Quercus alba L., also known as white oak, eastern white oak, or American white oak, is a quintessential North American species within the white oak section (Quercus) of the genus Quercus, subgenus Quercus. This species plays a vital role in local and regional economies and as a keystone species in eastern North American forests. As a long-lived woody perennial covering an extensive natural range, Q. alba’s biology is shaped by a myriad of adaptations accumulated throughout its natural history. Populations of Q. alba are crucial repositories of genetic, genomic, and evolutionary insights, capturing the essence of successful historical adaptations and ongoing responses to contemporary environmental challenges in the Anthropocene. This intersection offers an exceptional opportunity to integrate genomic knowledge with the discovery of climate-relevant traits, advancing tree improvement, forest ecology, and forest management strategies.
- Quercus alba
- adaptations
- environmental stress
- Anthropocene
- woody perennial
- climate-relevant traits
- research resources
1. Introduction
1.1. Quercus alba L.
1.2. Challenges for Oaks in the Anthropocene
2. Climate Change and Quercus alba Biology: Direct Impacts
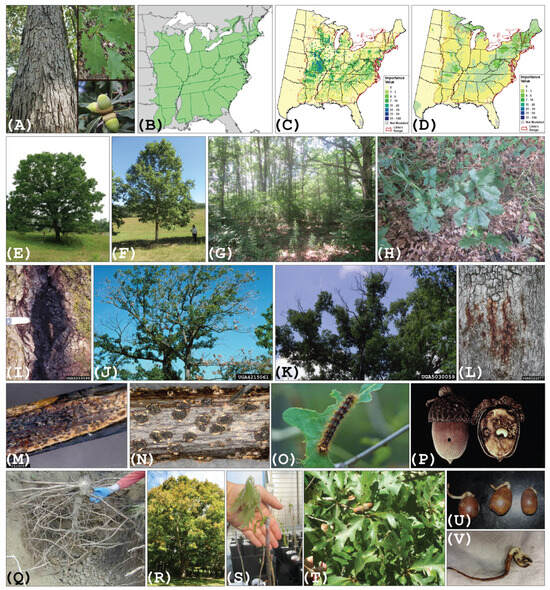
As sessile organisms, temperate trees have developmental and reproductive systems that are optimized for the annual cycle of changing environmental conditions. From studies of different plant species, these systems are sensitive to light/dark cycling, day/night temperatures, water availability, or a combination of the three. For a tree species in a particular climate zone, there are several key physiological systems adapted to the cyclic changing environmental conditions. These include but are not limited to: (1) tree water maintenance and transport systems; (2) juvenility and reproductive systems; (3) meristematic growth and development systems; and (4) abiotic and biotic cellular stress response systems. All these systems are composed of phenotypic traits that are critical for maintaining the species in its current environment. For Q. alba, as discussed above, of great interest are traits that maintain regeneration (flowering, pollination, seed development, and seedling survival and growth), enhance recruitment (drought and shade tolerance and sapling survival and growth) (Figure 1H), and tolerate or resist biotic (pathogens and pests) (Figure 1I–P) and abiotic stress. Adaptation can be a long-term process depending on the phenotypic plasticity and genetic basis for any trait, especially for species with long generation times. Therefore, rapid climate change poses a significant threat to the continued presence of such species in their current environment. To predict the future success and distribution of oaks in the changing temperate forest zones with the objective of potentially assisting its future success, we must know: (1) Which adaptive traits were key to the oaks’ success in their extant locations? (2) What is the degree and mechanism of phenotypic plasticity and/or genetic variation for these traits required by individuals or populations for adaptation? (3) What genes or molecular networks control the climate critical traits? (4) Which of these traits should be prioritized as targets for oak management, reforestation, and genetic improvement efforts in the future? The following are examples of oak traits likely to be important in their adaptive responses to rapid climate change.2.1. Water Relations: Control of Root Growth in Oaks
Predicting the future success and distribution of Q. alba in North America relies heavily on our understanding of the physiology and genetics underlying this species’ ability to adapt to the direct impacts of increased environmental warming. Although we have very limited knowledge of this in Q. alba, significant studies of drought tolerance and related traits, such as water-use efficiency in other models for annual and woody plant species [26][27][28][67,68,69], have provided research targets and foundational studies for deployment in the research on Q. alba. From the genetic perspective, several studies have revealed the sources of variation and candidate genes underlying drought tolerance and adaptation in European white oaks [29][30][31][32][33][70,71,72,73,74]. Unlike four other northeastern oaks studied by Reed and Kaye [34][75], Q. alba is responsive to rich soil composition and higher moisture levels, associated shale vs. sand bedrock. However, data and publications on the performance of Q. alba on different types of soil are scarce (for more information on site recommendations for Q. alba, refer to [35][76]). As Q. alba have a taproot architecture (Figure 1Q), presumably to enable deep soil water acquisition under drought conditions, studies focusing on the control of the development of this root form in woody plant species are particularly relevant. Oaks exhibit both shallow and deep rooting systems, allowing them to access water at multiple depths in the soil profile during the annual cycles of rain and drought. They successfully compete with species possessing rooting systems more specialized for deep soil (e.g., maples and pines) [36]. The rooting architecture of oaks in concert with stem vascular organization (ring porous) and leaf structural features (thickness, stomatal size) enable oaks to exploit niches across regional and climatic zones greatly differing in hydrologic conditions [36][37][36,37], from mesic to xeric. Equally important, oaks survive and prosper in a broad range of different edaphic conditions and drought stress, due to the structural features of oak vascular tissues such as tyloses (vascular structures arising post-differentiation that limit the diffusion of liquids through the wood sample [38][77], deep rooting, and the ability to form and sustain mycorrhizal associations with a high diversity of fungi; for review, see [39][78]). Quercus species distributions provide excellent examples of how these anatomical traits and their associated physiological systems have enabled the species to become dominant in North American forests across a large range of environments (for a review of physiological characteristics, see [36][37][40][41][42][32,36,37,40,79]). A recent review by Kościelniak et al. [43][80] lays out the current status of our understanding of taproot development and its control while highlighting several questions that need future study: “(1) how does organization and cellular signaling enable a taproot to grow and penetrate deep soil layers, (2) what internal factors enable taproots to grow rapidly and penetrate deep soil layers (Figure 1Q), and (3) how does soil water limitation induce the vertical growth of taproots (Figure 1Q). Aside from the unanswered questions above, how much does the genetic control of cell division explain the continued maintenance of root growth and apical dominance of taproot meristems?” As outlined by Kościelniak et al. [43][80], emerging concepts in the control of root growth come from studies of models such as Arabidopsis and other model tree species (e.g., Populus species). The control of root growth and architecture is related to phytohormone balance and pathway regulation, plant growth and development transcription factors, microRNAs, and signal transduction pathways. Additionally, emerging studies indicate that epigenetic factors likely also play a key role in the adaptive responses of trees to environmental change (for review, see [44][45][81,82]). In the case of epigenetics, work in Populus species on drought stress transcriptomic responses of clonally propagated material planted in differing geographic regions demonstrates specific DNA methylation patterning correlated with provenance and drought stress transcriptomic responses [45][82]. These types of studies linking local adaptation to epigenetic markers in reference to oak adaptation could unlock potential plasticity targets for the improvement of the species.2.2. Reproduction
In European white oaks, Caignard et al. [46][83] presented evidence that increasing temperatures associated with climate change are responsible for an observed increased seed production in environments that are historically cooler and currently not impacted by water deficit. This contrasts to studies of other oak species in regions experiencing both temperature increase and water deficit, whereby tree growth and survival were negatively impacted by a warming climate [47][84]. Effects on other aspects of oak reproduction (e.g., masting) and the effects of other environmental changes (e.g., drought) need to be established before any meaningful modelling about the future habitat of deciduous oaks in the temperate forests. Current predictions suggest that oaks will expand northward with changing climate zones and retreat in warmer drought-prone areas. A recent limited provenance study of Q. alba performance suggests that white oaks from northern temperate locations, when challenged with more southerly climate conditions, do not perform as well as trees from more southern provenances, suggesting that there may be a fitness cost for Q. alba as the climate zones shift further northward [48][85]. Research into the fundamental genetics and physiology of oak species regeneration in response to environmental conditions are needed to predict outcomes and produce strategies in order to promote the continued presence and establishment of white oaks.2.3. Pollination
Long-distance pollination is a well-established feature of the maintenance of genetic diversity within oak stands [49][86] (for review, see [7]). Multiple studies of isolated stands have revealed that long-distance pollen dispersal is evident and leads to the conclusion that the fragmentation of oak populations due to repurposed land use may not necessarily lead to local losses in genetic diversity [50][51][52][53][54][55][56][57][58][59][60][61][87,88,89,90,91,92,93,94,95,96,97,98]. However, other barriers may lead to loss of diversity or inbreeding, such as genetically determined fertilization incompatibilities and/or timing of flowering, which may play a significant role in determining the genetic architecture of oak forest stands, despite pollen dispersal [49][86]. Thus, climate change could lead to local maladaptation, due to incompatible phenology, making it difficult to predict future oak sustainability for species such as Q. alba, for which we know little regarding these traits and at what spatial scales these traits have adapted.2.4. Flowering
Oaks are monoecious, with staminate and pistillate flowers on the same tree. Little is known of the physiology or genetics of the regulation of flowering in oaks. Contrastingly, there is a substantial body of growing knowledge about the molecular basis of flowering control in some model forest and fruit tree species (for recent review, see [62][99]). Both floral and leaf bud dormancy are initiated and controlled by key environmental factors such as light (day length), cold (chilling requirement), and stress (abiotic: heat, cold, osmotic; biotic: pathogens and pests). All of these will likely be significantly impacted by climate change. The establishment, maintenance, and release of dormancy are regulated by gene networks that respond to these different environmental cues, depending on the individual adaptation of a particular species or population. For example, Prunus fruit trees, such as peach (P. persica), establish, maintain, and release bud dormancy via pathways that are sensitive to temperature [63][100], while in Populus species, these steps are regulated more by day length [64][101] (reviewed by [65][66][102,103]). In general, these networks involve light response networks, temperature response networks, hormone pathways, cell cycle control, epigenetics, and others [62][63][99,100]. Genomics-based research on dormancy in the European pedunculate oak (Q. robur) has highlighted some of the gene networks evident in these previous fruit and forest tree studies, suggesting that oaks may utilize similar genetic control mechanisms as other tree species [67][104]. However, it is difficult to predict and improve the performance of Q. alba in terms of flowering traits (Figure 1S) without first obtaining substantial knowledge of the physiological, ecological, and genetic systems that underpin these traits in this species.2.5. Masting
Masting is a population scale synchronous flowering event observed in certain tree species. However, as pointed out in a detailed review of the genetic control of masting [68][105], the manifestation of this event is dependent on the diversity in the flowering control mechanism among individual members of the species (diversity) and the coordinated response of these individuals within a population and year (synchrony). A resource-driven pollen limitation hypothesis was directly supported for two European oaks species, Q. petrea and Q. robur [69][106]. From studies of other perennial and annual plant species, the physiological and genetic control of masting could likely involve molecular networks controlling flowering and dormancy release [62][68][70][99,105,107]; abiotic and biotic stress response [71][108]; seed maturation, meristem growth, and developmental control [43][72][80,109]; root/shoot communication and sink-source physiology [73][74][110,111]; the epigenetic regulation of gene activity [75][112], and potentially others. Although not possible in the past, access to gene information in oaks is rapidly increasing (see Emerging tools and resources for oak biology and improvement, below) and the stage is ideally set to make significant progress in understanding the physiological and genetic underpinnings of this important climate critical trait in Q. alba (Figure 1R,T,U).2.6. Seed Germination
In oaks, there are contrasting climate-related seedling germination strategies. In general, white oaks do not exhibit a stratification requirement and germinate in the fall, concomitant with acorn drop (Figure 1U), while red oak acorns overwinter and germinate the following spring during more favorable growing temperatures [76][77][113,114]. In some red oaks, this stratification requirement is not absolute as acorns will germinate without prior cold treatment (e.g., Quercus pagoda Raf. [78][115]); however, the germination efficiency increases substantially with cold treatment [76][78][113,115], leading to the conclusion that red oaks exhibit a physiological dormancy, rather than true dormancy (endodormancy) which is associated with cell cycle arrest and chill requirement for dormancy release. Unfortunately, we know very little about the genetics and physiology of this climate critical trait in oaks in contrast to what is known regarding annual crop and model species plants. In recent years, through studies of annual crop models, Arabidopsis, and a few woody perennial species (e.g., peach, poplar, and grape), major advances have been made in our understanding of the genetic and physiological underpinnings of seed and bud dormancy in plants. For reviews on seed germination, see [79][116], and for bud dormancy, [62][99]. However, much of our knowledge of seed dormancy and germination control comes from studies of plants with orthodox (annual) or typical seeds, such as Arabidopsis, whereby seeds mature concomitant with desiccation. This is not the case for recalcitrant or intermediate seed species, including oaks or other nut-producing species like chestnut, for which the desiccation of seeds after maturity significantly negatively impacts seed viability and germination [76][80][113,117]. The relationship between the chemistry, germination timing, and dispersal of acorns has been an area of interest for quite some time. An early study on germination in oaks [76][113] suggested that the fat content of acorns may be related to the germination control in red oak acorns and not the tannin content of the seed. Subsequent reports on northern red oak (Quercus rubra) and Q. alba germination and dispersal over the following decades were synthesized into a Differential Dispersal Hypothesis (DDH) [81][118], based on the characteristics of acorns reported to affect germination, feeding, and dispersal by animals. The DDH summarized the primary features of Q. alba acorns affecting dispersal, such as low tannin and low-fat content, and early germination, predisposing Q. alba acorns to immediate and/or selective consumption (relative to northern red oak) in autumn, or embryo excision by squirrels prior to cashing. In northern red oak, acorns traits affecting dispersal included high tannin and high fat content, with delayed germination, associated with the selective caching of northern red oak acorns (relative to Q. alba) for later consumption during winter. Overall, the DDH predicted that northern red oak acorns would be selectively scatter-hoarded by animals across greater dispersal distances than Q. alba, especially smaller-seeded acorns, which jays could disperse over very long distances, primarily into open areas suitable for oak regeneration. More recently, studies in sawtooth white oak (Q. aliena var. accuserata) suggested that the acorn pericarp and cotyledons contain substances that inhibit germination, and that the removal of the pericarp and a portion of the cotyledon can increase germination efficiency [82][119]. The germination morphology of Q. alba seeds (the plumule separated from the cotyledons) (Figure 1U,V) promotes seedling establishment in case of pruning by rodents [83][120]. The comparison of red and white oak species acorns by maturity and germination demonstrates that transitions from maturation to germination show changes in cellular location and the metabolism of lipids, insoluble and soluble carbohydrates, and proteins (reviewed by [84][121]). Many features of the interaction of the genotype, phylogeny, ecotype, and physiology of seed germination remain to be clarified. For a more comprehensive history and detailed overview of ecology and biology research findings for oak seed dispersal, see [85][122]. Recent phylogenomic analyses have also highlighted the importance of seed structure and germination in the radiation of species, as well as introgression, within the Fagaceae family [86][123].2.7. Seedling Growth Control
In oaks, some studies examine the effect of seed size on seed germination and seedling survival (for review see [87][124]). Among species, the question of seed size versus seed abundance for optimal species survival seems not to be an issue of a simple tradeoff but may also incorporate the longevity of the large-seeded species and the consequences of continued reproduction over extended time periods. Among and within species studies suggest that the larger seeds may have a significant advantage in germination and subsequent seedling recruitment [76][88][89][90][91][113,125,126,127,128]. Llanderal-Mendoza et al. [89][126] suggest that decreases in acorn size along latitudinal climatic differences in Q. rugosa in Mexico effect successful recruitment whereby the northward expansion of the species range has led to smaller-sized acorns with a reduction in germination and recruitment. Conclusions from this work were supported and further extended to other Quercus species in Mexico [90][127], whereby they demonstrated that, in a common garden experiment for seven red oak species and three white oak species, acorn fresh weight was positively correlated with germination efficiency, and acorn dry matter was driving this correlation. Furthermore, they demonstrated that nutritional storage compounds and not water content were responsible for this result. This size effect was consistent when compared between red and white oak species, as well as within red and white oak species. Finally, a recent study [91][128] demonstrated that large seed size was positively correlated with seed viability in Q. robur acorns collected from multiple sites in Croatia over a ten-year period. Larger seedlings can improve the competitive status of oak regeneration relative to average-sized seedlings [92][93][94][34,52,129]. Extra-large seedlings [95][96][97][130,131,132] can be planted to enrich advanced natural regeneration and may be especially critical when harvesting occurs in years of poor mast production, which is a regular occurrence with Q. alba [98][133]. While these seedling size considerations are important, the genetic potential of the seedlings is also important for successful regeneration. Thus, the development of high-quality, well-performing Q. alba seedlings for artificial regeneration should be achievable through a combination of tree genetic improvement and good nursery and planting practices [97][132]. Unfortunately, it is difficult to predict how results from orthodox annual seed plants will translate to oaks and other nut-producing trees. However, we now have the genomic resources and materials in key oak species to bridge this knowledge gap. As stated in [99][134], “hardwood seed production, seed harvest, and seedling production must be approached as a coordinated system where all aspects from flower initiation to seed development, harvest, and storage to seedling production, transplanting and establishment are integrated. The best approach to insure predictable amounts of high-quality seeds and seedlings is to establish and manage seed orchards and use container production”. However, sustained regenerative success in the forest in the long term will rely on an understanding of the genetic and environmental interplay on climate-relevant traits that underpin seed production, germination, and seedling establishment (Figure 1H,R–V). The contrasting strategies of flowering, seed maturation, seed germination, and seedling growth control between sympatric white and red oaks species is ideal for delimiting the genetic architecture of these climate-relevant traits and predicting the impact of climate change on their manifestation. This can lead to optimized management and tree improvement strategies incorporating genetic and physiological knowledge-based inputs.3. Climate Change and Quercus alba Biology: Indirect Impacts
While the rapidly changing climate can directly impact tree growth and reproduction, it also indirectly impacts tree survival by altering the distributions of pests and pathogens, potentially leading to increased pest and pathogen pressure on native tree populations (for a review, see [100][101][102][135,136,137]). Oaks are susceptible to native and non-native pathogens (Table 1 and Figure 1I–P). Alone, many of these pests and pathogens are not necessarily lethal to the host; however, in concert with the plant stress imposed by a rapidly changing climate, these pathogens can significantly contribute to oak decline [103][138]. Therefore, the introduction of novel non-native pathogens or the climate-driven expansion of native pathogen ranges can be extremely detrimental to previously unexposed forest trees (for examples, see [104][139]). A case in point from a related Fagaceae species is the spread and subsequent impact on the American Chestnut of Phytophthora cinnamomi post its introduction to the eastern US [105][106][140,141]. This oomycete pathogen is hosted by over 5000 different plant species (for a review, see [107][142]) (see Figure 1I for Phytophthora root and crown rots in Q. alba) and is a major destructive pathogen of members of the Fagaceae. Its introduction into the southeastern US has created a major complication to the planned introduction of chestnut blight-resistant American chestnut from chestnut restoration programs. In oaks, its northward expansion with global warming is already impacting oaks and other forest trees as part of the oak decline syndrome in North America and Europe [108][109][110][143,144,145]. Another Phytophthora species, P. ramorum, is responsible for sudden oak death on California oaks (primarily Quercus agrifolia, coast live oak, in the red oak subgenus Erythrobalanus) and the related tanoak species Notholithocarpus densiflorus in the Fagaceae family, as well as other plant species in the western US. Its impact is predicted to mount with increased microclimate variability that is associated with climate change [111][112][113][146,147,148]. Many different diseases affect white oaks, including canker rots [114][115][149,150], oak anthracnose [116][117][151,152], leaf blisters [118][153], stem canker [119][154], oak wilt [120][155], oak decline [121][156], and stem decay [115][150] (Figure 1I–P for Q. alba images). Of all these diseases, oak decline and oak wilt are the two most devastating, with major impacts on oak survival and acorn production, resulting in altered forest structure and composition over time. Several additional diseases and insects that are rarely fatal can also impact acorn production and are expected to increase as climate change advances [122][157]. These relevant pathogens and pests are tabulated below (Table 1).|
Disease |
Pathogen/Pest |
Relevant Classification |
Key Features |
Q. alba Resistance? |
References |
|---|---|---|---|---|---|
|
Oak wilt |
Bretziella fagacearum (Bretz) (Microascales: Certocystidaceae) (formerly Ceratocystis fagacearum) |
Ascomycete fungus, Necrotrophic |
Vascular wilt, vectored through root grafts and sap-feeding beetles Scolytidae and Nitidulidae. |
Somewhat resistant, exhibiting slower fungal growth. |
[123][124][125][126][127][128][129][158,159,160,161,162,163,164, [130]165[131][132][133[134][135],166,167],168,169,170, [136]171[137][138][139],172,173,174] |
|
Oak decline |
Agrilus bilineatus Weber and Armillaria mellea [Vahl. Ex Fr.] and Phytophthora cinnamomi |
Coleptylebran beetle and oomycete hemibiotroph interaction. |
Caused by the interaction between severely stressed trees, secondary pests, such as the two-lined chestnut borer, and root diseases like armillaria root rot and ink disease. |
Less susceptible than other North American oak species and less severe in young (less than 70 years) and heterogenous stands. |
[140][141][142][143][144][145][146][175,176,177,178,179,180,181] |
|
Hypoxylon cankers |
Hypoxylon atropunctatum (Schw. ex Fr.) Cke |
Ascomycete fungus, Necrotrophic |
Less pathogenic fungal species that frequently accompanies dieback. |
Live healthy unstressed trees less susceptible. |
|
|
Root and crown rot |
Phytophthora cinnamomi |
Oomycete hemibiotrophic |
Extirpated American chestnut and a component of oak decline. |
White oaks less effected by this pathogen? |
[105][106][107][108][109][140,141,142,143,144, [110]145[111][112][113[122],146,147],148,157] |
|
Anthracnose |
Dendrostoma leiphaemia Senan. and K.D. Hyde (formerly Discula quercina (Westend.) Arx Anamorph of Ascomycete Apiognomonia quercina. |
Ascomycete fungus, hemibiotrophic |
One of the most damaging leaf and twig diseases, impacting reproduction and masting; widespread across North America. |
Leaves are less susceptible as they age due to thicker protective cuticle; large range of the pathogen across a variety of climates suggests adaptation to distinct climates |
|
|
Twig, branch and rots cankers |
Botryosphaeria spp. (including B. rhodina [Berk. and Curt.] von Arx, B. dothidea [Moug. ex Fr.] Ces. and de Not., B. obtusa [Schw.] Shoemaker, and B. quercum [Sch.: Fr.] Saccardo) and Botryodiplodia gallae (Schw.) Petrak and Sydow |
Ascomycete fungus, necrotrophic/hemi-biotrophic |
Can play role in oak decline syndrome. |
Most susceptible under drought or cold stress. |
|
|
Spongy moth (formally gypsy moth) |
Lymantia dispar |
Lepidopteran insect |
A significant insect pest of Q. alba forests, usually in low numbers but occasionally surging to severe outbreaks. |
Q. alba is preferred over the hundreds of tree species spongy moth caterpillars feed on. |
[149][150]][184[151,185][152][153,186,187,188, [154]189[155][156][157],190,191,192] |
|
Acorn weevil |
Curculio and Conotrachelus spp. |
Coleopteran insect |
The major oak seed predator and a factor in the reduced regeneration in the eastern United States. |
Unknown. |
