
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Md. Biddut Hossain | -- | 3352 | 2024-02-07 04:24:05 | | | |
| 2 | Peter Tang | Meta information modification | 3352 | 2024-02-07 06:33:48 | | |
Video Upload Options
Deep learning (DL) has been applied successfully in medical imaging such as reconstruction, classification, segmentation, and detection.
1. Introduction
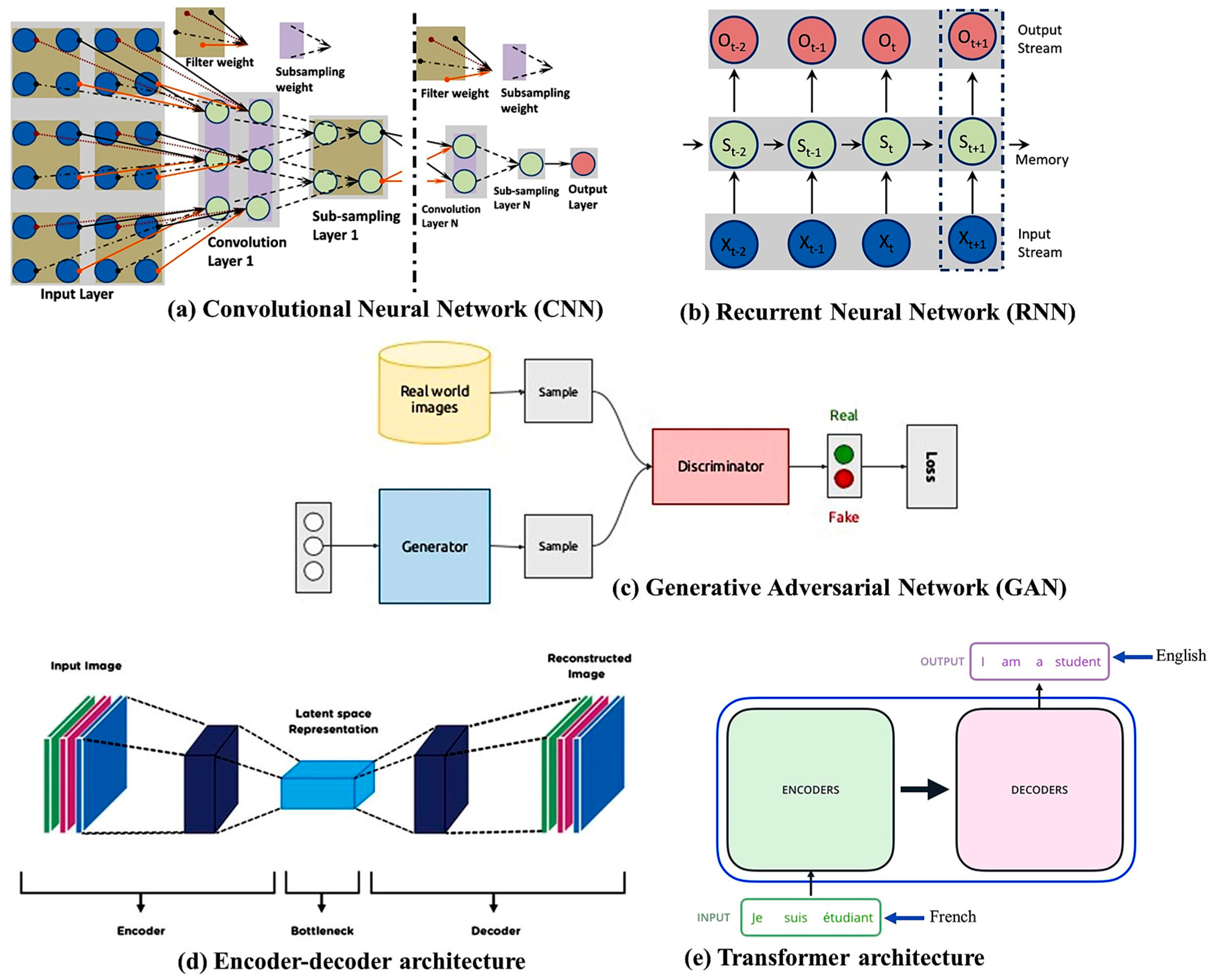
2. DL Architectures
3. DL Tools
|
Ref. |
Tool Name |
Description |
|---|---|---|
|
[41] |
Deeplearning4j |
Distributed deep learning library that allows for training models on Java interoperating with the Python environment. |
|
[42] |
Julia |
A flexible and dynamic framework that is more suitable for scientific and numerical computing. |
|
[43] |
Keras |
A Python-based library that is integrated with TensorFlow and used in different ML algorithms. |
|
[44] |
MatConvNet |
A MATLAB toolbox used for image reconstruction, segmentation, and classification by CNN. |
|
[45] |
MS cognitive toolkit |
Describes DNNs as a series of computationally directed graphs, where leaf nodes represent input parameters and other nodes indicate matrix operation. |
|
[46] |
Neural designer |
Data mining tool that was developed by the Artelnics company used in NNs. |
|
[47] |
PyTorch |
Developed by Facebook, works on complex data and is easy to learn. |
|
[48] |
Scikit-image |
Applied for histogram equalization of the input images on various image processing algorithms. |
|
[49] |
Sigpy |
The signal processing package operates on multi-dimensional array plotting and MRI reconstruction. |
|
[50] |
TensorFlow |
Open-source Python framework developed by Google Brain Team that is the most used tool for developing deep learning models. |
|
[51] |
TensorFlow Federated (TFF) |
An open-source framework developed by Google, TFF provides tools for FL. It allows developers to implement federated models and train them across distributed devices. |
|
[52] |
PySyft |
PySyft is a flexible and powerful library for encrypted privacy-preserving ML. It extends PyTorch and TensorFlow to enable the security of FL. |
|
[53] |
Substra |
In 2016, a multi-partner research project developed this FL framework. It concentrates on the medical industry to protect patient privacy and data ownership. It is currently utilized by the pharmaceutical industry for drug discovery. |
4. Network Training Strategies
4.1. Supervised and Unsupervised Learning
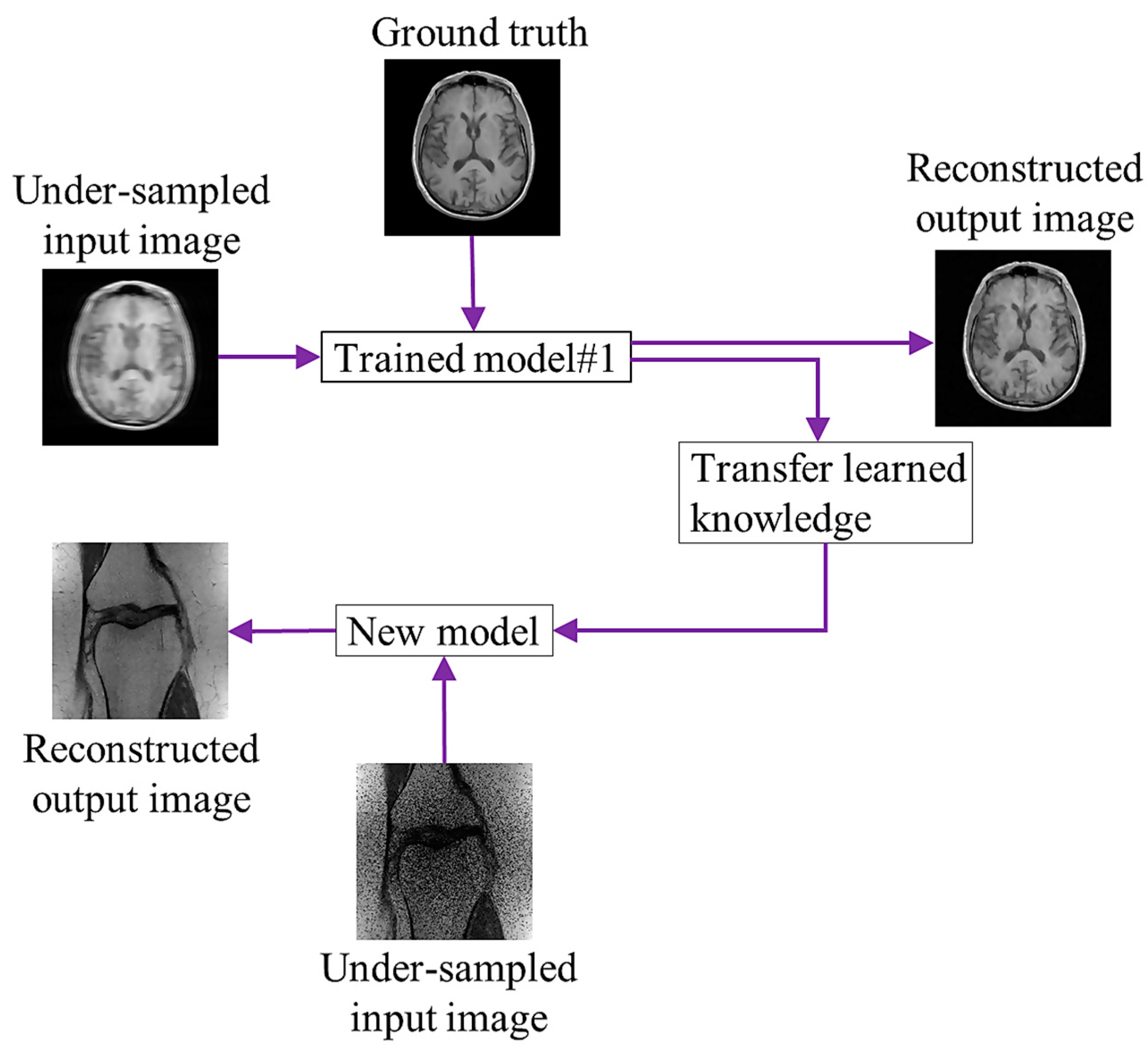
4.2. Transfer Learning
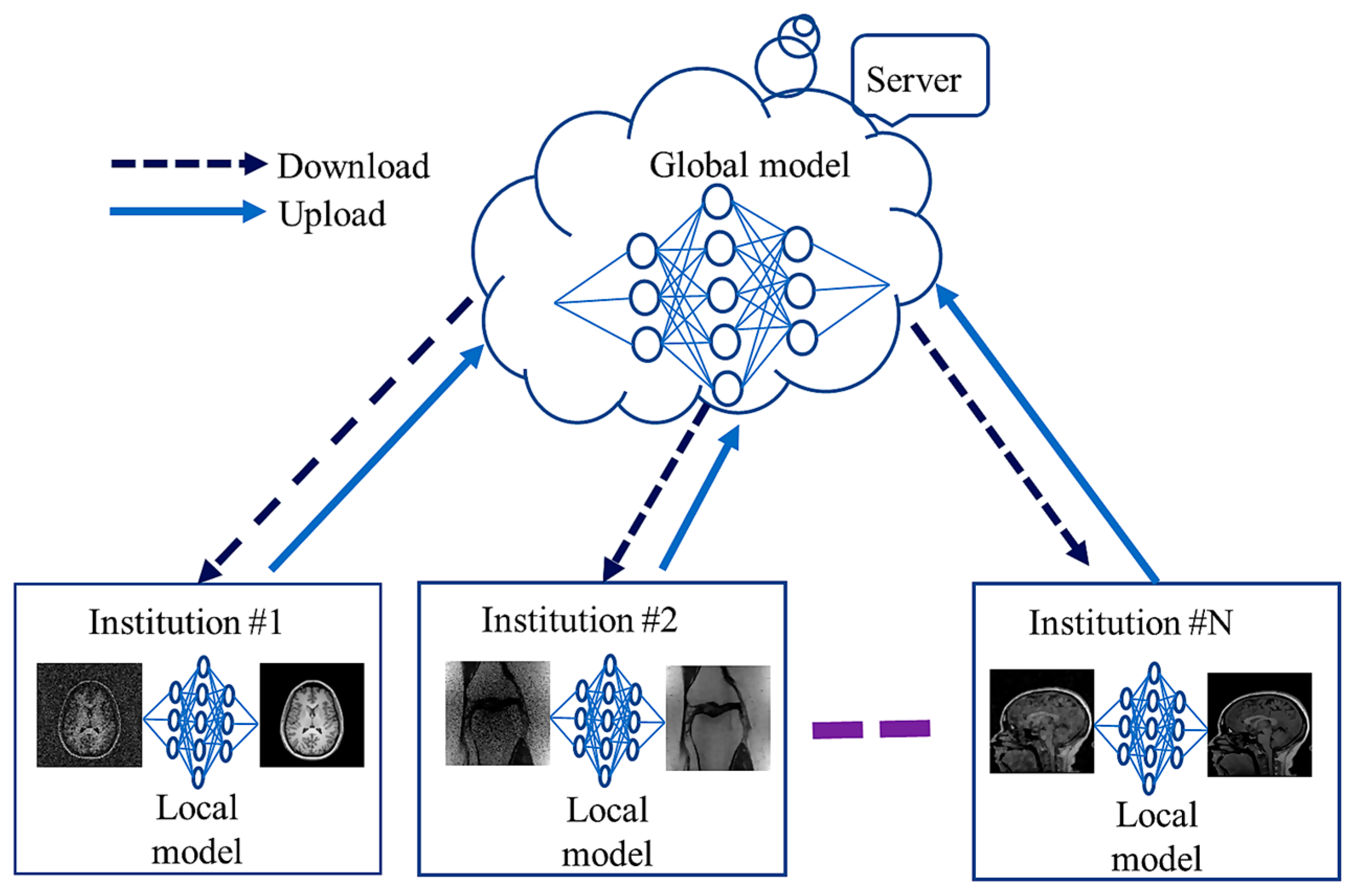
4.3. Federated Learning
References
- Brown, R.W.; Cheng, Y.-C.N.; Haacke, E.M.; Thompson, M.R.; Venkatesan, R. Magnetic Resonance Imaging: Physical Principles and Sequence Design, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2014; ISBN 9781118633953.
- Cercignani, M.; Dowell, N.G.; Tofts, P.S. Quantitative MRI of the Brain: Principles of Physical Measurement; CRC Press: Boca Raton, FL, USA, 2018; Volume 15, ISBN 9781315363578.
- Muckley, M.J.; Riemenschneider, B.; Radmanesh, A.; Kim, S.; Jeong, G.; Ko, J.; Jun, Y.; Shin, H.; Hwang, D.; Mostapha, M.; et al. Results of the 2020 FastMRI Challenge for Machine Learning MR Image Reconstruction. IEEE Trans. Med. Imaging 2021, 40, 2306–2317.
- Deshmane, A.; Gulani, V.; Griswold, M.A.; Seiberlich, N. Parallel MR Imaging. J. Magn. Reson. Imaging 2012, 36, 55–72.
- Lustig, M.; Donoho, D. Compressed Sensing MRI. Signal Process. Mag. 2008, 25, 72–82.
- Griswold, M.A.; Jakob, P.M.; Heidemann, R.M.; Nittka, M.; Jellus, V.; Wang, J.; Kiefer, B.; Haase, A. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn. Reson. Med. 2002, 47, 1202–1210.
- Pruessmann, K.P.; Weiger, M.; Scheidegger, M.B.; Boesiger, P. SENSE: Sensitivity Encoding for Fast MRI. Magn. Reson. Med. 1999, 42, 952–962.
- Hu, Z.; Zhao, C.; Zhao, X.; Kong, L.; Yang, J.; Wang, X.; Liao, J.; Zhou, Y. Joint Reconstruction Framework of Compressed Sensing and Nonlinear Parallel Imaging for Dynamic Cardiac Magnetic Resonance Imaging. BMC Med. Imaging 2021, 21, 182.
- Islam, R.; Islam, M.S.; Uddin, M.S. Compressed Sensing in Parallel MRI: A Review. Int. J. Image Graph. 2022, 22, 2250038.
- Lee, J.-G.; Jun, S.; Cho, Y.-W.; Lee, H.; Kim, G.B.; Seo, J.B.; Kim, N. Deep Learning in Medical Imaging: General Overview. Korean J. Radiol. 2017, 18, 570–584.
- Zhang, Y.; Gorriz, J.M.; Dong, Z. Deep Learning in Medical Image Analysis. J. Imaging 2021, 7, 74.
- Hossain, M.B.; Kwon, K.-C.; Shinde, R.K.; Imtiaz, S.M.; Kim, N. A Hybrid Residual Attention Convolutional Neural Network for Compressed Sensing Magnetic Resonance Image Reconstruction. Diagnostics 2023, 13, 1306.
- Badža, M.M.; Barjaktarović, M.C. Classification of Brain Tumors from Mri Images Using a Convolutional Neural Network. Appl. Sci. 2020, 10, 1999.
- Zhao, C.; Xiang, S.; Wang, Y.; Cai, Z.; Shen, J.; Zhou, S.; Zhao, D.; Su, W.; Guo, S.; Li, S. Context-Aware Network Fusing Transformer and V-Net for Semi-Supervised Segmentation of 3D Left Atrium. Expert Syst. Appl. 2023, 214, 119105.
- Kim, S.; Park, S.; Na, B.; Yoon, S. Spiking-YOLO: Spiking Neural Network for Energy-Efficient Object Detection. Proc. AAAI Conf. Artif. Intell. 2020, 34, 11270–11277.
- Ahishakiye, E.; Van Gijzen, M.B.; Tumwiine, J.; Wario, R.; Obungoloch, J. A Survey on Deep Learning in Medical Image Reconstruction. Intell. Med. 2021, 1, 118–127.
- Montalt-Tordera, J.; Muthurangu, V.; Hauptmann, A.; Steeden, J.A. Machine Learning in Magnetic Resonance Imaging: Image Reconstruction. Phys. Medica 2021, 83, 79–87.
- Zhang, H.M.; Dong, B. A Review on Deep Learning in Medical Image Reconstruction. J. Oper. Res. Soc. China 2020, 8, 311–340.
- He, Z.; Quan, C.; Wang, S.; Zhu, Y.; Zhang, M.; Zhu, Y.; Liu, Q. A Comparative Study of Unsupervised Deep Learning Methods for MRI Reconstruction. Investig. Magn. Reson. Imaging 2020, 24, 179.
- Knoll, F.; Hammernik, K.; Zhang, C.; Moeller, S.; Pock, T.; Sodickson, D.K.; Akcakaya, M. Deep-Learning Methods for Parallel Magnetic Resonance Imaging Reconstruction: A Survey of the Current Approaches, Trends, and Issues. IEEE Signal Process. Mag. 2020, 37, 128–140.
- O’Shea, K.; Nash, R. An Introduction to Convolutional Neural Networks. arXiv 2015, arXiv:1511.08458.
- Khan, A.; Sohail, A.; Zahoora, U.; Qureshi, A.S. A Survey of the Recent Architectures of Deep Convolutional Neural Networks. Artif. Intell. Rev. 2020, 53, 5455–5516.
- Krizhevsky, A.; Sutskever, I.; Hinton, G.E. ImageNet Classification with Deep Convolutional Neural Networks. Adv. Neural Inf. Process. Syst. 2012, 25, 145–151.
- Shafiq, M.; Gu, Z. Deep Residual Learning for Image Recognition: A Survey. Appl. Sci. 2022, 12, 8972.
- Shinde, R.K.; Alam, S.; Hossain, B.; Imtiaz, S.; Kim, J. Squeeze-MNet: Precise Skin Cancer Detection Model for Low Computing IoT Devices Using Transfer Learning. Cancers 2023, 14, 12.
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation; Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 2015; Volume 9351, pp. 234–241. ISBN 9783319245737.
- Ravi, D.; Wong, C.; Deligianni, F.; Berthelot, M.; Andreu-Perez, J.; Lo, B.; Yang, G.-Z. Deep Learning for Health Informatics. IEEE J. Biomed. Health Inform. 2017, 21, 4–21.
- Sherstinsky, A. Fundamentals of Recurrent Neural Network (RNN) and Long Short-Term Memory (LSTM) Network. Phys. D Nonlinear Phenom. 2020, 404, 132306.
- Ramadevi, R.; Marshiana, D.; Bestley, J.S.; Jamuna, R.D. Recurrent Neural Network (RNN) Analysis for Brain Tumor Classification Using Decision Tree Classifiers. J. Crit. Rev. 2020, 7, 2202–2205.
- Alam, M.S.; Kwon, K.-C.; Md Imtiaz, S.; Hossain, M.B.; Kang, B.-G.; Kim, N. TARNet: An Efficient and Lightweight Trajectory-Based Air-Writing Recognition Model Using a CNN and LSTM Network. Hum. Behav. Emerg. Technol. 2022, 2022, 6063779.
- Creswell, A.; White, T.; Dumoulin, V.; Arulkumaran, K.; Sengupta, B.; Bharath, A.A. Generative Adversarial Networks: An Overview. IEEE Signal Process. Mag. 2018, 35, 53–65.
- Yoon, J.; Jordon, J.; Van Der Schaar, M. Supplementary Materials—RadialGAN: Leveraging Multiple Datasets to Improve Target-Specific Predictive Models Using Generative Adversarial Networks. Int. Conf. Mach. Learn. ICML 2018, 13, 9069–9071.
- Choi, Y.; Choi, M.; Kim, M.; Ha, J.W.; Kim, S.; Choo, J. StarGAN: Unified Generative Adversarial Networks for Multi-Domain Image-to-Image Translation. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 8789–8797.
- Asadi, A.; Safabakhsh, R. The Encoder-Decoder Framework and Its Applications. In Deep Learning: Concepts and Architectures; Springer: Berlin/Heidelberg, Germany, 2020; pp. 133–167.
- Zhai, J.; Zhang, S.; Chen, J.; He, Q. Autoencoder and Its Various Variants. In Proceedings of the 2018 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Miyazaki, Japan, 7–10 October 2018; pp. 415–419.
- Kingma, D.P.; Welling, M. An Introduction to Variational Autoencoders. Found. Trends Mach. Learn. 2019, 12, 307–392.
- Patwardhan, N.; Marrone, S.; Sansone, C. Transformers in the Real World: A Survey on NLP Applications. Information 2023, 14, 242.
- Carion, N.; Massa, F.; Synnaeve, G.; Usunier, N.; Kirillov, A.; Zagoruyko, S. End-to-End Object Detection with Transformers. In Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2020; Volume 12346, pp. 213–229.
- Dosovitskiy, A.; Beyer, L.; Kolesnikov, A.; Weissenborn, D.; Zhai, X.; Unterthiner, T.; Dehghani, M.; Minderer, M.; Heigold, G.; Gelly, S.; et al. An Image Is Worth 16x16 Words: Transformers for Image Recognition at Scale. arXiv 2020, arXiv:2010.11929.
- Huang, J.; Wu, Y.; Wu, H.; Yang, G. Fast MRI Reconstruction: How Powerful Transformers Are? In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 2066–2070.
- Deeplearning4j. Available online: https://deeplearning4j.org/ (accessed on 4 July 2021).
- Julia. Available online: https://julialang.org/ (accessed on 4 July 2021).
- Keras. Available online: https://keras.io/ (accessed on 5 July 2021).
- MatConvNet. Available online: https://www.vlfeat.org/matconvnet/ (accessed on 5 July 2021).
- MS Cognitive Toolkit (CNTK). Available online: https://docs.microsoft.com/en-us/cognitive-toolkit/ (accessed on 5 July 2021).
- Neural Designer. Available online: https://www.neuraldesigner.com/ (accessed on 5 July 2021).
- PyTorch. Available online: https://pytorch.org/ (accessed on 6 July 2021).
- Scikit-Image. Available online: https://scikit-image.org/ (accessed on 6 July 2021).
- Sigpy. Available online: https://sigpy.readthedocs.io/en/latest/ (accessed on 6 July 2021).
- TensorFlow. Available online: https://www.tensorflow.org/ (accessed on 6 July 2021).
- TensorFlow Federated (TFF). Available online: https://www.tensorflow.org/federated (accessed on 15 November 2023).
- PySyft. Available online: https://blog.openmined.org/tag/pysyft/ (accessed on 20 November 2023).
- Substra. Available online: https://www.substra.ai/ (accessed on 10 December 2023).
- Ghahramani, Z. Unsupervised Learning. In Summer School on Machine Learning; Springer: Berlin/Heidelberg, Germany, 2004; pp. 72–112.
- Gong, K.; Han, P.; El Fakhri, G.; Ma, C.; Li, Q. Arterial Spin Labeling MR Image Denoising and Reconstruction Using Unsupervised Deep Learning. NMR Biomed. 2022, 35, e4224.
- Aggarwal, H.K.; Pramanik, A.; John, M.; Jacob, M. ENSURE: A General Approach for Unsupervised Training of Deep Image Reconstruction Algorithms. IEEE Trans. Med. Imaging 2023, 42, 1133–1144.
- Wei, R.; Chen, J.; Liang, B.; Chen, X.; Men, K.; Dai, J. Real-time 3D MRI Reconstruction from Cine-MRI Using Unsupervised Network in MRI-guided Radiotherapy for Liver Cancer. Med. Phys. 2023, 50, 3584–3596.
- Yurt, M.; Dalmaz, O.; Dar, S.; Ozbey, M.; Tinaz, B.; Oguz, K.; Cukur, T. Semi-Supervised Learning of MRI Synthesis without Fully-Sampled Ground Truths. IEEE Trans. Med. Imaging 2022, 41, 3895–3906.
- Hu, C.; Li, C.; Wang, H.; Liu, Q.; Zheng, H.; Wang, S. Self-Supervised Learning for MRI Reconstruction with a Parallel Network Training Framework. In Medical Image Computing and Computer Assisted Intervention—MICCAI 2021; Springer: Cham, Switzerland, 2021; pp. 382–391.
- Torrey, L.; Shavlik, J. Transfer Learning. In Handbook of Research on Machine Learning Applications and Trends; IGI Global: Hershey, PA, USA, 2010; pp. 242–264.
- Dar, S.U.H.; Özbey, M.; Çatlı, A.B.; Çukur, T. A Transfer-Learning Approach for Accelerated MRI Using Deep Neural Networks. Magn. Reson. Med. 2020, 84, 663–685.
- Arshad, M.; Qureshi, M.; Inam, O.; Omer, H. Transfer Learning in Deep Neural Network Based Under-Sampled MR Image Reconstruction. Magn. Reson. Imaging 2021, 76, 96–107.
- Lv, J.; Li, G.; Tong, X.; Chen, W.; Huang, J.; Wang, C.; Yang, G. Transfer Learning Enhanced Generative Adversarial Networks for Multi-Channel MRI Reconstruction. Comput. Biol. Med. 2021, 134, 104504.
- Yaqub, M.; Jinchao, F.; Ahmed, S.; Arshid, K.; Bilal, M.A.; Akhter, M.P.; Zia, M.S. GAN-TL: Generative Adversarial Networks with Transfer Learning for MRI Reconstruction. Appl. Sci. 2022, 12, 8841.
- Park, S.J.; Ahn, C.-B. Blended-Transfer Learning for Compressed-Sensing Cardiac CINE MRI. Investig. Magn. Reson. Imaging 2021, 25, 10.
- Cheng, C.; Lin, D. MRI Reconstruction Based on Transfer Learning Dynamic Dictionary Algorithm. In Proceedings of the 2023 2nd International Conference on Big Data, Information and Computer Network (BDICN), Xishuangbanna, China, 6–8 January 2023; pp. 1–4.
- Gulamhussene, G.; Rak, M.; Bashkanov, O.; Joeres, F.; Omari, J.; Pech, M.; Hansen, C. Transfer-Learning Is a Key Ingredient to Fast Deep Learning-Based 4D Liver MRI Reconstruction. Sci. Rep. 2023, 13, 11227.
- Yang, Q.; Liu, Y.; Cheng, Y.; Kang, Y.; Chen, T.; Yu, H. Federated Learning; Synthesis Lectures on Artificial Intelligence and Machine Learning Series; Springer: Cham, Switzerland, 2019; Volume 13, pp. 1–207.
- Li, X.; Gu, Y.; Dvornek, N.; Staib, L.H.; Ventola, P.; Duncan, J.S. Multi-Site FMRI Analysis Using Privacy-Preserving Federated Learning and Domain Adaptation: ABIDE Results. Med. Image Anal. 2020, 65, 101765.
- Guo, P.; Wang, P.; Zhou, J.; Jiang, S.; Patel, V.M. Multi-Institutional Collaborations for Improving Deep Learning-Based Magnetic Resonance Image Reconstruction Using Federated Learning. In Proceedings of the 2021 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Nashville, TN, USA, 20–25 June 2021; pp. 2423–2432.
- Feng, C.M.; Yan, Y.; Wang, S.; Xu, Y.; Shao, L.; Fu, H. Specificity-Preserving Federated Learning for MR Image Reconstruction. IEEE Trans. Med. Imaging 2022, 26, 2010–2021.
- Elmas, G.; Dar, S.U.; Korkmaz, Y.; Ceyani, E.; Susam, B.; Ozbey, M.; Avestimehr, S.; Cukur, T. Federated Learning of Generative Image Priors for MRI Reconstruction. IEEE Trans. Med. Imaging 2022, 9, 1996–2009.
- Levac, B.R.; Arvinte, M.; Tamir, J.I. Federated End-to-End Unrolled Models for Magnetic Resonance Image Reconstruction. Bioengineering 2023, 10, 364.
- Feng, C.-M.; Li, B.; Xu, X.; Liu, Y.; Fu, H.; Zuo, W. Learning Federated Visual Prompt in Null Space for MRI Reconstruction. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Vancouver, BC, Canada, 18–22 June 2023.
- Sandhu, S.S.; Gorji, H.T.; Tavakolian, P.; Tavakolian, K.; Akhbardeh, A. Medical Imaging Applications of Federated Learning. Diagnostics 2023, 13, 3140.
- Li, T.; Sahu, A.K.; Talwalkar, A.; Smith, V. Federated Learning: Challenges, Methods, and Future Directions. IEEE Signal Process. Mag. 2020, 37, 50–60.




