Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Josimar Ribeiro | -- | 2349 | 2024-02-05 15:52:13 | | | |
| 2 | Wendy Huang | + 1 word(s) | 2350 | 2024-02-07 07:41:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Barcelos, M.M.; Vasconcellos, M.D.L.S.; Ribeiro, J. Electrocatalysts for CO2 Reduction. Encyclopedia. Available online: https://encyclopedia.pub/entry/54773 (accessed on 07 February 2026).
Barcelos MM, Vasconcellos MDLS, Ribeiro J. Electrocatalysts for CO2 Reduction. Encyclopedia. Available at: https://encyclopedia.pub/entry/54773. Accessed February 07, 2026.
Barcelos, Marcela Miranda, Maria De Lourdes Soprani Vasconcellos, Josimar Ribeiro. "Electrocatalysts for CO2 Reduction" Encyclopedia, https://encyclopedia.pub/entry/54773 (accessed February 07, 2026).
Barcelos, M.M., Vasconcellos, M.D.L.S., & Ribeiro, J. (2024, February 05). Electrocatalysts for CO2 Reduction. In Encyclopedia. https://encyclopedia.pub/entry/54773
Barcelos, Marcela Miranda, et al. "Electrocatalysts for CO2 Reduction." Encyclopedia. Web. 05 February, 2024.
Copy Citation
Given the environmental problems caused by burning fossil fuels, it is believed that converting carbon dioxide (CO2) into chemical inputs is a great ally to generating clean energy. In this way, investigative studies related to electrochemical CO2 reduction (CO2RE) concerning the behavior of metal catalysts have received attention about the processes involved. CO2RE can be an important tool to mitigate the presence of this gas in the Earth’s atmosphere.
CO2
electrochemical
reduction
electrocatalysts
electrode
1. Introduction
Over the years, there has been an increase in the use of fossil fuels (oil, natural gas, and coal), which are responsible for the greenhouse effect [1][2][3]. Recent research, presented by Mikkelsen, Jørgensen, and Krebs, points out that the atmospheric concentration of greenhouse gases, including CO2, nitrous oxide (N2O), methane (CH4), and chlorofluorocarbons (CFCs), reveals an excess of approximately 3.9% in relation to the natural cycle carbon [4].
CO2 is naturally present in the atmosphere in quantities less than 0.035%, being essential to the life of the planet as it is one of the fundamental elements for carrying out photosynthesis, the process in which photosynthetic organisms transform solar energy into chemical energy, absorbing atmospheric CO2 and transforming it into glucose and releasing oxygen (O2), thus contributing to the chemical energy of living beings [5].
CO2 can be captured directly from industrial sources through three methods: post-combustion capture, pre-combustion capture, and combustion of fossil fuels in a pure oxygen environment. Post-combustion capture, which uses chemical solvents such as monoethanolamine, separates CO2 from the exhaust gas, which is composed mainly of a mixture of N2 and CO2 [6]. Researchers are directing efforts to reduce costs and improve the efficiency of post-combustion capture. This involves exploring more effective chemical solvents and membranes for separating CO2 from N2. At the same time, materials are being developed to reduce investment costs in large separation equipment required for industrial-scale capture. Research also focuses on the development of new materials capable of resisting higher temperatures and pressures, aiming to improve efficiency in energy generation with CO2 capture [6].
One of the alternatives for reducing CO2 emissions into the atmosphere is the conversion of atmospheric CO2 into low-carbon fuels. It can be used as an energy storage carrier, demonstrating great importance in alleviating energy shortages and global environmental pollution [7]. Currently, several methods for CO2 conversion are being investigated, such as photocatalytic reduction, electrocatalytic reduction, and photoelectrocatalytic CO2 reduction. Photocatalytic reduction of CO2, similar to the photosynthetic process in plants, seeks to convert atmospheric CO2 into oxygen, a function performed by green plants and photosynthetic bacteria. Over the years, scientists have developed and designed several types of photocatalysts, covering metal oxides, metal nitrides, metal phosphides, and semiconductors, among other materials [7]. Although there are a variety of photocatalytic materials, their practical efficiency has not yet reached an ideal level. Given this, researchers have explored techniques to improve photocatalytic performance, involving control of morphology and size, manipulation of the crystalline face, doping, application of noble metals, recombination of semiconductors, sensitization by dyes, and introduction of defects, among other approaches [7].
The other two methods to reduce carbon dioxide emissions are electrocatalytic reduction of CO2 and photoelectrocatalytic reduction of CO2. The first method is the process that uses the external electric field as the main source of energy to induce the redox reaction in the electrodes and the photoelectrocatalytic reduction of CO2, as the two previous techniques present limitations for their application. Another method is the photoelectrocatalytic reduction of CO2, which refers to the process in which the semiconductor photoelectrode generates electrons by photoexcitation, and then electrons migrate to the electrode surface under the guidance of an external voltage to carry out the catalytic reduction of CO2 [7].
Concerning other processes that involve CO2 capture, it is adsorption, because a good adsorbent should present high selectivity, high adsorption capacity at low pressure, fast adsorption/desorption kinetics, good mechanical properties, high hydrothermal and chemical stability, high regeneration capacity, and low synthesis costs. Some examples that have been studied are zeolites, metal-organic frameworks (MOFs), and carbon-based adsorbents [8].
CO2 captured from various sources can be reused in a process known as carbon capture and utilization. This not only reduces the CO2 concentration in the atmosphere but also reduces dependence on fossil fuel raw materials. Research focuses on CO2 storage and capture techniques, particularly the effective and long-term use of catalysts in various CO2 conversion reactions. Transforming this pollutant into value-added products represents a significant challenge, but it also offers many opportunities to reduce CO2 emissions [8].
Furthermore, other approaches to dealing with CO2 include capturing and storing it or converting it into valuable chemicals. Another method that is being extensively studied and researched is the recycling of the CO2 molecules by electrochemical reduction [9]. The electrochemical route is the most promising of the alternatives available for CO2 reduction, as it does not require high temperatures or high pressures for efficient reduction, uses water as a source of protons, and allows greater product selectivity than that obtained with other reduction methods. In addition to having greater operational flexibility, it can be easily installed in places with difficult access and/or the availability of cheap energy [10][11][12].
2. Electrocatalysts Types in the Electrochemical Reduction of CO2
An electrocatalyst participates in an electron transfer reaction (at an electrode) and facilitates the acceleration of a chemical reaction [13][14]. Electron transfer reactions are the most important processes at electrochemical interfaces. They are determined by the interaction between the interaction of the reagent with the solvent and the electronic levels of the electrode surface. Both electron transfer and kinetic chemistry must be fast for an efficient electrocatalyst [15]. Furthermore, an ideal electrocatalyst must present a good thermodynamic correspondence between the redox potential for the electron transfer reaction and the chemical reaction being catalyzed (e.g., reduction of CO2 to CO) [13][14].
The effectiveness of the electrochemical reduction of CO2 is directly linked to the electrocatalyst, making it possible to adjust its activity and selectivity by modifying its structure. To improve electrocatalysts, two engineering protocols are widely employed: (1) increase the number of active sites on an electrode, exposing more active sites per gram through optimization of the catalyst structure; and (2) improve the intrinsic activity of each active site. These strategies are not mutually exclusive and can be combined to achieve significant improvements. Several approaches, such as nanostructures, the use of supports, modeling, alloy formation, and doping with heteroatoms, among others, are used in the manufacture of high-performance catalysts [16].
The electrocatalyst used and the applied potential electrode have a great influence on the final reduction products [10][11][17]. Metallic electrodes, such as Cu, Au, and Sn, have been extensively explored in recent decades for CO2 reduction. The generation of intermediate CO2 is crucial to the rate of CO2 reduction in most cases. Therefore, the main function of these electrocatalysts is to stabilize this intermediate to achieve high energy efficiency in reducing CO2. Metal electrodes can be classified into three groups, depending on the binding tendency of intermediates and final products, as shown in Figure 1. Group I has difficulty binding to the CO2 intermediate, resulting in the formation of formate or formic acid by an outer sphere mechanism. Group II, the intermediate *CO obtained, is weakly bound to the metal surface, being easily dissolved and emerging as the predominant product. Group III, represented only by Cu, is capable of binding and converting the *CO intermediate into higher value-added products, such as hydrocarbons and alcohols, through *COH or *CHO intermediates [18].
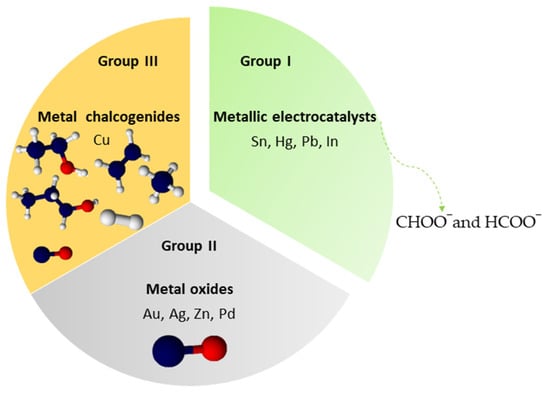
Figure 1. Classification of metal electrodes with their respective products.
To implement practical applications in the electrochemical reduction of CO2, it is necessary to develop electrocatalysts efficient in electron transfer that operate close to the thermodynamic potential of the reaction to be conducted. Therefore, it is essential to choose a catalyst that contributes to accelerating the reaction of interest as well as the supporting electrolyte that favors the processes [19][20].
Electrocatalysts can be classified into three types, such as metallic, non-metallic, and molecular. The materials used in the preparation of the catalysts are what designate their characteristics. Therefore, metallic catalysts can have a metal in their composition or a combination of two metals, termed monometallic or bimetallic. For non-metallic catalysts, the most commonly used materials are carbon nanofiber, nitrogen-doped carbon, metal-organic structure, known as MOF, and covalent-organic structure, Covalent-Organic Framework (COF). On the other hand, molecular catalysts present in their composition the formation of macrocyclic complexes linked to some metals [21][22]. The electrocatalyst material involves the conversion of CO2 into various products, as listed in Table 1.
Table 1. Main electrocatalysts described in the literature with their respective Faradaic efficiency, stability, and final products.
| Electrocatalyst | Electrolyte | Main Product | Faradaic Efficiency | Stability | Ref. |
|---|---|---|---|---|---|
| FeF20TPP/CNT-CF/CC | 0.5 M NaHCO3 | HCOOH | 95% | 50 h | [23] |
| CuSn-4 | 0.5 M KHCO3 | HCOOH | 93.7% | - | [24] |
| Ni@HNC | 0.1 M KHCO3 | CO | 98.7% | - | [25] |
| Ag-NP | 2 M KOH | CO | 99.9% | - | [26] |
| Cu-polyamine | 1 M KOH | C2H4 | 72% | 3 h | [27] |
| Cu/PTFE | 0.1M KHCO3 | C2+ | 80% | 24 h | [28] |
| Cu-12 | 0.1M KHCO3 | C2H4 | 64% | 190 h | [29] |
| Cu/Ni(OH) | 0.5 M NaHCO3 | CO | 92% | 22 h | [30] |
| 4H/fcc Au-MMT | 1.0 M KHCO3 | HCOOH | 92.3% | 12 h | [31] |
| CNT@mC/Ni | 0.5 M KHCO3 | CO | 98% | 24 h | [32] |
| Ag75/C | 1 M KOH | CO, H2, and HCOOH | 90.1% | 30 h | [33] |
| Pd/PdOx | 0.5 M KHCO3 | CO | 90% | 24 h | [34] |
| ZnO | 1 M KOH | CO | 91.6% | 18 h | [35] |
| Ni-N2-C | 0.5 M KHCO3 | HCOOH | 98% | 10 h | [36] |
| Bi-N4 | 0.1 M NaHCO3 | CO | 97% | 4 h | [37] |
| CuNNs | 5 M NaOH | C2H4 | 52% | 6 h | [38] |
| Sn | 0.1 M Na2SO4 | HCOOH | 95% | 10 h | [39] |
| Cu–In | 0.1 M KHCO3 | CO | 55% | 24 h | [40] |
| CuO–Sn | 0.1 M KHCO3 | CO | 90% | 14 h | [41] |
| Cu nanowire | 0.1 M KHCO3 | CO, HCOOH, C2H4 | 17.5% | 5 h | [42] |
| Cu (dendrite) | [EMIM](BF4)/H2O (85/15 v/v) | HCOOH | 87% | 8 h | [43] |
| Ag | 0.5 M KHCO3 | CO | 30–80% | 285 min | [44] |
| Sn | 0.5 M KHCO3 + 2 M KCl | HCOOH | 70% | 4 h | [45] |
| Cu-based metal–organic porous materials | 0.5 M KHCO3 | CH3OH, C2H5OH | 56% | 90 min | [46] |
| Cu2O/ZnO | 0.5 M KHCO3 | CH4 C2H4 | 31.4% | 90 min | [47] |
Analyzing the work presented in Table 1, it can be observed that several types of electrocatalysts enable the reduction of CO2 with high efficiency. Furthermore, it is noted that the most enterprising venture is metallic [22][48], with copper being used. This factor is related to its characteristic of continuing the reduction of CO2 into aldehydes, hydrocarbons, and alcohol with high efficiency [49].
In previous publications regarding CO2, Hori et al. studied the intermediate products by reducing CO at Cu electrodes, which is subsequently reduced to hydrocarbons and alcohols. CO is also formed on Ni and Pt electrodes and subsequently adsorbed on the electrode. And in this way, the adsorbed CO prevents further reduction of CO2 in Ni and Pt. These points lead the metal electrodes to be classified into two groups: CO formation metals (Cu, Au, Ag, Zn, Pd, Ga, Ni, and Pt) and HCOO− (Pb, Hg, In, Sn, Cd, and Tl) [50][51][52].
In 2019, Zhou and collaborators [53] highlighted the need to recycle CO2 in the atmosphere due to the greenhouse effect and the energy crisis. It addresses electrocatalytic CO2 reduction as a viable solution but highlights the challenge of developing electrocatalysts with high activity, selectivity, and durability for this reaction. Explores recent advances in nanostructures of different dimensions as promising catalysts to accelerate CO2 conversion, discussing the challenges and prospects for achieving high efficiency in this process.
Moreover, in 2020, Tang and collaborators [54] discussed the carbon dioxide reduction electrochemistry along transition metals, addressing a complex network of reactions. The research combines experimental observations from the literature with theoretical analysis to explain that not all intermediates in CO2 reduction are formed by direct protonation steps. A selectivity map for two-electron products (carbon monoxide and formate) on pure metal surfaces is derived, using only the CO and OH binding energies as descriptors. The analysis rationalizes the experimentally observed product distributions in CO2RE in pure metal systems, highlighting the need for additional descriptors for screening materials in CO2 reduction and the importance of considering the competition in the elementary steps of the hydrogen evolution reaction.
Johnson and collaborators [55] highlighted the significant role of carbon dioxide in global warming, with the burning of fossil fuels being the main source of pollution. The capture and reduction of CO2 for the production of valuable chemicals using renewable energy sources is an important area of research. Catalysts, support structures, and electrolytes are key factors affecting the electrochemistry of CO2 reduction for the production of value-added chemicals and fuels. The research covers the field of CO2RE electrocatalysis, focusing on non-precious transition metal-based catalysts and highlighting design, synthesis, characterization, and mechanisms. Advanced catalyst design, including two-dimensional metal carbide and nitride materials, and state-of-the-art in situ/operando spectroelectrochemical techniques are emphasized. The text concludes by highlighting the remaining challenges and perspectives for future research and opportunities.
In 2022, Jiang and collaborators [56] highlighted the electrochemistry of CO reduction as a promising approach to address the energy crisis and reduce carbon emissions. Cu-based electrocatalysts have been considered to generate hydrocarbons and alcohols in CO2RE, but face challenges of high initial potential and low selectivity. The research proposes a series of Cu-based single-atom alloy catalysts (SAACs), TM1/Cu (111), designed by isolated doping of transition metal (TM) atoms on the Cu (111) surface. TM1/Cu (111) demonstrated, theoretically, greater stability and efficiency in the hydrogenation of CO2, avoiding hydrogen evolution. Theoretical calculations suggest that the initial hydrogenation of CO2 in SAACs would form the *CO intermediate, which could be subsequently hydrogenated to produce methane. The bond angle of adsorbed *CO2 and the binding energy of *OH were identified as important descriptors of the activity and activation capacity of TM1/Cu (111) in CO2RE. It is speculated that V/Cu (111) may present the best activity and selectivity among all TM1/Cu doped with 3d-TM (111). The research provides rational guidance for the efficient design of new single-atom catalysts for CO2RE.
Clark and collaborators [57] address electrosynthesis, such as CO2 reduction, which involves the formation of dipolar and polarizable transition states during the rate determination step. Highlights the need for systematic and independent control of surface reactivity and electric field strength to accelerate the discovery of highly active electrocatalysts. The research shows that intermetallic alloys allow independent control over the d-band energetics and work function by varying the alloy composition and the identity of the oxophilic constituent. Identifies intermetallic phases with the potential for greater intrinsic activity in CO reduction compared to conventional Cu-based electrocatalysts. However, it highlights the propensity of these alloys to segregate in the air as a significant challenge for investigating their electrocatalytic activity.
References
- Larkum, A.; Orth, R.J.; Duarte, C. Seagrasses: Biology, Ecology and Conservation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 140202942X.
- Jones, A.M. Environmental Biology; Routledge: London, UK, 2006; ISBN 9781134777662.
- Grace, J. Understanding and Managing the Global Carbon Cycle. J. Ecol. 2004, 92, 189–202.
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The Teraton Challenge. A Review of Fixation and Transformation of Carbon Dioxide. Energy Environ. Sci. 2010, 3, 43–81.
- Pacheco, M.R.P.D.S.; Helene, E.M. Atmosphere, carbon fluxes, and CO2 fertilization. Estud. Avançados 1990, 4, 204–220. (In Portuguese)
- Benson, S.M.; Orr, F.M., Jr. Carbon Dioxide Capture and Storage. MRS Bull. 2008, 33, 303–305.
- Chen, P.; Zhang, Y.; Zhou, Y.; Dong, F. Photoelectrocatalytic Carbon Dioxide Reduction: Fundamental, Advances and Challenges. Nano Mater. Sci. 2021, 3, 344–367.
- Simões, L.A.A.; Rodrigues, S.J.; Araujo, H.M.; Vieira, S.S. The Chemistry Involved in CO2 Conversion: Challenges and Opportunities. Rev. Virtual Quim. 2022, 14, 468–483.
- Albo, J.; Alvarez-Guerra, M.; Castaño, P.; Irabien, A. Towards the Electrochemical Conversion of Carbon Dioxide into Methanol. Green Chem. 2015, 17, 2304–2324.
- Nguyen, D.L.T.; Kim, Y.; Hwang, Y.J.; Won, D.H. Progress in Development of Electrocatalyst for CO2 Conversion to Selective CO Production. Carbon Energy 2019, 2, 72–98.
- Bevilacqua, M.; Filippi, J.; Miller, H.A.; Vizza, F. Recent Technological Progress in CO2 Electroreduction to Fuels and Energy Carriers in Aqueous Environments. Energy Technol. 2015, 3, 197–210.
- Birhanu, M.K.; Tsai, M.; Kahsay, A.W.; Chen, C.; Zeleke, T.S.; Ibrahim, K.B.; Huang, C.; Su, W.; Hwang, B. Copper and Copper-Based Bimetallic Catalysts for Carbon Dioxide Electroreduction. Adv. Mater. Interfaces 2018, 5, 1800919.
- Ishiki, N.A.; Lima, F.H.B.; Ticianelli, E. AElectrochemical CO2 Reduction: Remaking Our Carbon Footprints. Quim. Nova Esc. 2023, 45, 109–116. (In Portuguese)
- Raciti, D.; Wang, C. Recent Advances in CO2 Reduction Electrocatalysis on Copper. Energy Lett. 2018, 3, 1545–1556.
- El Aisnada, A.N.; Miyauchi, M.; Liu, M.; Yamaguchi, A. Recent Update on Electrochemical CO2 Reduction Catalyzed by Metal Sulfide Materials. Mater. Rep. Energy 2023, 3, 100190.
- Santos, E.; Nazmutdinov, R.; Schmickler, W. Electron Transfer at Different Electrode Materials: Metals, Semiconductors, and Graphene. Curr. Opin. Electrochem. 2020, 19, 106–112.
- Amos, P.I.; Louis, H.; Adegoke, K.A.; Eno, E.A.; Udochukwu, A.O.; Magub, T.O. Understanding the Mechanism of Electrochemical Reduction of CO2 Using Cu/Cu-Based Electrodes: A Review. Asian J. Nanosci. Mater 2022, 4, 252–293.
- Wang, G.; Chen, J.; Ding, Y.; Cai, P.; Yi, L.; Li, Y.; Tu, C.; Hou, Y.; Wen, Z.; Dai, L. Electrocatalysis for CO2 conversion: From Fundamentals to Value-Added Products. Chem. Soc. Rev. 2021, 50, 4993–5061.
- Martens, J.A.; Bogaerts, A.; De Kimpe, N.; Jacobs, P.A.; Marin, G.B.; Rabaey, K.; Saeys, M.; Verhelst, S. The Chemical Route to a Carbon Dioxide Neutral World. ChemSusChem 2017, 10, 1039–1055.
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452.
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and Homogeneous Approaches to Conversion of CO2 to Liquid Fuels. Chem. Soc. Rev. 2009, 38, 89–99.
- Long, C.; Li, X.; Guo, J.; Shi, Y.; Liu, S.; Tang, Z. Electrochemical Reduction of CO2 over Heterogeneous Catalysts in Aqueous Solution: Recent Progress and Perspectives. Small Methods 2019, 3, 1800369.
- Lu, X.; Ahsaine, H.A.; Dereli, B.; Garcia-Esparza, A.T.; Reinhard, M.; Shinagawa, T.; Li, D.; Adil, K.; Tchalala, M.R.; Kroll, T.; et al. Operando Elucidation on the Working State of Immobilized Fluorinated Iron Porphyrin for Selective Aqueous Electroreduction of CO2 to CO. Catalysis 2021, 11, 6499–6509.
- Li, Y.; Huang, G.; Chen, Z.; Xiong, Y.; Huang, Q.; Xu, X.; Huo, Z. Effects of Irrigation and Fertilization on Grain Yield, Water and Nitrogen Dynamics and Their Use Efficiency of Spring Wheat Farmland in an Arid Agricultural Watershed of Northwest China. Agric. Water Manag. 2022, 260, 107277.
- Zhou, L.; Qu, Z.; Fu, L. Rational Design of Hollow Nitrogen-Doped Carbon Supported Nickel Nanoparticles for Efficient Electrocatalytic CO2 Reduction. J. Environ. Chem. Eng. 2023, 11, 109427.
- Verma, S.; Lu, S.; Kenis, P.J.A. Co-Electrolysis of CO2 and Glycerol as a Pathway to Carbon Chemicals with Improved Technoeconomics Due to Low Electricity Consumption. Nat. Energy 2019, 4, 466–474.
- Chen, X.; Chen, J.; Alghoraibi, N.M.; Henckel, D.A.; Zhang, R.; Nwabara, U.O.; Madsen, K.E.; Kenis, P.J.A.; Zimmerman, S.C.; Gewirth, A.A. Electrochemical CO2-to-Ethylene Conversion on Polyamine-Incorporated Cu Electrodes. Nat. Catal. 2021, 4, 20–27.
- Gabardo, C.M.; O’Brien, C.P.; Edwards, J.P.; McCallum, C.; Xu, Y.; Dinh, C.T.; Li, J.; Sargent, E.H.; Sinton, D. Continuous Carbon Dioxide Electroreduction to Concentrated Multi-Carbon Products Using a Membrane Electrode Assembly. Joule 2019, 3, 2777–2791.
- Li, F.; Thevenon, A.; Rosas-Hernández, A.; Wang, Z.; Li, Y.; Gabardo, C.M.; Ozden, A.; Dinh, C.T.; Li, J.; Wang, Y.; et al. Molecular Tuning of CO2-to-Ethylene Conversion. Nature 2020, 577, 509–513.
- Dai, L.; Qin, Q.; Wang, P.; Zhao, X.; Hu, C.; Liu, P.; Qin, R.; Chen, M.; Ou, D.; Xu, C.; et al. Ultrastable Atomic Copper Nanosheets for Selective Electrochemical Reduction of Carbon Dioxide. Sci. Adv. 2017, 3, e1701069.
- Wang, J.; Yu, J.; Sun, M.; Liao, L.; Zhang, Q.; Zhai, L.; Zhou, X.; Li, L.; Wang, G.; Meng, F.; et al. Surface Molecular Functionalization of Unusual Phase Metal Nanomaterials for Highly Efficient Electrochemical Carbon Dioxide Reduction under Industry-Relevant Current Density. Small 2022, 18, e2106766.
- Du, J.; Chen, A.; Hou, S.; Guan, J. CNT Modified by Mesoporous Carbon Anchored by Ni Nanoparticles for CO2 Electrochemical Reduction. Carbon Energy 2022, 4, 1274–1284.
- Hong, J.; Park, K.T.; Kim, Y.E.; Tan, D.; Jeon, Y.E.; Park, J.E.; Youn, M.H.; Jeong, S.K.; Park, J.; Ko, Y.N.; et al. Ag/C Composite Catalysts Derived from Spray Pyrolysis for Efficient Electrochemical CO2 Reduction. Chem. Eng. J. 2022, 431, 133384.
- Lu, H.; Zhang, L.; Zhong, J.H.; Yang, H.G. Partially Oxidized Palladium Nanodots for Enhanced Electrocatalytic Carbon Dioxide Reduction. Chem. Asian J. 2018, 13, 2800–2804.
- Luo, W.; Zhang, Q.; Zhang, J.; Moioli, E.; Zhao, K.; Züttel, A. Electrochemical Reconstruction of ZnO for Selective Reduction of CO2 to CO. Appl. Catal. B 2020, 273, 119060.
- Gong, Y.N.; Jiao, L.; Qian, Y.; Pan, C.Y.; Zheng, L.; Cai, X.; Liu, B.; Yu, S.H.; Jiang, H.L. Regulating the Coordination Environment of MOF-Templated Single-Atom Nickel Electrocatalysts for Boosting CO2 Reduction. Angew. Chem.-Int. Ed. 2020, 59, 2705–2709.
- Zhang, E.; Wang, T.; Yu, K.; Liu, J.; Chen, W.; Li, A.; Rong, H.; Lin, R.; Ji, S.; Zheng, X.; et al. Bismuth Single Atoms Resulting from Transformation of Metal-Organic Frameworks and Their Use as Electrocatalysts for CO2 Reduction. J. Am. Chem. Soc. 2019, 141, 16569–16573.
- Xiang, K.; Zhu, F.; Liu, Y.; Pan, Y.; Wang, X.; Yan, X.; Liu, H. A Strategy to Eliminate Carbon Deposition on a Copper Electrode in Order to Enhance Its Stability in CO2RR Catalysis by Introducing Crystal Defects. Electrochem. Commun. 2019, 102, 72–77.
- Wu, J.; Risalvato, F.G.; Ke, F.-S.; Pellechia, P.J.; Zhou, X.-D. Electrochemical Reduction of Carbon Dioxide I. Effects of the Electrolyte on the Selectivity and Activity with Sn Electrode. J. Electrochem. Soc. 2012, 159, F353–F359.
- Larrazábal, G.O.; Martín, A.J.; Mitchell, S.; Hauert, R.; Pérez-Ramírez, J. Enhanced Reduction of CO2 to CO over Cu-In Electrocatalysts: Catalyst Evolution Is the Key. ACS Catal. 2016, 6, 6265–6274.
- Sarfraz, S.; Garcia-Esparza, A.T.; Jedidi, A.; Cavallo, L.; Takanabe, K. Cu-Sn Bimetallic Catalyst for Selective Aqueous Electroreduction of CO2 to CO. ACS Catal. 2016, 6, 2842–2851.
- Ma, M.; Djanashvili, K.; Smith, W.A. Controllable Hydrocarbon Formation from the Electrochemical Reduction of CO2 over Cu Nanowire Arrays. Angew. Chem. 2016, 128, 6792–6796.
- Huan, T.N.; Simon, P.; Rousse, G.; Génois, I.; Artero, V.; Fontecave, M. Porous Dendritic Copper: An Electrocatalyst for Highly Selective CO2 Reduction to Formate in Water/Ionic Liquid Electrolyte. Chem. Sci. 2016, 8, 742–747.
- Delacourt, C.; Ridgway, P.L.; Kerr, J.B.; Newman, J. Design of an Electrochemical Cell Making Syngas (CO + H2) from CO2 and H2O Reduction at Room Temperature. J. Electrochem. Soc. 2008, 155, B42.
- Oloman, C.; Li, H. Electrochemical Processing of Carbon Dioxide. ChemSusChem 2008, 1, 385–391.
- Albo, J.; Vallejo, D.; Beobide, G.; Castillo, O.; Castaño, P.; Irabien, A. Copper-Based Metal–Organic Porous Materials for CO2 Electrocatalytic Reduction to Alcohols. ChemSusChem 2017, 10, 1100–1109.
- Albo, J.; Irabien, A. Cu2O-Loaded Gas Diffusion Electrodes for the Continuous Electrochemical Reduction of CO2 to Methanol. J. Catal. 2016, 343, 232–239.
- Gu, Z.; Shen, H.; Shang, L.; Lv, X.; Qian, L.; Zheng, G. Nanostructured Copper-Based Electrocatalysts for CO2 Reduction. Small Methods 2018, 2, 1800121.
- Vasileff, A.; Xu, C.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Surface and Interface Engineering in Copper-Based Bimetallic Materials for Selective CO2 Electroreduction. Chem 2018, 4, 1809–1831.
- Hori, Y.; Murata, A.; Takahashi, R. Formation of Hydrocarbons in the Electrochemical Reduction of Carbon Dioxide at a Copper Electrode in Aqueous Solution. J. Chem. Soc. Faraday Trans. 1989, 85, 2309.
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic Process of CO Selectivity in Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Media. Electrochim. Acta 1994, 39, 1833–1839.
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Springer: New York, NY, USA, 2008; pp. 89–189.
- Zhou, H.; Liu, K.; Li, H.; Cao, M.; Fu, J.; Gao, X.; Hu, J.; Li, W.; Pan, H.; Zhan, J.; et al. Recent Advances in Different-Dimension Electrocatalysts for Carbon Dioxide Reduction. J. Colloid Interface Sci. 2019, 550, 17–47.
- Tang, M.T.; Peng, H.; Lamoureux, P.S.; Bajdich, M.; Abild-Pedersen, F. From Electricity to Fuels: Descriptors for C1 Selectivity in Electrochemical CO2 Reduction. Appl. Catal. B Environ. 2020, 279, 119384.
- Johnson, D.; Qiao, Z.; Djire, A. Progress and Challenges of Carbon Dioxide Reduction Reaction on Transition Metal Based Electrocatalysts. ACS Appl. Energy Mater. 2021, 4, 8661–8684.
- Jiang, J.-C.; Chen, J.-C.; Zhao, M.; Yu, Q.; Wang, Y.-G.; Li, J. Rational Design of Copper-Based Single-Atom Alloy Catalysts for Electrochemical CO2 Reduction. Nano Res. 2022, 15, 7116–7123.
- Clark, E.L.; Nielsen, R.; Sørensen, J.E.; Needham, J.L.; Seger, B.; Chorkendorff, I. Tuning Surface Reactivity and Electric Field Strength via Intermetallic Alloying. ACS Energy Lett. 2023, 8, 4414–4420.
More
Information
Subjects:
Electrochemistry
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
836
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
07 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




