Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tie Zhou | -- | 2500 | 2024-02-15 16:43:14 | | | |
| 2 | Sirius Huang | Meta information modification | 2500 | 2024-02-17 02:03:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhou, W.; Zhang, W.; Yan, S.; Zhang, K.; Wu, H.; Chen, H.; Shi, M.; Zhou, T. Therapies for Bone Metastasis in Prostate Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/55074 (accessed on 07 February 2026).
Zhou W, Zhang W, Yan S, Zhang K, Wu H, Chen H, et al. Therapies for Bone Metastasis in Prostate Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/55074. Accessed February 07, 2026.
Zhou, Wenhao, Wei Zhang, Shi Yan, Kaixuan Zhang, Han Wu, Hongyu Chen, Minfeng Shi, Tie Zhou. "Therapies for Bone Metastasis in Prostate Cancer" Encyclopedia, https://encyclopedia.pub/entry/55074 (accessed February 07, 2026).
Zhou, W., Zhang, W., Yan, S., Zhang, K., Wu, H., Chen, H., Shi, M., & Zhou, T. (2024, February 15). Therapies for Bone Metastasis in Prostate Cancer. In Encyclopedia. https://encyclopedia.pub/entry/55074
Zhou, Wenhao, et al. "Therapies for Bone Metastasis in Prostate Cancer." Encyclopedia. Web. 15 February, 2024.
Copy Citation
In the absence of early detection and initial treatment, prostate cancer often progresses to an advanced stage, frequently spreading to the bones and significantly impacting patients’ well-being and healthcare resources. Therefore, managing patients with prostate cancer that has spread to the bones often involves using bone-targeted medications like bisphosphonates and denosumab to enhance bone structure and minimize skeletal complications. Additionally, researchers are studying the tumor microenvironment and biomarkers to understand the mechanisms and potential treatment targets for bone metastases in prostate cancer.
prostate cancer
bone metastasis
bone-targeted therapies
skeletal-related event
1. Introduction
Prostate cancer (PCa) is a prevalent malignant tumor in the United States, ranking second in terms of mortality rate after lung cancer [1]. There exists a significant disparity in the occurrence rate of prostate cancer between China (10.2/100,000) and North America (73.0/100,000), with both the incidence and mortality rates showing a consistent upward trend in recent years [2][3]. The 2014 China Multicenter Report revealed that a significant proportion of Chinese patients (approximately 30.5%) diagnosed with prostate cancer had already developed distant metastases at the time of initial diagnosis, which is considerably higher compared to the rates observed in North America [4]. Nowadays, the treatment options for patients diagnosed with metastatic prostate cancer (mPCa) have shown significant advancements in recent years. Androgen deprivation therapy (ADT) serves as the primary treatment for this condition. Additional treatment options encompass chemotherapy, new generation hormone therapy, radium-223, and, more recently, radioligand therapy. Special considerations should be directed toward the management of bone health and the prevention of treatment-induced bone loss in these patients [5]. Among individuals diagnosed with castration-resistant prostate cancer (CRPC), bone metastasis is commonly detected in 70% to 90% of patients through imaging examinations [6]. Bone metastases give rise to the occurrence of skeletal-related events (SREs), which encompass severe pain, pathological fracture, spinal cord/intervertebral nerve compression, and hypercalcemia [5]. Preventing and reducing the occurrence of SREs, relieving pain caused by bone metastases, and improving patients’ quality of life are the goals of treatment. The management of bone metastases in prostate cancer has undergone significant advancements due to the enhanced comprehension of the disease’s progression, signaling pathways, mutational characteristics, and mechanisms of drug resistance. Table 1 and Figure 1 summarize the main pathways and mechanisms of action of the principal PC therapeutic agents. However, there exists a dearth of data analysis pertaining to drug trials and their progression over the previous decade.
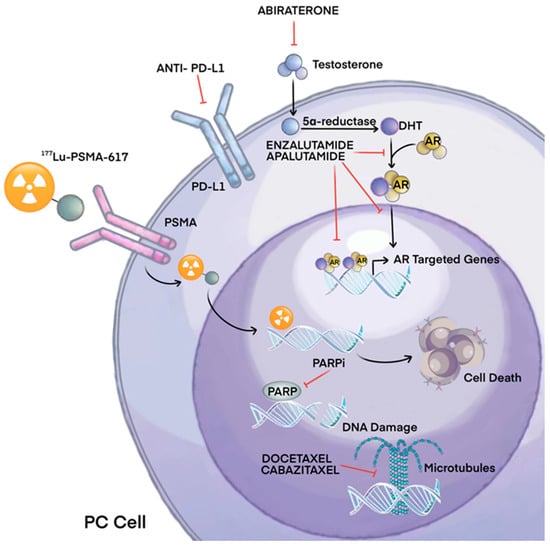
Figure 1. Main mechanisms of therapeutic agents for prostate cancer.
Table 1. Drugs and their mechanisms of actions against prostate cancer.
| Drug | Action | Mechanism |
|---|---|---|
| Abiraterone | Inhibition of androgen synthesis | Inhibits CYP17, reduces androgen production |
| Enzalutamide | Antagonization of androgen action | Androgen receptor inhibitor, blocks testosterone effects |
| Bicalutamide | Blockade of the AR | |
| Apalutamide | Prevent AR translocation, DNA binding, and AR–mediated transcription | |
| Docetaxel Cabazitaxel |
Inhibition of mitosis | Tubulin inhibition |
| Radium-223 | Alpha radiation, gamma rays | Targets bone metastases, emits alpha particles |
| 177Lu-PSMA-617 MEDI3726 |
Inhibition of growth signals | Binding and internalization of the PSMA ligands triggers cell death |
| Ipilimumab | Checkpoint (CTLA-4) inhibitor | Increases antitumor T-cell responses |
| Olaparib Rucaparib |
PARP inhibitor | Inhibition of DNA repair |
| Pembrolizumab | PD-1 inhibitor | Regulates T cell activation |
| Sipuleucel-T | Immunotherapy | Autologous vaccine |
| CAR-T | Targeted PSMA |
2. Hormonal Therapy
Enzalutamide is a second-generation androgen receptor antagonist and was initially reported in 2009 [7]. In men with metastatic hormone-sensitive prostate cancer (mHSPC), the ARCHES study demonstrated that enzalutamide exhibited a significant reduction in the risk of mortality when compared to placebo (HR: 0.39, 95% CI 0.30–0.50; p < 0.001). Additionally, enzalutamide was found to be effective in reducing the occurrence of the first symptomatic skeletal events, castration resistance, and pain progression [8]. A post hoc analysis of the ARCHES study provided additional clarification on the effects of enzalutamide in reducing the risk of radiographic progression of bone metastases (HR: 0.33, 95% CI 0.22–0.49) and the risk of bone metastases with lymph node metastases (HR: 0.31, 95% CI 0.21–0.47) when compared to placebo. Still, there was no significant reduction in the risk of lymph node metastasis [9]. PREVAIL, a double-blind, phase III study [10], met its primary endpoint, radiographic progression-free survival, with a significant advantage in the enzalutamide arm (65% versus 14%, p < 0.001). The occurrence of SREs, which was assessed as a secondary endpoint, demonstrated improvement in the enzalutamide group (32% vs. 37%, p < 0.001). Additionally, enzalutamide has shown efficacy in patients with low baseline PSA levels (i.e., <10 ng/mL), including those with ≥4 bone metastases and/or visceral disease and <4 bone metastases without visceral disease [11].
Abiraterone, a CYP17 inhibitor that targets a crucial enzyme involved in androgen synthesis, was assessed in the LATITUDE trial [12]. In this phase III trial, a total of 1199 mCSPC patients were randomly assigned in a 1:1 ratio to receive ADT + abiraterone acetate + prednisone versus ADT + dual placebo. Treatment with abiraterone was associated with a statistically significant survival advantage (not reached vs. 34.7 months), and the median length of radiographic progression-free survival was 33.0 months in the abiraterone group and 14.8 months in the placebo group. A post hoc analysis revealed that abiraterone acetate led to improvements in bone pain, fatigue symptoms, and overall health-related quality of life. Patients in the abiraterone group had a longer median time to worst pain intensity, worst fatigue intensity, and functional deterioration condition [13]. The STAMPEDE trial examined the efficacy of abiraterone acetate in combination with prednisolone and ADT versus ADT alone in patients with locally advanced or metastatic PC. After 3 years of treatment, survival improved (83% versus 76%, HR 0.63; p < 0.001) and the risk of SREs decreased (12% versus 22%, HR 0.46, p < 0.001) in the combination group [14]. Abiraterone was assessed in the COU-AA-302 trial, which examined the efficacy of abiraterone acetate in combination with prednisone compared to placebo and prednisone in mCRPC patients who had not previously received chemotherapy. The pain progression was 26.7 months in the abiraterone group and 18.4 months in the prednisone group (HR, 0.82; 95% CI, 0.67 to 1.00; p < 0.05), and the advantage in radiographic progression-free survival reached statistical significance (16.5 months versus 8.3 months; p < 0.001) [15].
Apalutamide was first described in 2012 as a novel antiandrogen for prostate cancer [16]. The TITAN trial evaluated the therapeutic efficacy of adding oral apalutamide to ADT for the treatment of adult men with metastatic castration-sensitive prostate cancer (mCSPC). The 24-month OS rates were 82.4% in apalutamide plus ADT recipients and 73.5% in placebo plus ADT recipients (p = 0.005). There was no substantial difference between the two groups in the time to the skeletal-related events of prostate cancer [16]. The primary objective of the TITAN study was to assess the therapeutic effectiveness of combining oral apalutamide with ADT (not reached, HR 0.80) [17].
Approximately 8% of CRPC patients have the androgen receptor splice variant-7 (AR-V7) blood biomarker [18], which has been linked to resistance against enzalutamide and abiraterone [19]. Galeterone has been reported to inhibit AR signaling through multiple mechanisms: CYP17 inhibition, AR competitive antagonism, and induction of AR and AR-V7 protein degradation [20]. In a randomized phase III trial [21], CRPC patients with AR-V7 expression were randomly assigned to receive either galeterone or enzalutamide in an open-label manner. However, this trial did not achieve its primary objective due to a high number of patients discontinuing the study as a result of disease progression. Therefore, there is an urgent need for alternative treatments for circulating tumor cells expressing AR-V7 in mCRPC.
Bipolar androgen therapy (BAT) is an emerging treatment option for patients with CRPC. BAT has shown promise in restoring drug sensitivity in some patients, particularly to medications like Abiraterone and Enzalutamide. This therapy has demonstrated significant advantages in the treatment of CRPC patients [22]. The RESTORE trial was a single-arm, multicohort study, focused on CRPC patients. The results from this trial indicated that patients who had previously experienced progression on enzalutamide showed a 52% PSA50 response rate to enzalutamide after undergoing BAT. Similarly, patients who had previously progressed on abiraterone demonstrated a 16% PSA50 response to abiraterone after BAT. However, this study did not investigate the effects of BAT on SREs [23][24].
More than 50% of prostate cancer patients receiving long-term ADT have significant associated metabolic consequences, such as insulin resistance and metabolic syndrome [25][26]. A phase II trial observed that the use of high-dose metformin in mCRPC reduced PSA secretion and delayed the progression of prostate cancer [27].A cohort study based on a prostate cancer population showed that patients with hyperlipidemia may have prolonged survival with metformin and statins while undergoing radiotherapy [28]. Ongoing clinical trials will help elucidate the role of metformin in the treatment of locally advanced or metastatic prostate cancer [29][30].
3. Chemotherapy
In 2004, the United States Food and Drug Administration (FDA) approved the use of Docetaxel, a taxane drug that binds to tubulin, for the treatment of mCRPC [31]. Another taxane drug called cabazitaxel was also approved by the FDA in 2010 as a second-line salvage chemotherapy for prostate cancer [32]. A phase II trial found that a weekly treatment schedule of 10 mg/m2 of docetaxel resulted in a 34.9% prostate-specific antigen (PSA) response rate, with lower toxicity rates of 14.2% neutropenia and 35.7% diarrhea [33]. However, the GETUG-AFU 15 trial suggested that the addition of docetaxel to ADT should not be used as a first-line treatment for mCSPC as it did not improve overall survival [34]. On the other hand, the CHAARTED trial showed that adding docetaxel to ADT in early-stage prostate cancer improved overall survival, particularly in high-volume disease, but did not show a clear survival benefit in low-volume disease [35][36]. One potential reason for the discrepancy between the GETUG-AFU 15 and CHAARTED trials is the lack of statistical power in the former. The STAMPEDE trial reported a survival benefit with upfront docetaxel in patients with mCSPC, regardless of metastatic burden [37]. According to the National Comprehensive Cancer Network (NCCN) guidelines, patients with high-volume metastatic disease who are suitable for chemotherapy should receive ADT in combination with docetaxel, along with either abiraterone or darolutamide. The ARASENS trial found that adding darolutamide to ADT and docetaxel improved the overall survival of mHSPC patients with a similar rate of side effects compared to using a placebo with ADT and docetaxel [38]. The PEACE-1 trial demonstrated that using abiraterone in combination with ADT improved overall survival and progression-free survival in patients with de novo mCSPC, with only slight increases in treatment-related side effects [39]. Additionally, the findings from the ENZAMET trial suggested that adding enzalutamide should be considered for patients with mCSPC who are treated with docetaxel [40][41]. While studies have shown the benefits of doublet therapy with ADT plus androgen receptor signaling inhibitors (ARSIs), as well as the benefits of triplet therapy with ADT plus docetaxel and ARSIs, a direct comparison between doublet therapy and triplet therapy for mCSPC has not been conducted [38][39]. However, patients with low-volume disease appear to have increased treatment benefit from ARSI doublet therapy compared to docetaxel and ADT [42]. It is noteworthy that the overall survival (OS) rate is comparatively lower in African American individuals than in Caucasian individuals among patients diagnosed with prostate cancer. However, after administration of docetaxel, the OS rate in African American patients approached parity with that of Caucasian patients. This phenomenon may be attributed to racial disparities in drug responsiveness. The documented benefits of docetaxel or cabazitaxel in terms of OS are well established. However, there is currently no conclusive evidence regarding their impact on pain management and the potential delay or prevention of SREs in patients with mCRPC [43].
4. Bone-Modifying Agents
Osteoporosis is commonly observed in patients with prostate cancer. Studies have shown that a significant percentage of hormone-naïve PC patients (ranging from 3.9% to 37.8%) develop osteoporosis even before receiving any oncological treatment. This suggests that PC itself may be a risk factor for the loss of bone mineral density (BMD) due to its promotion of bone resorption [44]. ADT is designed to reduce testosterone by up to 95% and lower estrogen, but it also causes an increase in bone resorption to altering the balance between osteoblasts and osteoclasts and results in a rapid decline in BMD. The decline in BMD begins shortly after the initiation of ADT and continues throughout the treatment period [45]. The duration of ADT is directly proportional to the risk of osteoporotic fractures [46].
The efficacy of bone health agents, such as zoledronic acid and denosumab, in reducing the occurrence of SREs and delaying their onset in patients with bone metastases from prostate cancer has been extensively studied. The NCCN guidelines for the treatment of osteoporosis in prostate cancer patients receiving ADT recommend several strategies. They suggest calcium and vitamin D3 supplementation as a standard approach. Additionally, for men aged 50 years and older who have low bone mass in the femoral neck (with T values falling between −1.0 and −2.5), the NCCN advises considering further therapy options such as denosumab or zoledronic acid. Zoledronic acid is the most commonly used bisphosphonate for managing bone metastasis in prostate cancer patients due to its reported ability to prolong the time to SREs and alleviate bone pain [47]. Despite having similar rates of overall survival and SREs, zoledronic acid demonstrated superior efficacy in managing pain compared to clodronate [48] (Table S1). However, the effectiveness of zoledronic acid varies among studies, and some have yielded inconclusive results. For instance, a phase III clinical trial demonstrated that patients with mCSPC and bone metastases treated with zoledronic acid and ADT experienced a significantly shorter time to the first SRE (18.8 months) compared to those treated with ADT alone [49]. Conversely, the ALLIANCE 90202 trial found no association between zoledronic acid use and a reduced risk of SREs in men with mCSPC [50]. In TROG 03.04 RADAR trail [51], 18 months of androgen suppression plus radiotherapy is a more effective regimen for treating locally advanced prostate cancer, but the addition of zoledronic acid to this regimen does not significantly improve OS. Similarly, the TRAPEZE study reported that zoledronic acid did not prolong OS [52]. Moreover, in patients at high risk for localized PCa, zoledronic acid proved to be ineffective in preventing bone metastases [53]. Zoledronic acid has been shown to improve BMD when administered at various dosing intervals. In the United States, the approved use of zoledronic acid specifies that it should be used when prostate cancer has progressed despite hormone therapy. For patients with mCRPC and skeletal metastases, zoledronic acid has been utilized in accordance with the EAU guidelines to mitigate the occurrence of SREs [54]. The currently approved dose in most clinical trials is 4 mg intravenously every 3–4 weeks [55][56][57].
Numerous trials have examined the effectiveness of zoledronic acid in preventing BMD decline, but none of these trials were designed to detect a difference in fracture risk [58]. Denosumab, on the other hand, is a fully humanized monoclonal antibody that binds to and neutralizes RANKL, a protein involved in bone resorption. By inhibiting signaling through its target RANK, denosumab suppresses bone resorption by osteoclasts [59]. A post hoc analysis of three phase III trials compared denosumab to zoledronic acid in terms of reducing the risk of SREs, including both first-time and subsequent events [60]. The analysis found that denosumab was more effective than zoledronic acid in preventing SREs, regardless of factors such as Eastern Cooperative Oncology Group performance status, location and number of bone metastases, presence or absence of visceral metastases, and urinary N-telopeptide level. The standard dosage for denosumab is 120 mg administered subcutaneously every 4 weeks and there is evidence to suggest that administering bone-modifying agents every 12 weeks instead of every 4 weeks may be equally effective in preventing SREs [61][62]. Thus, prolonging the interval between doses of bone-modifying agents may help avoid the risk of adverse events such as osteonecrosis of the jaw (ONJ) without compromising SRE prevention.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48.
- Zhu, Y.; Mo, M.; Wei, Y.; Wu, J.; Pan, J.; Freedland, S.J.; Zheng, Y.; Ye, D. Epidemiology and genomics of prostate cancer in Asian men. Nat. Rev. Urol. 2021, 18, 282–301.
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132.
- Chen, R.; Ren, S.; Yiu, M.K.; Fai, N.C.; Cheng, W.S.; Ian, L.H.; Naito, S.; Matsuda, T.; Kehinde, E.; Kural, A.; et al. Prostate cancer in Asia: A collaborative report. Asian J. Urol. 2014, 1, 15–29.
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663.
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425.
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790.
- Armstrong, A.J.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; Shore, N.D.; et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J. Clin. Oncol. 2019, 37, 2974–2986.
- Armstrong, A.J.; Shore, N.D.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; et al. Efficacy of Enzalutamide plus Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer by Pattern of Metastatic Spread: ARCHES Post Hoc Analyses. J. Urol. 2021, 205, 1361–1371.
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433.
- Taplin, M.E.; Armstrong, A.J.; Lin, P.; Krivoshik, A.; Phung, D.; Parli, T.; Tombal, B.; Beer, T.M. Clinical Outcomes of Chemotherapy Naïve Men with Metastatic Castration Resistant Prostate Cancer and Low Baseline Prostate Specific Antigen Treated with Enzalutamide vs Placebo. J. Urol. 2017, 198, 1324–1332.
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360.
- Chi, K.N.; Protheroe, A.; Rodríguez-Antolín, A.; Facchini, G.; Suttman, H.; Matsubara, N.; Ye, Z.; Keam, B.; Damião, R.; Li, T.; et al. Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): An international, randomised phase 3 trial. Lancet Oncol. 2018, 19, 194–206.
- James, N.D.; de Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351.
- Ryan, C.J.; Smith, M.R.; de Bono, J.S.; Molina, A.; Logothetis, C.J.; de Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Ng, S.; et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 2013, 368, 138–148.
- Clegg, N.J.; Wongvipat, J.; Joseph, J.D.; Tran, C.; Ouk, S.; Dilhas, A.; Chen, Y.; Grillot, K.; Bischoff, E.D.; Cai, L.; et al. ARN-509: A novel antiandrogen for prostate cancer treatment. Cancer Res. 2012, 72, 1494–1503.
- Chi, K.N.; Agarwal, N.; Bjartell, A.; Chung, B.H.; Pereira de Santana Gomes, A.J.; Given, R.; Juárez Soto, Á.; Merseburger, A.S.; Özgüroğlu, M.; Uemura, H.; et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2019, 381, 13–24.
- Antonarakis, E.S.; Lu, C.; Luber, B.; Wang, H.; Chen, Y.; Zhu, Y.; Silberstein, J.L.; Taylor, M.N.; Maughan, B.L.; Denmeade, S.R.; et al. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men with Metastatic Castration-Resistant Prostate Cancer Treated with First- and Second-Line Abiraterone and Enzalutamide. J. Clin. Oncol. 2017, 35, 2149–2156.
- Antonarakis, E.S.; Lu, C.; Wang, H.; Luber, B.; Nakazawa, M.; Roeser, J.C.; Chen, Y.; Mohammad, T.A.; Chen, Y.; Fedor, H.L.; et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 2014, 371, 1028–1038.
- Bastos, D.A.; Antonarakis, E.S. Galeterone for the treatment of advanced prostate cancer: The evidence to date. Drug Des. Dev. Ther. 2016, 10, 2289–2297.
- Taplin, M.E.; Antonarakis, E.S.; Ferrante, K.J.; Horgan, K.; Blumenstein, B.; Saad, F.; Luo, J.; de Bono, J.S. Androgen Receptor Modulation Optimized for Response-Splice Variant: A Phase 3, Randomized Trial of Galeterone versus Enzalutamide in Androgen Receptor Splice Variant-7-expressing Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 76, 843–851.
- Denmeade, S.R. Bipolar androgen therapy in the treatment of prostate cancer. Clin. Adv. Hematol. Oncol. 2018, 16, 408–411.
- Markowski, M.C.; Wang, H.; Sullivan, R.; Rifkind, I.; Sinibaldi, V.; Schweizer, M.T.; Teply, B.A.; Ngomba, N.; Fu, W.; Carducci, M.A.; et al. A Multicohort Open-label Phase II Trial of Bipolar Androgen Therapy in Men with Metastatic Castration-resistant Prostate Cancer (RESTORE): A Comparison of Post-abiraterone Versus Post-enzalutamide Cohorts. Eur. Urol. 2021, 79, 692–699.
- Teply, B.A.; Wang, H.; Luber, B.; Sullivan, R.; Rifkind, I.; Bruns, A.; Spitz, A.; DeCarli, M.; Sinibaldi, V.; Pratz, C.F.; et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: An open-label, phase 2, multicohort study. Lancet Oncol. 2018, 19, 76–86.
- Faris, J.E.; Smith, M.R. Metabolic sequelae associated with androgen deprivation therapy for prostate cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 240–246.
- Saylor, P.J.; Smith, M.R. Metabolic complications of androgen deprivation therapy for prostate cancer. J. Urol. 2013, 189, S34–S42; discussion S43–S44.
- Rothermundt, C.; Hayoz, S.; Templeton, A.J.; Winterhalder, R.; Strebel, R.T.; Bärtschi, D.; Pollak, M.; Lui, L.; Endt, K.; Schiess, R.; et al. Metformin in chemotherapy-naive castration-resistant prostate cancer: A multicenter phase 2 trial (SAKK 08/09). Eur. Urol. 2014, 66, 468–474.
- Li, K.; Si-Tu, J.; Qiu, J.; Lu, L.; Mao, Y.; Zeng, H.; Chen, M.; Lai, C.; Chang, H.J.; Wang, D. Statin and metformin therapy in prostate cancer patients with hyperlipidemia who underwent radiotherapy: A population-based cohort study. Cancer Manag. Res. 2019, 11, 1189–1197.
- Crawley, D.; Chandra, A.; Loda, M.; Gillett, C.; Cathcart, P.; Challacombe, B.; Cook, G.; Cahill, D.; Santa Olalla, A.; Cahill, F.; et al. Metformin and longevity (METAL): A window of opportunity study investigating the biological effects of metformin in localised prostate cancer. BMC Cancer 2017, 17, 494.
- Gillessen, S.; Gilson, C.; James, N.; Adler, A.; Sydes, M.R.; Clarke, N. Repurposing Metformin as Therapy for Prostate Cancer within the STAMPEDE Trial Platform. Eur. Urol. 2016, 70, 906–908.
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512.
- de Bono, J.S.; Oudard, S.; Ozguroglu, M.; Hansen, S.; Machiels, J.P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154.
- Climent, M.; Pérez-Valderrama, B.; Mellado, B.; Fernández Parra, E.M.; Fernández Calvo, O.; Ochoa de Olza, M.; Muinelo Romay, L.; Anido, U.; Domenech, M.; Hernando Polo, S.; et al. Weekly cabazitaxel plus prednisone is effective and less toxic for ‘unfit’ metastatic castration-resistant prostate cancer: Phase II Spanish Oncology Genitourinary Group (SOGUG) trial. Eur. J. Cancer 2017, 87, 30–37.
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 149–158.
- Sweeney, C.J.; Chen, Y.H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746.
- Kyriakopoulos, C.E.; Chen, Y.H.; Carducci, M.A.; Liu, G.; Jarrard, D.F.; Hahn, N.M.; Shevrin, D.H.; Dreicer, R.; Hussain, M.; Eisenberger, M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J. Clin. Oncol. 2018, 36, 1080–1087.
- Clarke, N.W.; Ali, A.; Ingleby, F.C.; Hoyle, A.; Amos, C.L.; Attard, G.; Brawley, C.D.; Calvert, J.; Chowdhury, S.; Cook, A.; et al. Corrigendum to Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: Long-term survival results from the STAMPEDE trial. Ann. Oncol. 2020, 31, 442.
- Smith, M.R.; Hussain, M.; Saad, F.; Fizazi, K.; Sternberg, C.N.; Crawford, E.D.; Kopyltsov, E.; Park, C.H.; Alekseev, B.; Montesa-Pino, Á.; et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2022, 386, 1132–1142.
- Fizazi, K.; Foulon, S.; Carles, J.; Roubaud, G.; McDermott, R.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; Ronchin, P.; et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2022, 399, 1695–1707.
- Sweeney, C.J.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Cheung, L.; Chi, K.N.; Chowdhury, S.; Frydenberg, M.; Horvath, L.G.; Joshua, A.M.; et al. Testosterone suppression plus enzalutamide versus testosterone suppression plus standard antiandrogen therapy for metastatic hormone-sensitive prostate cancer (ENZAMET): An international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 323–334.
- Davis, I.D.; Martin, A.J.; Stockler, M.R.; Begbie, S.; Chi, K.N.; Chowdhury, S.; Coskinas, X.; Frydenberg, M.; Hague, W.E.; Horvath, L.G.; et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N. Engl. J. Med. 2019, 381, 121–131.
- Riaz, I.B.; Naqvi, S.A.A.; He, H.; Asghar, N.; Siddiqi, R.; Liu, H.; Singh, P.; Childs, D.S.; Ravi, P.; Hussain, S.A.; et al. First-line Systemic Treatment Options for Metastatic Castration-Sensitive Prostate Cancer: A Living Systematic Review and Network Meta-analysis. JAMA Oncol. 2023, 9, 635–645.
- Zustovich, F.; Pastorelli, D. Therapeutic management of bone metastasis in prostate cancer: An update. Expert Rev. Anticancer Ther. 2016, 16, 1199–1211.
- Lassemillante, A.C.; Doi, S.A.; Hooper, J.D.; Prins, J.B.; Wright, O.R. Prevalence of osteoporosis in prostate cancer survivors II: A meta-analysis of men not on androgen deprivation therapy. Endocrine 2015, 50, 344–354.
- Lee, H.; McGovern, K.; Finkelstein, J.S.; Smith, M.R. Changes in bone mineral density and body composition during initial and long-term gonadotropin-releasing hormone agonist treatment for prostate carcinoma. Cancer 2005, 104, 1633–1637.
- Walsh, P.C. Risk of fracture after androgen deprivation for prostate cancer. J. Urol. 2005, 174, 929–930.
- Finianos, A.; Aragon-Ching, J.B. Zoledronic acid for the treatment of prostate cancer. Expert Opin. Pharmacother. 2019, 20, 657–666.
- Wang, F.; Chen, W.; Chen, H.; Mo, L.; Jin, H.; Yu, Z.; Li, C.; Liu, Q.; Duan, F.; Weng, Z. Comparison between zoledronic acid and clodronate in the treatment of prostate cancer patients with bone metastases. Med. Oncol. 2013, 30, 657.
- Kamba, T.; Kamoto, T.; Maruo, S.; Kikuchi, T.; Shimizu, Y.; Namiki, S.; Fujimoto, K.; Kawanishi, H.; Sato, F.; Narita, S.; et al. A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: Results of the ZAPCA trial. Int. J. Clin. Oncol. 2017, 22, 166–173.
- Smith, M.R.; Halabi, S.; Ryan, C.J.; Hussain, A.; Vogelzang, N.; Stadler, W.; Hauke, R.J.; Monk, J.P.; Saylor, P.; Bhoopalam, N.; et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: Results of CALGB 90202 (alliance). J. Clin. Oncol. 2014, 32, 1143–1150.
- Denham, J.W.; Joseph, D.; Lamb, D.S.; Spry, N.A.; Duchesne, G.; Matthews, J.; Atkinson, C.; Tai, K.H.; Christie, D.; Kenny, L.; et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol 2019, 20, 267–281.
- James, N.D.; Pirrie, S.J.; Pope, A.M.; Barton, D.; Andronis, L.; Goranitis, I.; Collins, S.; Daunton, A.; McLaren, D.; O’Sullivan, J.; et al. Clinical Outcomes and Survival Following Treatment of Metastatic Castrate-Refractory Prostate Cancer with Docetaxel Alone or With Strontium-89, Zoledronic Acid, or Both: The TRAPEZE Randomized Clinical Trial. JAMA Oncol. 2016, 2, 493–499.
- Wirth, M.; Tammela, T.; Cicalese, V.; Gomez Veiga, F.; Delaere, K.; Miller, K.; Tubaro, A.; Schulze, M.; Debruyne, F.; Huland, H.; et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: Efficacy and safety results of the Zometa European Study (ZEUS). Eur. Urol. 2015, 67, 482–491.
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282.
- Okegawa, T.; Higaki, M.; Matsumoto, T.; Kase, H.; Murata, A.; Noda, K.; Noda, H.; Asaoka, H.; Oshi, M.; Tomoishi, J.; et al. Zoledronic acid improves clinical outcomes in patients with bone metastatic hormone-naïve prostate cancer in a multicenter clinical trial. Anticancer Res. 2014, 34, 4415–4420.
- Nozawa, M.; Inagaki, T.; Nagao, K.; Nishioka, T.; Komura, T.; Esa, A.; Kitagawa, M.; Imanishi, M.; Uekado, Y.; Ogawa, T.; et al. Phase II trial of zoledronic acid combined with androgen-deprivation therapy for treatment-naïve prostate cancer with bone metastasis. Int. J. Clin. Oncol. 2014, 19, 693–701.
- Saad, F.; Segal, S.; Eastham, J. Prostate-specific antigen kinetics and outcomes in patients with bone metastases from castration-resistant prostate cancer treated with or without zoledronic acid. Eur. Urol. 2014, 65, 146–153.
- Joseph, J.S.; Lam, V.; Patel, M.I. Preventing Osteoporosis in Men Taking Androgen Deprivation Therapy for Prostate Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. Oncol. 2019, 2, 551–561.
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176.
- Lipton, A.; Fizazi, K.; Stopeck, A.T.; Henry, D.H.; Smith, M.R.; Shore, N.; Martin, M.; Vadhan-Raj, S.; Brown, J.E.; Richardson, G.E.; et al. Effect of denosumab versus zoledronic acid in preventing skeletal-related events in patients with bone metastases by baseline characteristics. Eur. J. Cancer 2016, 53, 75–83.
- Himelstein, A.L.; Foster, J.C.; Khatcheressian, J.L.; Roberts, J.D.; Seisler, D.K.; Novotny, P.J.; Qin, R.; Go, R.S.; Grubbs, S.S.; O’Connor, T.; et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients with Bone Metastases: A Randomized Clinical Trial. JAMA 2017, 317, 48–58.
- Clemons, M.; Liu, M.; Stober, C.; Pond, G.; Jemaan Alzahrani, M.; Ong, M.; Ernst, S.; Booth, C.; Mates, M.; Abraham Joy, A.; et al. Two-year results of a randomised trial comparing 4- versus 12-weekly bone-targeted agent use in patients with bone metastases from breast or castration-resistant prostate cancer. J. Bone Oncol. 2021, 30, 100388.
More
Information
Subjects:
Urology & Nephrology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
687
Revisions:
2 times
(View History)
Update Date:
17 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




