnesota. 2020. Available online: https://hdl.handle.net/11299/214055 (accessed on 29 November 2023).
- Kościelniak, P.; Glazińska, P.; Kȩsy, J.; Zadworny, M. Formation and Development of Taproots in Deciduous Tree Species. Front. Plant Sci. 2021, 12, 772567.
- Bräutigam, K.; Vining, K.J.; Lafon-Placette, C.; Fossdal, C.G.; Mirouze, M.; Marcos, J.G.; Fluch, S.; Fraga, M.F.; Guevara, M.Á.; Abarca, D.; et al. Epigenetic Regulation of Adaptive Responses of Forest Tree Species to the Environment. Ecol. Evol. 2013, 3, 399–415.
- Raj, S.; Bräutigam, K.; Hamanishi, E.T.; Wilkins, O.; Thomas, B.R.; Schroeder, W.W.; Mansfield, S.D.; Plant, A.L.; Campbell, M.M. Clone History Shapes Populus Drought Responses. Proc. Natl. Acad. Sci. USA 2011, 108, 12521–12526.
- Caignard, T.; Kremer, A.; Firmat, C.; Nicolas, M.; Venner, S.; Delzon, S. Increasing Spring Temperatures Favor Oak Seed Production in Temperate Areas. Sci. Rep. 2017, 7, 8555.
- Petritan, A.M.; Petritan, I.C.; Hevia, A.; Walentowski, H.; Bouriaud, O.; Sánchez-Salguero, R. Climate Warming Predispose Sessile Oak Forests to Drought-Induced Tree Mortality regardless of Management Legacies. For. Ecol. Manag. 2021, 491, 119097.
- Thomas, A.M.; Coggeshall, M.V.; O’Connor, P.A.; Nelson, D.C. Climate Adaptation in White Oak (Quercus alba, L.): A Forty-Year Study of Growth and Phenology. Forests 2024. submitted.
- Ashley, M.V. Answers Blowing in the Wind: A Quarter Century of Genetic Studies of Pollination in Oaks. Forests 2021, 12, 575.
- Dow, B.D.; Ashley, M.V. Microsatellite Analysis of Seed Dispersal and Parentage of Saplings in Bur Oak, Quercus Macrocarpa. Mol. Ecol. 1996, 5, 615–627.
- Dow, B.D. High Levels of Gene Flow in Bur Oak Revealed by Paternity Analysis Using Microsatellites. J. Hered. 1998, 89, 62–70.
- Dow, B.D.; Ashley, M.V. Factors influencing male mating success in bur oak, Quercus macrocarpa. New For. 1998, 15, 161–180.
- Streiff, R.; Ducousso, A.; Lexer, C.; Steinkellner, H.; Gloessl, J.; Kremer, A. Pollen dispersal inferred from paternity analysis in a mixed oak stand of Quercus robur L. and Q. petraea (Matt.) Liebl. Mol. Ecol. 1999, 8, 831–841.
- Valbuena-Carabãa, M.; González-Martínez, S.C.; Sork, V.L.; Collada, C.; Soto, A.; Goicoechea, P.G.; Gil, L. Gene flow and hybridisation in a mixed oak forest (Quercus pyrenaica Willd. and Quercus petraea (Matts.) Liebl.) in central Spain. Heredity 2005, 95, 457–465.
- Craft, K.J.; Ashley, M.V. Landscape genetic structure of bur oak (Quercus macrocarpa) savannas in Illinois. For. Ecol. Manag. 2007, 239, 13–20.
- Craft, K.J.; Ashley, M.V. Pollen-mediated gene flow in isolated and continuous stands of bur oak, Quercus macrocarpa (Fagaceae). Am. J. Bot. 2010, 97, 1999–2006.
- Nakanishi, A.; Tomaru, N.; Yoshimaru, H.; Kawahara, T.; Manabe, T.; Yamamoto, S. Patterns of pollen flow and genetic differentiation among pollen pools in Quercus salicina in a warm temperate old-growth evergreen broad-leaved forest. Silvae Genet. 2004, 53, 258–264.
- Nakanishi, A.; Tomaru, N.; Yoshimaru, H.; Manabe, T.; Yamamoto, S. Effects of seed- and pollen-mediated gene dispersal on genetic structure among Quercus salicina saplings. Heredity 2009, 102, 182–189.
- Abraham, S.T.; Zaya, D.N.; Koenig, W.D.; Ashley, M.V. Interspecific and intraspecific pollination patterns of valley oak, Quercus lobata, in a mixed stand in Coastal Central California. Int. J. Plant Sci. 2011, 172, 691–699.
- Hampe, A.; Pemonge, M.-H.; Petit, R.J. Efficient mitigation of founder effects during the establishment of a leading-edge oak population. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131070.
- Gerber, S.; Chadoeuf, J.; Gugerli, F.; Lascoux, M.; Buiteveld, J.; Cottrell, J.; Dounavi, A.; Fineschi, S.; Forrest, L.L.; Fogelqvist, J.; et al. High rates of gene flow by pollen and seed in oak populations across Europe. PLoS ONE 2014, 9, e85130.
- Yang, Q.; Gao, Y.; Wu, X.; Moriguchi, T.; Bai, S.; Teng, Y. Bud endodormancy in deciduous fruit trees: Advances and prospects. Hortic. Res. 2021, 8, 139.
- Abbott, A.G.; Zhebentyayeva, T.; Barakat, A.; Liu, Z. The genetic control of bud-break in trees. In Advances in Botanical Research; Plomion, C., Blondon, A.-F., Eds.; Academic Press: New York, NY, USA, 2015; pp. 201–228.
- Böhlenius, H.; Huang, T.; Charbonnel-Campaa, L.; Brunner, A.M.; Jansson, S.; Strauss, S.H.; Nilsson, O. CO/FT Regulatory Module Controls Timing of Flowering and Seasonal Growth Cessation in Trees. Science 2006, 312, 1040–1043.
- Singh, R.K.; Maurya, J.P.; Azeez, A.; Miskolczi, P.; Tylewicz, S.; Stojkovič, K.; Delhomme, N.; Busov, V.; Bhalerao, R.P. A genetic network mediating the control of bud break in hybrid aspen. Nat. Commun. 2018, 9, 4173.
- Azeez, A.; Zhao, Y.C.; Singh, R.K.; Yordanov, Y.S.; Dash, M.; Miskolczi, P.; Stojkovič, K.; Strauss, S.H.; Bhalerao, R.P.; Busov, V.B. EARLY BUD-BREAK 1 and EARLY BUD-BREAK 3 control resumption of poplar growth after winter dormancy. Nat. Commun. 2021, 12, 1123.
- Lesur, I.; Le Provost, G.; Bento, P.; Da Silva, C.; Leplé, J.-C.; Murat, F.; Ueno, S.; Bartholomé, J.; Lalanne, C.; Ehrenmann, F.; et al. The oak gene expression atlas: Insights into Fagaceae genome evolution and the discovery of genes regulated during bud dormancy release. BMC Genom. 2015, 16, 112.
- Satake, A.; Kelly, D. Supplementary material from “Studying the genetic basis of masting”. R. Soc. Collect. 2021, 376, 20210116.
- Schermer, É.; Bel-Venner, M.C.; Fouchet, D.; Siberchicot, A.; Boulanger, V.; Caignard, T.; Thibaudon, M.; Oliver, G.; Nicolas, M.; Gaillard, J.M.; et al. Pollen limitation as a main driver of fruiting dynamics in oak populations. Ecol. Lett. 2019, 22, 98–107.
- Yu, J.; Conrad, A.O.; Decroocq, V.; Zhebentyayeva, T.; Williams, D.E.; Bennett, D.; Roch, G.; Audergon, J.-M.; Dardick, C.; Liu, Z.; et al. Distinctive Gene Expression Patterns Define Endodormancy to Ecodormancy Transition in Apricot and Peach. Front. Plant Sci. 2020, 11, 180.
- Kobayashi, M.J.; Takeuchi, Y.; Kenta, T.; Kume, T.; Diway, B.; Shimizu, K.K. Mass flowering of the tropical tree Shorea beccariana was preceded by expression changes in flowering and drought responsive genes. Mol. Ecol. 2013, 22, 4767–4782.
- Hacket-Pain, A.J.; Friend, A.D.; Lageard, J.G.A.; Thomas, P.A. The influence of masting phenomenon on growth–climate relationships in trees: Explaining the influence of previous summers’ climate on ring width. Tree Physiol. 2015, 35, 319–330.
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent Advances in Carbon and Nitrogen Metabolism in C3 Plants. Int. J. Mol. Sci. 2021, 22, 318.
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53.
- Kurpisz, B.; Pawłowski, T.A. Epigenetic Mechanisms of Tree Responses to Climatic Changes. Int. J. Mol. Sci. 2022, 23, 13412.
- Korstian, C.F. Factors Controlling Germination and Early Survival in Oaks; Yale University School of Forestry Bulletin No. 19; Yale University Press: New Haven, CT, USA, 1927; 115p.
- Olsen, D.F.; Boyce, S.G. Factors Affecting Acorn Production and Germination and Early Growth of Seedlings and Seedling Sprouts. In Proceedings of the Oak Symposium Proceedings, Morgantown, WV, USA, 16–20 August 1971; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Upper Darby, PA, USA, 1971; pp. 44–48.
- Hawkins, T.S. The Influence of Dormancy Break Requirements on Germination and Viability Responses to Winter Submergence in Acorns of Three Bottomland Red Oak (Sect. Lobatae) Species. For. Sci. 2019, 65, 556–561.
- Carrera-Castaño, G.; Calleja-Cabrera, J.; Pernas, M.; Gómez, L.; Oñate-Sánchez, L. An Updated Overview on the Regulation of Seed Germination. Plants 2020, 9, 703.
- Sung, S.-J.S.; Kormanik, P.P.; Cook, C.D.; Zarnoch, S.J.; Kormanik, T.L. Effect of Acorn Moisture Content at Sowing on Germination and Seedling Growth of White Oak and Northern Red Oak. In Proceedings of the 13th Biennial Southern Silvicultural Research Conference, Memphis, TN, USA, 28 February–4 March 2005; Kristina, F.C., Ed.; Gen. Tech. Rep. SRS-92. U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2006; pp. 241–246.
- Steele, M.A.; Smallwood, P.; Terzaghi, W.B.; Carlson, J.E.; Contreras, T.; McEuen, A. Oak dispersal syndromes: Do red and white oaks exhibit different dispersal strategies? In Upland Oak Ecology Symposium; Gen. Tech. Rep. SRS–73; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2002; pp. 72–77.
- Liu, Y.; Liu, G.; Li, Q.; Liu, Y.; Hou, L.; Li, G.L. Influence of pericarp, cotyledon and inhibitory substances on sharp tooth oak (Quercus aliena var. acuteserrata) germination. PLoS ONE 2012, 7, 47682.
- Yi, X.; Yang, Y.; Curtis, R.; Bartlow, A.W.; Agosta, S.J.; Steele, M.A. Alternative strategies of seed predator escape by early-germinating oaks in Asia and North America. Ecol. Evol. 2012, 2, 487–492.
- Bonner, F.T.; Vozzo, J.A. Seed Biology and Technology of Quercus; Gen. Tech. Rep. SO-66; U.S. Department of Agriculture, Forest Service, Southern Forest Experiment Station: New Orleans, LA, USA, 1987.
- Steele, M.A. Oak Seed Dispersal: A Study in Plant-Animal Interactions; The Johns Hopkins University Press: Baltimore, MD, USA, 2021.
- Zhou, B.F.; Yuan, S.; Crowl, A.A.; Liang, Y.Y.; Shi, Y.; Chen, X.Y.; An, Q.Q.; Kang, M.; Manos, P.S.; Wang, B. Phylogenomic analyses highlight innovation and introgression in the continental radiations of Fagaceae across the Northern Hemisphere. Nat. Commun. 2022, 13, 1320.
- Moles, A.T.; Westoby, M. Seedling survival and seed size: A synthesis of the literature. J. Ecol. 2004, 92, 372–383.
- Long, T.; Jones, R. Seedling growth strategies and seed size effects in fourteen oak species native to different soil moisture habitats. Trees 1996, 11, 1–8.
- Llanderal-Mendoza, J.; Gugger, P.F.; Oyama, K.; Uribe-Salas, D.; Gonzales-Rodriguez, A. Climatic determinants of acorn size and germination percentage of Quercus rugosa (Fagaceae) along a latitudinal gradient in Mexico. Bot. Sci. 2017, 95, 37–45.
- Sánchez-Montes de Oca, E.J.; Badano, E.I.; Silva-Alvarado, L.-E.; Flores, J.; Barragán-Torres, F.; Flores-Cano, J.A. Acorn weight as determinant of germination in red and white oaks: Evidences from a common-garden greenhouse experiment. Ann. For. Sci. 2018, 75, 12.
- Gavranović Markić, A.; Bogdan, S.; Gradečki Poštenjak, M.; Lanšćak, M.; Vujnović, Z.; Bogunović, S.; Ivanković, M. Acorn Yields and Seed Viability of Pedunculate Oak in a 10-year Period in Forest Seed Objects across Croatia. South-East Eur. For. 2022, 13, 27–36.
- Johnson, P.S.; Shifley, S.R.; Rogers, R.; Dey, D.C.; Kabrick, J.M. The Ecology and Silviculture of Oaks; CABI: Wallingford, UK, 2019.
- Loftis, D.L. Upland oak regeneration and management. In Upland Oak Ecology Symposium: History, Current Conditions, and Sustainability; Spetich, M.A., Ed.; USDA Forest Service Gen. Tech. Rep. SRS-73; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2004; pp. 163–167.
- Brose, P.H.; Van Lear, D.H. Survival of hardwood regeneration during prescribed fires: The importance of root development and root collar location. In Upland Oak Ecology Symposium: History, Current Conditions, and Sustainability; Spetich, M.A., Ed.; SRS-73, Gen. Tech. Rep; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2004; pp. 123–127.
- Kormanik, P.P.; Sung, S.-J.; Zarnoch, S.J.; Tibbs, G.T. Artificial regeneration of northern red oak and white oak on high-quality sites: Effect of root morphology and relevant biological characteristics. In Proceedings of the 2001 National Silviculture Workshop; Parker, S., Hummel, S.S., Eds.; Gen. Tech. Rep. PNW-GTR-546. USDA Forest Service Pacific Northwest Research Station: Portland, OR, USA, 2002; pp. 83–91.
- Kormanik, P.P.; Sung, S.-J.; Kormanik, T.L. Growing, selecting, and establishing 1-0 Quercus rubra and Q. alba seedlings for rapid growth and early acorn production on forested lands in the southeastern United States. J. Int. Oak Soc. 2004, 15, 119–125.
- Sung, S.-J.; Kormanik, P.P.; Zarnoch, S.J. Growth and development of first-year nursery-grown white oak seedlings of individual mother trees. In Proceedings of the Eleventh Biennial Southern Silvicultural Research Conference, Knoxville, TN, USA, 20–22 March 2022; Outcalt, K.W., Ed.; Gen. Tech. Rep., SRS-48. USDA Forest Service, Southern Research Station: Asheville, NC, USA, 2002; Volume 622, pp. 346–351.
- Greenberg, C.H.; Parresol, B.R. Dynamics of acorn production by five species of southern Appalachian oaks. In Oak Forest Ecosystems: Ecology and Management for Wildlife; McShea, W.J., Healy, W.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2002; pp. 149–172.
- Struve, D.K. Seed Conditioning of Red Oak: A Recalcitrant North American Seed. Sci. Agric. Piracicaba 1998, 55, 67–73.
- Sturrock, R. Climate Change and Forest Diseases: Using Today’s Knowledge to Address Future Challenges. For. Syst. 2012, 21, 329–336.
- Tiberi, R.; Branco, M.; Bracalini, M.; Croci, F.; Panzavolta, T. Cork Oak Pests: A Review of Insect Damage and Management. Ann. For. Sci. 2016, 73, 219–232.
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of Forest Insect Pests to Climate Change: Not so Simple. Curr. Opin. Insect Sci. 2019, 35, 103–108.
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent Insects, Pathogens and Drought Shape Changing Patterns in Oak Decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205.
- Loo, J.A. Ecological Impacts of Non-Indigenous Invasive Fungi as Forest Pathogens. Biol. Invasions 2008, 11, 81–96.
- Anagnostakis, S.L. The Effect of Multiple Importations of Pests and Pathogens on a Native Tree. Biol. Invasions 2001, 3, 245–254.
- Sena, K.; Crocker, E.; Vincelli, P.; Barton, C. Phytophthora cinnamomi as a Driver of Forest Change: Implications for Conservation and Management. For. Ecol. Manag. 2018, 409, 799–807.
- Hardham, A.R.; Blackman, L.M. Phytophthora cinnamomi . Mol. Plant Pathol. 2018, 19, 260–285.
- Garbelotto, M.; Hüberli, D.; Shaw, D. First Report on an Infestation of Phytophthora cinnamomi in Natural Oak Woodlands of California and Its Differential Impact on Two Native Oak Species. Plant Dis. 2006, 90, 685.
- McConnell, M.E.; Balci, Y. Phytophthora cinnamomias a Contributor to White Oak Decline in Mid-Atlantic United States Forests. Plant Dis. 2014, 98, 319–327.
- Moricca, S.; Linaldeddu, B.T.; Ginetti, B.; Scanu, B.; Franceschini, A.; Ragazzi, A. Endemic and Emerging Pathogens Threatening Cork Oak Trees: Management Options for Conserving a Unique Forest Ecosystem. Plant Dis. 2016, 100, 2184–2193.
- Garbelotto, M.; Hayden, K.J. Sudden Oak Death: Interactions of the Exotic Oomycete Phytophthora ramorum with Naïve North American Hosts. Eukaryot. Cell 2012, 11, 1313–1323.
- DiLeo, M.V.; Bostock, R.M.; Rizzo, D.M. Microclimate Impacts Survival and Prevalence of Phytophthora ramorum in Umbellularia Californica, a Key Reservoir Host of Sudden Oak Death in Northern California Forests. PLoS ONE 2014, 9, e98195.
- Kozanitas, M.; Metz, M.R.; Osmundson, T.W.; Serrano, M.S.; Garbelotto, M. The Epidemiology of Sudden Oak Death Disease Caused by Phytophthora ramorum in a Mixed Bay Laurel-Oak Woodland Provides Important Clues for Disease Management. Pathogens 2022, 11, 250.
- Banerjee, S. An Oak (Quercus Robur L.) Canker Caused by Stereum Rugosum (Pers.) Fr. Trans. Br. Mycol. Soc. 1956, 39, 267-IN5.
- Davidson, R.W.; Campbell, W.A.; Vaughn, D.B. Fungi Causing Decay of Living Oaks in the Eastern United States and Their CuItural Identification; Technical Bulletin No. 785; US Department of Agriculture: Washington, DC, USA, 1942.
- Fergus, C.L. Relation of weather to the severity of white oak anthracnose. Phytopathology 1953, 43, 103–105.
- Neely, D.; Himelick, E.B. Characteristics and Nomenclature of the Oak Anthracnose Fungus. Phytopathology 1967, 57, 1230–1236.
- Goode, M.J. Control of oak leaf-blister in Mississippi. Phytopathology 1953, 43, 472.
- Reed, S.E.; EngLish, J.T.; Muzika, R.M. Investigation of Rapid White Oak (Quercus alba) Mortality within the Ozark Plateau and Adjacent Forest-Prairie Transition Ecoregion. In Forest Health Monitoring: National Status, Trends, and Analysis; Gen. Tech. Rep.; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2015; p. 127.
- Gibbs, J.N. Oak Wilt. Arboric. J. 1978, 3, 351–356.
- Oak, S.W.; Spetich, M.A.; Morin, R.S. Oak decline in central hardwood forests: Frequency, spatial extent, and scale. In Natural Disturbances and Historic Range of Variation: Type, Frequency, Severity, and Post-Disturbance Structure in Central Hardwood Forests USA; Greenberg, C., Collins, B., Eds.; Springer: Cham, Switzerland, 2015; Volume 32, pp. 49–71.
- Serrano, M.S.; Romero, M.Á.; Homet, P.; Gómez-Aparicio, L. Climate Change Impact on the Population Dynamics of Exotic Pathogens: The Case of the Worldwide Pathogen Phytophthora Cinnamomi. Agric. For. Meteorol. 2022, 322, 109002.
- De Beer, Z.W.; Marincowitz, S.; Duong, T.A.; Wingfield, M.J. Bretziella, a New Genus to Accommodate the Oak Wilt Fungus, Ceratocystis Fagacearum (Microascales, Ascomycota). MycoKeys 2017, 27, 1–19.
- Henry, B.W. Oak Wilt: Its Significance, Symptoms and Cause. Phytopathology 1944, 34, 636–647.
- Gibbs, J.N. The Transmission of Oak Wilt; North Central Forest Experiment Station, Forest Service, U.S. Department of Agriculture: St. Paul, MN, USA, 1980.
- True, R.P. Oak Wilt in West Virginia. Oak Wilt West Virginia, 448t. 1960. Available online: https://researchrepository.wvu.edu/wv_agricultural_and_forestry_experiment_station_bulletins/649 (accessed on 28 November 2023).
- Bretz, T.W. Plant Diseases: The Yearbook of Agriculture: Oak Wilt, a New Threat; USDA: Washington, DC, USA, 1953.
- Juzwik, J.; Harrington, T.C.; MacDonald, W.L.; Appel, D.N. The Origin of Ceratocystis Fagacearum, the Oak Wilt Fungus. Annu. Rev. Phytopathol. 2008, 46, 13–26.
- Cervenka, V.J.; Skalbeck, T.C.; Kyhl, J.F.; Blackford, D.C.; Juzwik, J.; Seybold, S.J. How to Identify Common Nitidulid Beetles Associated with Oak Wilt Mats in Minnesota; USDA Forest Service, North Central Research Station: St. Paul, MN, USA, 2001; Volume 71.
- Epstein, A.H. Root graft transmission of tree pathogens. Annu. Rev. Phytopathol. 1978, 16, 181–192.
- Oak, S.W. Native Diseases and Insects That Impact Oaks. In Oak Forest Ecosystems: Ecology and Management for Wildlife; Johns Hopkins University Press: Baltimore, MD, USA, 2002; pp. 80–99.
- Pokorny, J. How to collect field samples and identify the oak wilt fungus in the laboratory. In Proceedings of the Shade Tree Wilt Diseases: A National Conference, St. Paul, MN, USA, 25–28 August 1999; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2001; p. 139.
- O’Brien, J.G.; Mielke, M.E.; Starkey, D.; Juzwik, J. How to Prevent and Control Oak Wilt; USDA Forest Service, State and Private Forestry: St. Paul, MN, USA, 2000.
- Young, R.A. Studies in oak wilt caused by Chalara quercina. Phytopathology 1949, 38, 425–441.
- Anderson, R.L.; Skilling, D.D. Oak Wilt Damage: A Survey in Central Wisconsin; Station Paper; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1955; p. 11.
- Liese, W.; Ruetze, M. Development of a Fumigation Treatment of Oak Logs against Ceratocystis Fagacearum. EPPO Bull. 1985, 15, 29–36.
- MacDonald, W.L.; Schmidt, E.L.; Harner, E.J. Methyl Bromide Eradication of the Oak Wilt Fungus from Red and White Oak Logs. For. Prod. J. 1985, 35, 11–16.
- Schmidt, E.L.; Ruetze, M.M.; French, D.W. Methyl bromide treatment of oak wilt infected logs: Laboratory and preliminary field fumigations . For. Prod. J. 1982, 32, 46–49.
- Yang, A.; Seabright, K.; Juzwik, J.; Myers, S.W.; Taylor, A. Survival of the Oak Wilt Fungus in Logs Fumigated with Sulfuryl Fluoride and Methyl Bromide. For. Prod. J. 2019, 69, 87–95.
- Manion, P.D. Tree Disease Concepts; Prentice-Hall, Inc.: Hoboken, NJ, USA, 1981; p. 399.
- Lawrence, R.; Moltzan, B.; Moser, W.K. Oak decline and the future of Missouri’s forests. Mo. Conserv. 2002, 63, 11–18.
- Wargo, P.M.; Houston, D.R. Infection of defoliated sugar maple trees by Armillaria mellea. Phytopathology 1974, 64, 817–822.
- Wargo, P.M.; Houston, D.R.; LaMadeleine, L.S. Oak Decline; Forest Insect and Disease Leaflet 165; U.S. Department of Agriculture Forest Service: Washington, DC, USA, 1983; 8p.
- Starkey, D.A.; Oliveria, F.; Mangini, A.; Mielke, M. Oak Decline and Red Oak Borer in the Interior Highlands of Arkansas and Missouri: Natural Phenomena, Severe Occurrences; Gen. Tech. Rep. SRS-73; U.S. Department of Agriculture, Forest Service, Southern Research Station: Asheville, NC, USA, 2004; pp. 217–222.
- Wargo, P.M. Armillariella mellea and Agrilus bilineatus and Mortality of Defoliated Oak Trees. For. Sci. 1977, 23, 485–492.
- Starkey, D.A.; Oak, S.W.; Ryan, G.W.; Tainter, F.H.; Redmond, C.; Brown, H.C. Evaluation of Oak Decline Areas in the South; Protection Report R8-PR (USA); U.S. Department of Agriculture, Forest Service: Atlanta, GA, USA, 1989.
- Senanayake, I.C.; Jeewon, R.; Chomnunti, P.; Wanasinghe, D.N.; Norphanphoun, C.; Karunarathna, A.; Pem, D.; Perera, R.H.; Camporesi, E.; McKenzie, E.H.C.; et al. Taxonomic Circumscription of Diaporthales Based on Multigene Phylogeny and Morphology. Fungal Divers. 2018, 93, 241–443.
- Sinclair, W.A.; Lyon, H.H.; Johnson, W.T. Diseases of Trees and Shrubs; Cornell University Press: Ithaca, NY, USA, 1987.
- Elkinton, J.S.; Healy, W.M.; Liebhold, A.M.; Buonaccorsi, J.P. Gypsy moths and forest dynamics. In Oak Forest Ecosystems: Ecology and Management for Wildlife; Johns Hopkins University Press: Baltimore, MD, USA, 2002; pp. 100–112.
- EDDMapS. Early Detection & Distribution Mapping System. The University of Georgia—Center for Invasive Species and Ecosystem Health. Available online: http://www.eddmaps.org/ (accessed on 27 November 2023).
- Elkinton, J.S.; Liebhold, A.M. Population Dynamics of Gypsy Moth in North America. Annu. Rev. Entomol. 1990, 35, 571–596.
- Williams, D.W.; Fuester, R.W.; Metterhouse, W.W.; Balaam, R.J.; Bullock, R.H.; Chianese, R.J.; Reardo, R.C. Incidence and Ecological Relationships of Parasitism in Larval Populations of Lymantria Dispar (Lepidoptera: Lymantriidae). Biol. Control 1992, 2, 35–43.
- Williams, D.W.; Liebhold, A.M. Influence of Weather on the Synchrony of Gypsy Moth (Lepidoptera: Lymantriidae) Outbreaks in New England. Environ. Entomol. 1995, 24, 987–995.
- Drake, W.E. Evaluation of an approach to improve acorn production during thinning. In Proceedings of the Eighth Biennial Southern Silvicultural Research Conference, Auburn, AL, USA, 1–3 November 1994; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station: Radnor, PA, USA, 1995; pp. 429–441.
- Hough, J.A.; Pimentel, D. Influence of Host Foliage on Development, Survival, and Fecundity of the Gypsy Moth. Environ. Entomol. 1978, 7, 97–102.
- Gottschalk, K.W. Gypsy moth effects on mast production. In Proceedings of Southern Appalachian Mast Management Workshop, Knoxville, TN, USA, 14–16 August 1989; McGee, C.E., Ed.; University of Tennessee: Knoxville, TN, USA, 1989; pp. 42–50.
- Forbush, E.H.; Fernald, C.H. The Gypsy Moth: Porthetria dispar (linn.). A Report of the Work of Destroying the Insect in the Commonwealth of Massachusetts, Together with an Account of Its History and Habits Both in Massachusetts and Europe; Wright & Potter Printing Company: Boston, MA, USA, 1896.
- Christisen, D.M. Yield of Seed by Oaks in the Missouri Ozarks. J. For. 1955, 53, 439–441.
- Gibson, L.P. Insects That Damage Red Oak Acorns; Research Paper NE-492; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 1982.
- Riccardi, C.L.; McCarthy, B.C.; Long, R.P. Oak seed production, weevil (Coleoptera: Curculionidae) populations, and predation rates in mixed-oak forests of southeast Ohio. In Proceedings of the 14th Central Hardwood Forest Conference, Wooster, OH, USA, 16–19 March 2004; Yaussy, D.L., Hix, D.M., Long, R.P., Goebel, C.P., Eds.; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2004; pp. 10–21.
- Miller, D.R.; Schlarbaum, S.E. Acorn Fall and Weeviling in a Northern Red Oak Seedling Orchard. J. Entomol. Sci. 2005, 40, 31–38.




